Differences in Effects of Length-Dependent Regulation of Force and Ca2+ Transient in the Myocardial Trabeculae of the Rat Right Atrium and Ventricle
Abstract
:1. Introduction
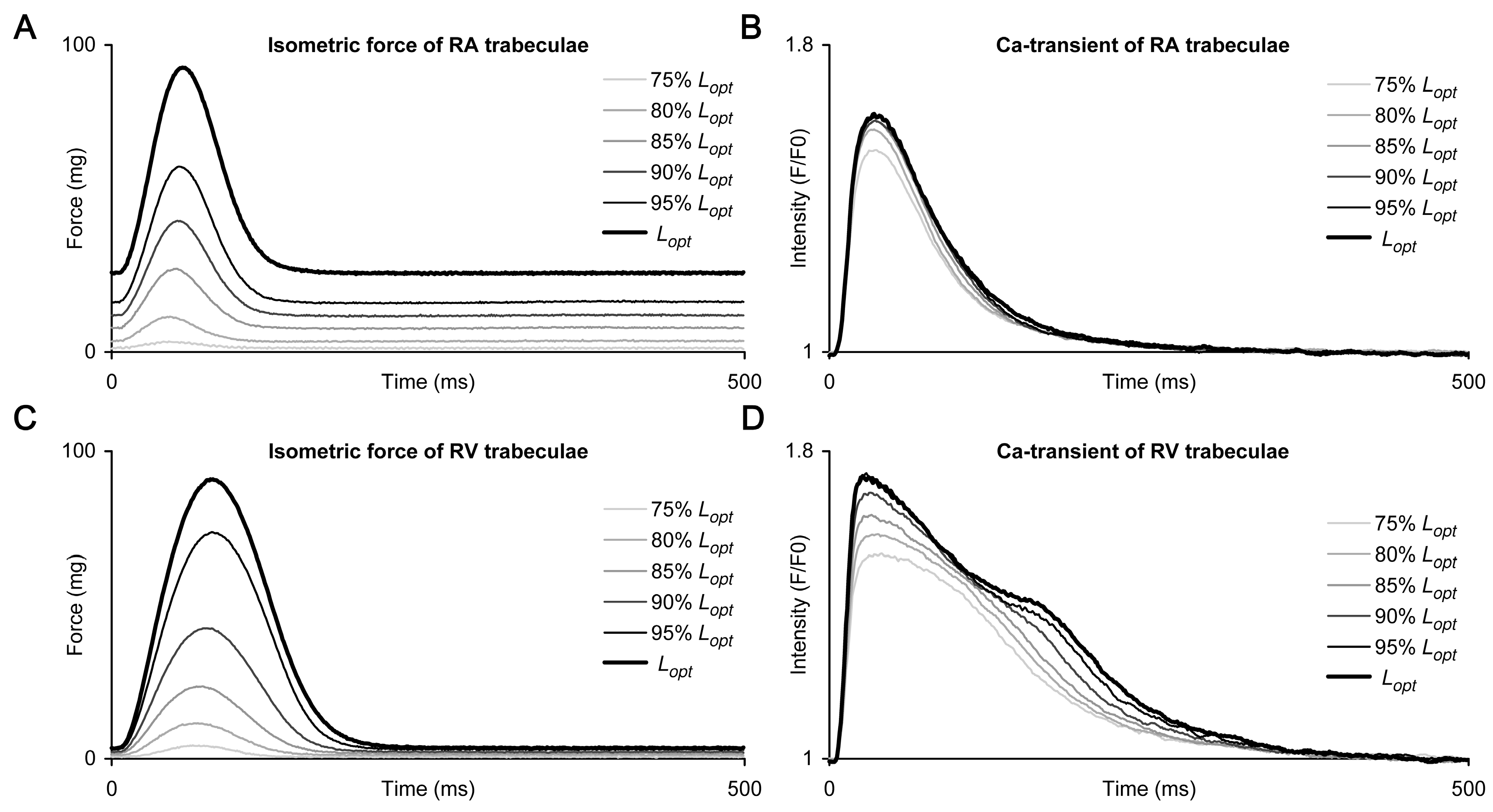
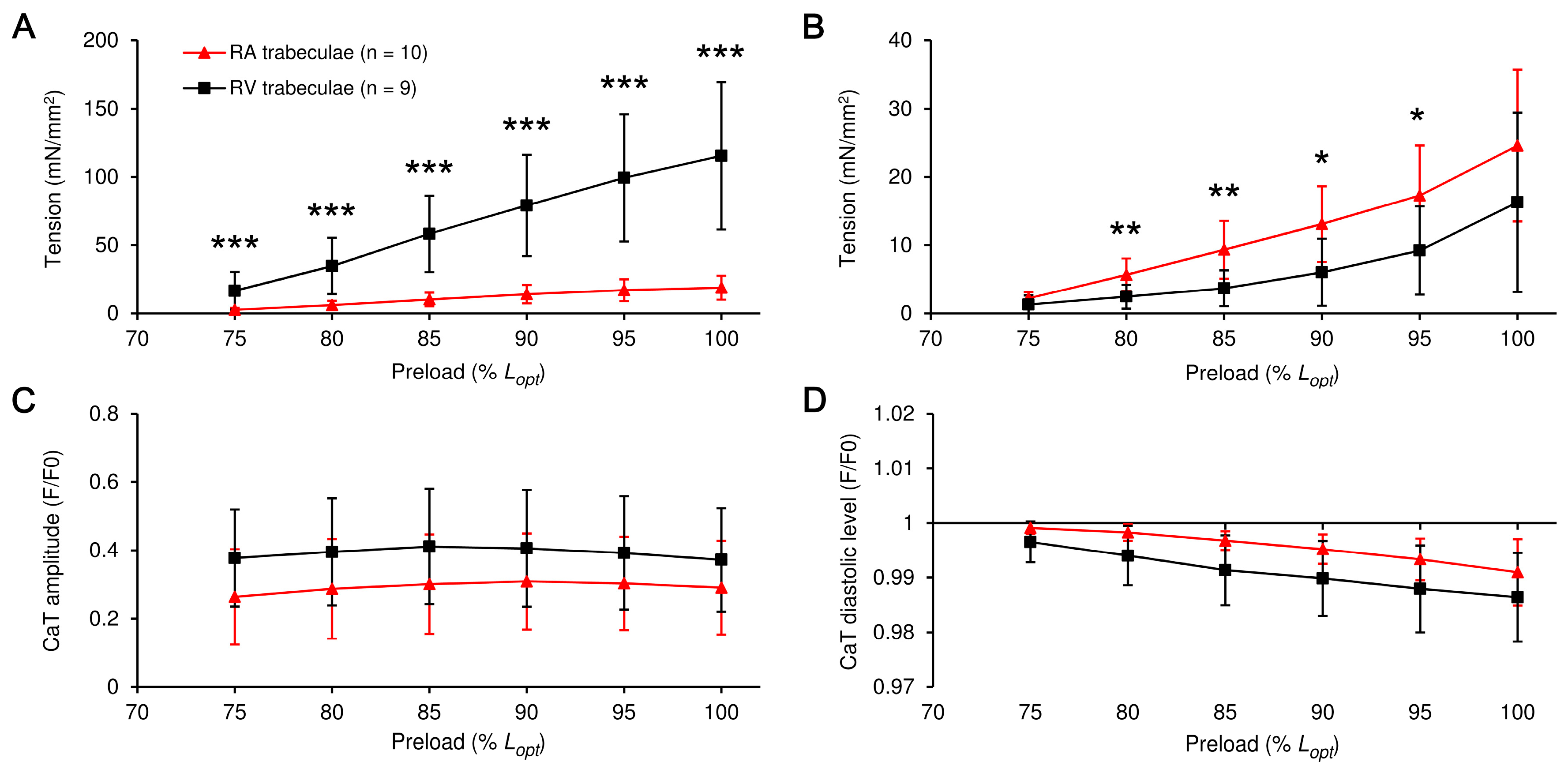
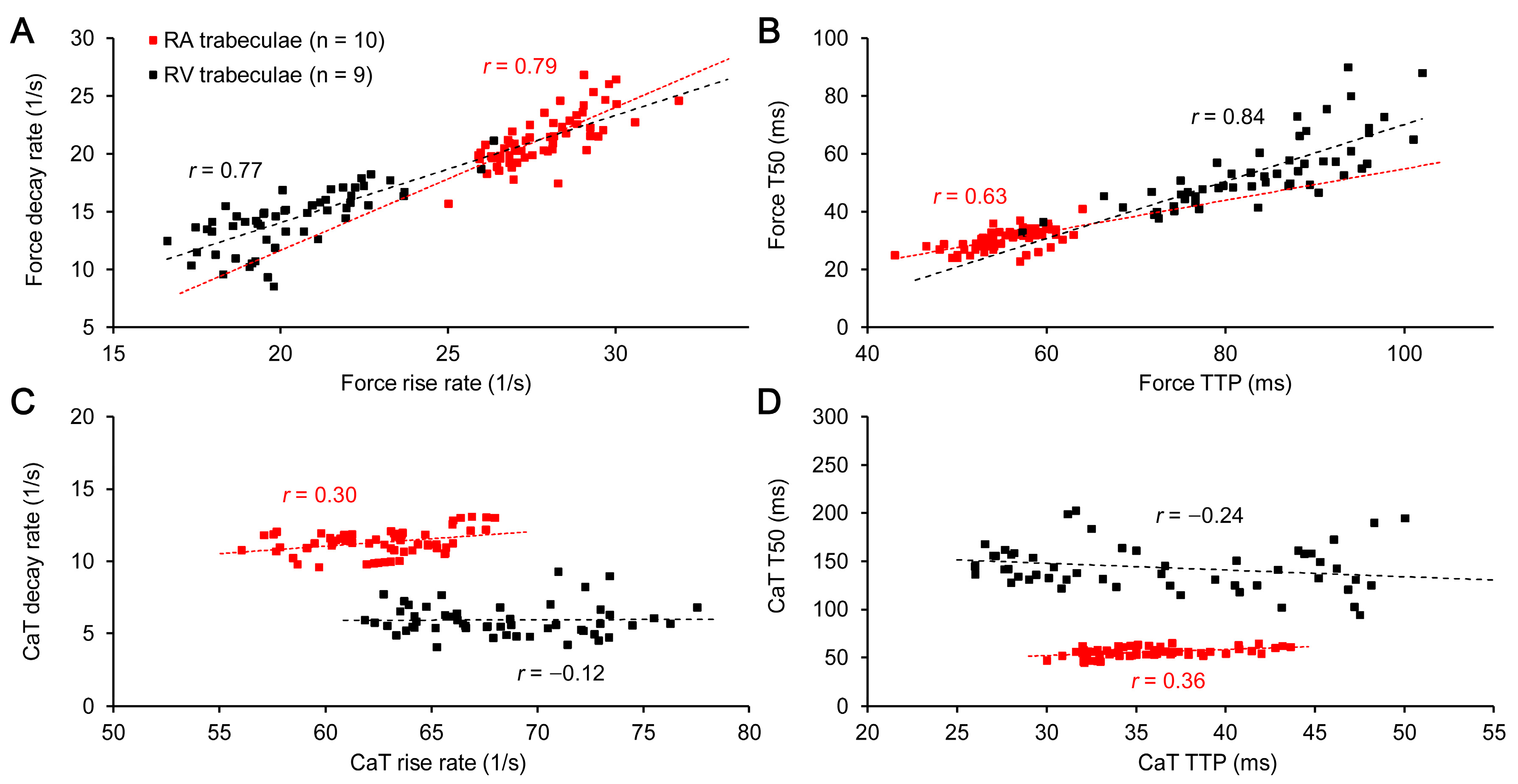
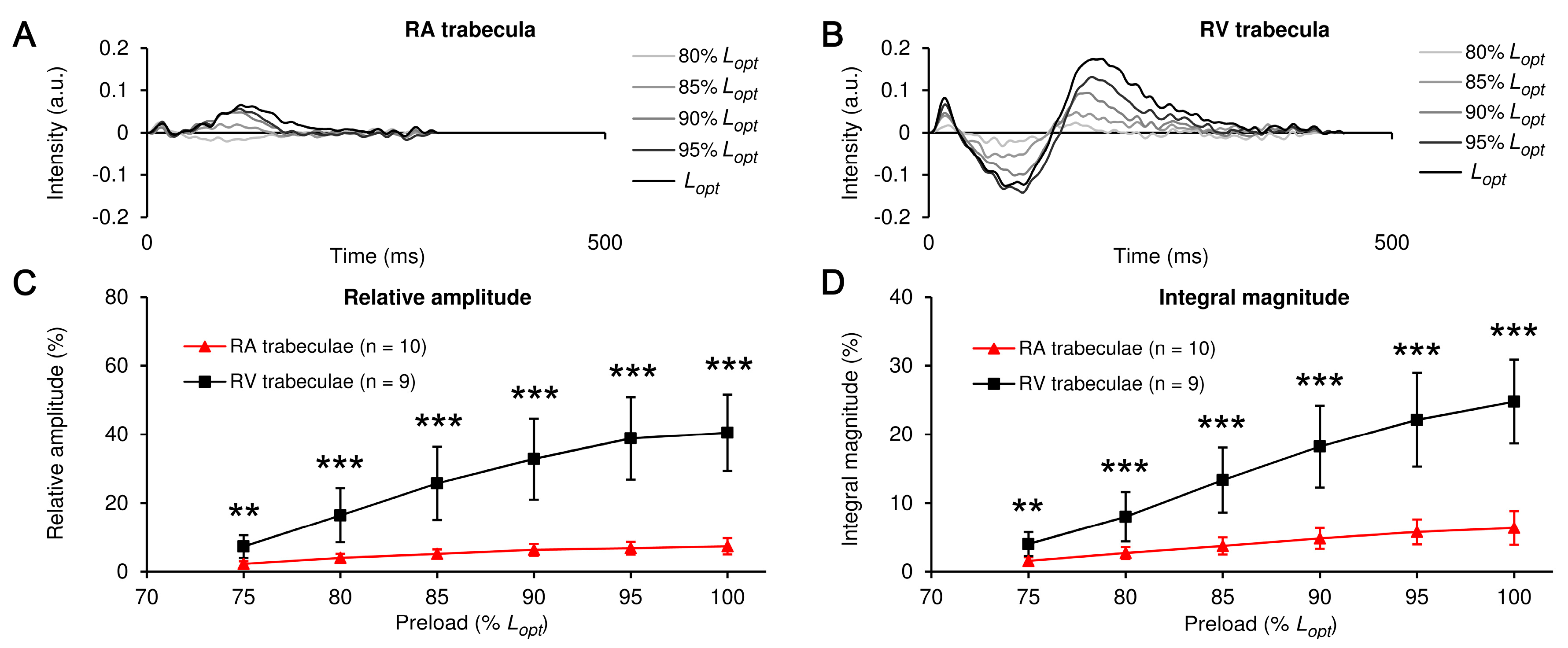
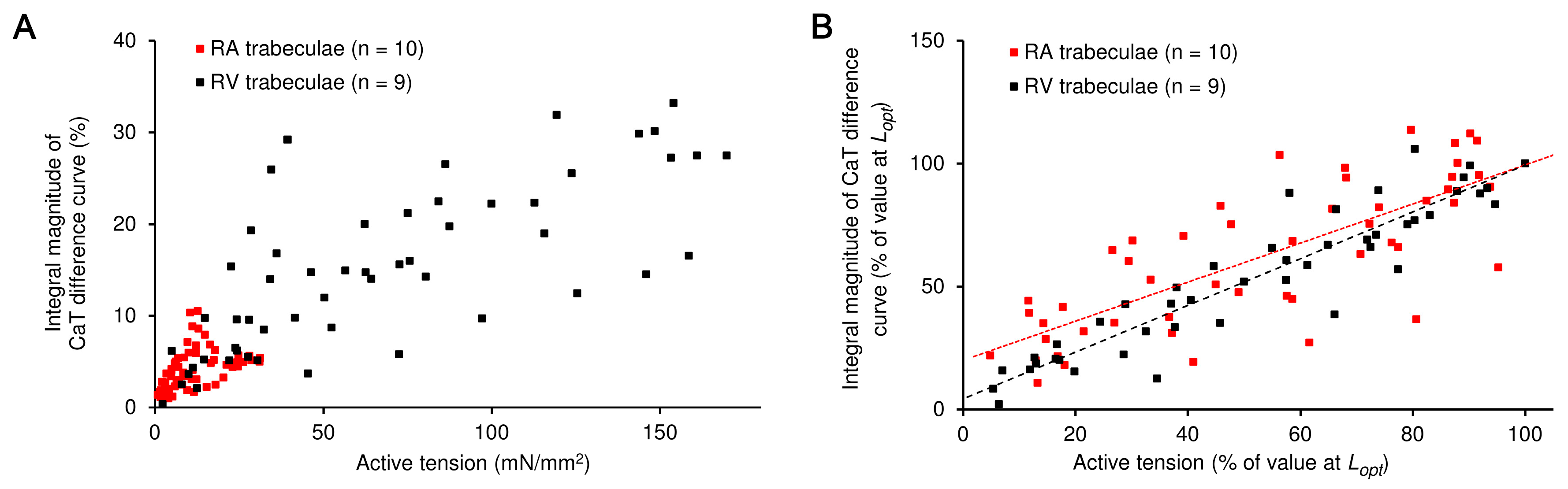
2. Results
3. Discussion
3.1. RA vs. RV: The Preload-Induced Effects on the Isometric Twitch Characteristics and Frank-Starling Mechanism
3.2. RA vs. RV: The Preload-Induced Effects on the Characteristics of Ca2+ Transient and Frank-Starling Mechanism
4. Materials and Methods
4.1. Ethical Approval
4.2. Muscle Preparations
4.3. Data Acquisition
4.4. Force-Length Protocol
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korte, F.S.; McDonald, K.S. Sarcomere Length Dependence of Rat Skinned Cardiac Myocyte Mechanical Properties: Dependence on Myosin Heavy Chain. J. Physiol. 2007, 581, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.M.; Geleijnse, M.L.; Soliman, O.I.; Nemes, A.; ten Cate, F.J. Left Atrial Frank–Starling Law Assessed by Real-Time, Three-Dimensional Echocardiographic Left Atrial Volume Changes. Heart 2007, 93, 1393–1397. [Google Scholar] [CrossRef] [PubMed]
- Rosca, M.; Lancellotti, P.; Popescu, B.A.; Pierard, L. Left Atrial Function: Pathophysiology, Echocardiographic Assessment, and Clinical Applications. Heart 2011, 97, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Walklate, J.; Ferrantini, C.; Johnson, C.A.; Tesi, C.; Poggesi, C.; Geeves, M.A. Alpha and Beta Myosin Isoforms and Human Atrial and Ventricular Contraction. Cell. Mol. Life Sci. 2021, 78, 7309–7337. [Google Scholar] [CrossRef]
- Wang, J.; Schwinger, R.H.G.; Frank, K.; Müller-Ehmsen, J.; Martín-Vasallo, P.; Pressley, T.; Xiang, A.; Erdmann, E.; McDonough, A. Regional Expression of Sodium Pump Subunit Isoforms and Na+-Ca++ Exchanger in the Human Heart. J. Clin. Investig. 1996, 98, 1650–1658. [Google Scholar] [CrossRef]
- Lüss, I.; Boknik, P.; Jones, L.R.; Kirchhefer, U.; Knapp, J.; Linck, B.; Lüss, H.; Meissner, A.; Müller, F.U.; Schmitz, W.; et al. Expression of Cardiac Calcium Regulatory Proteins in Atrium v Ventricle in Different Species. J. Mol. Cell. Cardiol. 1999, 31, 1299–1314. [Google Scholar] [CrossRef]
- Schram, G.; Pourrier, M.; Melnyk, P.; Nattel, S. Differential Distribution of Cardiac Ion Channel Expression as a Basis for Regional Specialization in Electrical Function. Circ. Res. 2002, 90, 939–950. [Google Scholar] [CrossRef]
- Walden, A.P.; Dibb, K.M.; Trafford, A.W. Differences in Intracellular Calcium Homeostasis between Atrial and Ventricular Myocytes. J. Mol. Cell. Cardiol. 2009, 46, 463–473. [Google Scholar] [CrossRef]
- Ng, S.; Wong, C.; Tsang, S. Differential Gene Expressions in Atrial and Ventricular Myocytes: Insights into the Road of Applying Embryonic Stem Cell-Derived Cardiomyocytes for Future Therapies. Am. J. Physiol-Cell. Physiol. 2010, 299, C1234–C1249. [Google Scholar] [CrossRef]
- Nollet, E.; Manders, E.; Goebel, M.; Jansen, V.; Brockmann, C.; Osinga, J.; van der Velden, J.; Helmes, M.; Kuster, D. Large-Scale Contractility Measurements Reveal Large Atrioventricular and Subtle Interventricular Differences in Cultured Unloaded Rat Cardiomyocytes. Front. Physiol. 2020, 11, 815. [Google Scholar] [CrossRef]
- Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Conway, S.J.; Bootman, M.D. The Spatial Pattern of Atrial Cardiomyocyte Calcium Signalling Modulates Contraction. J. Cell. Sci. 2004, 117, 6327–6337. [Google Scholar] [CrossRef]
- Smyrnias, I.; Mair, W.; Wachten, D.; Walker, S.; Roderick, H.; Bootman, M. Comparison of the T-Tubule System in Adult Rat Ventricular and Atrial Myocytes, and Its Role in Excitation–Contraction Coupling and Inotropic Stimulation. Cell. Calcium 2010, 47, 210–223. [Google Scholar] [CrossRef]
- Ferrantini, C.; Crocini, C.; Coppini, R.; Vanzi, F.; Tesi, C.; Cerbai, E.; Poggesi, C.; Pavone, F.; Sacconi, L. The Transverse-Axial Tubular System of Cardiomyocytes. Cell. Mol. Life Sci. CMLS 2013, 70, 4695–4710. [Google Scholar] [CrossRef]
- Yue, X.; Zhang, R.; Kim, B.; Ma, A.; Philipson, K. Heterogeneity of Transverse-Axial Tubule System in Mouse Atria: Remodeling in Atrial-Specific Na(+)-Ca(2+) Exchanger Knockout Mice. J. Mol. Cell. Cardiol. 2017, 108, 50–60. [Google Scholar] [CrossRef]
- Wagner, E.; Brandenburg, S.; Kohl, T.; Lehnart, S. Analysis of Tubular Membrane Networks in Cardiac Myocytes from Atria and Ventricles. J. Vis. Exp. JoVE 2014, 92, e51823. [Google Scholar] [CrossRef]
- Kanaporis, G.; Blatter, L.A. Alternans in Atria: Mechanisms and Clinical Relevance. Medicina 2017, 53, 139–149. [Google Scholar] [CrossRef]
- Marchena, M.; Echebarria, B. Influence of the Tubular Network on the Characteristics of Calcium Transients in Cardiac Myocytes. PLoS ONE 2020, 15, e0231056. [Google Scholar] [CrossRef]
- Berlin, J.R. Spatiotemporal Changes of Ca2+ during Electrically Evoked Contractions in Atrial and Ventricular Cells. Am. J. Physiol.-Heart Circ. Physiol. 1995, 269, H1165–H1170. [Google Scholar] [CrossRef]
- Fearnley, C.J.; Roderick, H.L.; Bootman, M.D. Calcium Signaling in Cardiac Myocytes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004242. [Google Scholar] [CrossRef]
- Díaz, M.E.; Trafford, A.W.; Eisner, D.A. The Role of Intracellular Ca Buffers in Determining the Shape of the Systolic Ca Transient in Cardiac Ventricular Myocytes. Pflügers Arch. 2001, 442, 96–100. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium Cycling and Signaling in Cardiac Myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Kreutziger, K.L.; Piroddi, N.; McMichael, J.T.; Tesi, C.; Poggesi, C.; Regnier, M. Calcium Binding Kinetics of Troponin C Strongly Modulate Cooperative Activation and Tension Kinetics in Cardiac Muscle. J. Mol. Cell. Cardiol. 2011, 50, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.E.; Trafford, A.W.; Eisner, D.A. The Effects of Exogenous Calcium Buffers on the Systolic Calcium Transient in Rat Ventricular Myocytes. Biophys. J. 2001, 80, 1915–1925. [Google Scholar] [CrossRef] [PubMed]
- Ferrantini, C.; Coppini, R.; Sacconi, L.; Tosi, B.; Zhang, M.L.; Wang, G.L.; de Vries, E.; Hoppenbrouwers, E.; Pavone, F.; Cerbai, E. Impact of Detubulation on Force and Kinetics of Cardiac Muscle Contraction. J. Gen. Physiol. 2014, 143, 783–797. [Google Scholar] [CrossRef]
- Kobirumaki-Shimozawa, F.; Inoue, T.; Shintani, S.A.; Oyama, K.; Terui, T.; Minamisawa, S.; Ishiwata, S.; Fukuda, N. Cardiac Thin Filament Regulation and the Frank–Starling Mechanism. J. Physiol. Sci. 2014, 64, 221–232. [Google Scholar] [CrossRef]
- Lookin, O. The Use of Ca-Transient to Evaluate Ca2+ Utilization by Myofilaments in Living Cardiac Muscle. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1824–1833. [Google Scholar] [CrossRef]
- Minajeva, A.; Kaasik, A.; Paju, K.; Seppet, E.N.N.; Lompré, A.-M.; Veksler, V.; Ventura-Clapier, R. Sarcoplasmic Reticulum Function in Determining Atrioventricular Contractile Differences in Rat Heart. Am. J. Physiol. 1997, 273, H2498–H2507. [Google Scholar] [CrossRef]
- Freestone, N.S.; Ribaric, S.; Scheuermann, M.; Mauser, U.; Paul, M.; Vetter, R. Differential Lusitropic Responsiveness to β-Adrenergic Stimulation in Rat Atrial and Ventricular Cardiac Myocytes. Pflügers Arch. 2000, 441, 78–87. [Google Scholar] [CrossRef]
- Marino, P.; Degiovanni, A.; Zanaboni, J. Complex Interaction between the Atrium and the Ventricular Filling Process: The Role of Conduit. Open. Heart 2019, 6, e001042. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Arakawa, M.; Tanaka, T.; Takaya, T.; Nagano, T.; Hirakawa, S. Study on Left Atrial Contractile Performance. Participation of Frank-Starling Mechanism. Jpn. Circ. J. 1987, 51, 1001–1009. [Google Scholar] [CrossRef]
- Kayhan, N.; Bodem, J.P.; Vahl, C.F.; Hagl, S. The Positive Staircase (Force-Frequency Relationship) and the Frank-Starling Mechanism Are Altered in Atrial Myocardium of Patients in End-Stage Heart Failure Transplanted for Dilative Cardiomyopathy. In Transplantation Proceedings; Elsevier Science: New York, NY, USA, 2002; Volume 34, pp. 2185–2191. [Google Scholar]
- Glower, D.D.; Spratt, J.A.; Snow, N.D.; Kabas, J.S.; Davis, J.W.; Olsen, C.O.; Tyson, G.S.; Sabiston, D.C., Jr.; Rankin, J.S. Linearity of the Frank-Starling Relationship in the Intact Heart: The Concept of Preload Recruitable Stroke Work. Circulation 1985, 71, 994–1009. [Google Scholar] [CrossRef]
- Kosta, S.; Dauby, P.C. Frank-Starling Mechanism, Fluid Responsiveness, and Length-Dependent Activation: Unravelling the Multiscale Behaviors with an in Silico Analysis. PLoS Comput. Biol. 2021, 17, e1009469. [Google Scholar] [CrossRef]
- De Tombe, P.P.; Mateja, R.D.; Tachampa, K.; Mou, Y.A.; Farman, G.P.; Irving, T.C. Myofilament Length Dependent Activation. J. Mol. Cell. Cardiol. 2010, 48, 851–858. [Google Scholar] [CrossRef]
- Tavi, P.; Han, C.; Weckström, M. Mechanisms of Stretch-Induced Changes in [Ca2+]i in Rat Atrial Myocytes: Role of Increased Troponin C Affinity and Stretch-Activated Ion Channels. Circ. Res. 1998, 83, 1165–1177. [Google Scholar] [CrossRef]
- Couttenye, M.M.; De Clerck, N.M.; Goethals, M.A.; Brutsaert, D.L. Relaxation Properties of Mammalian Atrial Muscle. Circ. Res. 1981, 48, 352–356. [Google Scholar] [CrossRef]
- Guilbeau-Frugier, C.; Cauquil, M.; Karsenty, C.; Lairez, O.; Dambrin, C.; Payré, B.; Cassard, H.; Josse, C.; Seguelas, M.-H.; Allart, S. Structural Evidence for a New Elaborate 3D-Organization of the Cardiomyocyte Lateral Membrane in Adult Mammalian Cardiac Tissues. Cardiovasc. Res. 2019, 115, 1078–1091. [Google Scholar] [CrossRef]
- Jiang, Y.; Patterson, M.F.; Morgan, D.L.; Julian, F.J. Basis for Late Rise in Fura 2 R Signal Reporting [Ca2+] i during Relaxation in Intact Rat Ventricular Trabeculae. Am. J. Physiol.-Cell. Physiol. 1998, 274, C1273–C1282. [Google Scholar] [CrossRef]
- Lookin, O.; Protsenko, Y. The Lack of Slow Force Response in Failing Rat Myocardium: Role of Stretch-Induced Modulation of Ca–TnC Kinetics. J. Physiol. Sci. 2019, 69, 345–357. [Google Scholar] [CrossRef]
- Koss, K.L.; Grupp, I.L.; Kranias, E.G. The Relative Phospholamban and SERCA2 Ratio: A Critical Determinant of Myocardial Contractility. In Alterations of Excitation-Contraction Coupling in the Failing Human Heart; Springer: Berlin/Heidelberg, Germany, 1998; pp. 25–37. [Google Scholar]
- Ferrantini, C.; Coppini, R.; Scellini, B.; Ferrara, C.; Pioner, J.M.; Mazzoni, L.; Priori, S.; Cerbai, E.; Tesi, C.; Poggesi, C. R4496C RyR2 Mutation Impairs Atrial and Ventricular Contractility. J. Gen. Physiol. 2016, 147, 39–52. [Google Scholar] [CrossRef]
- Weiwad, W.; Linke, W.; Wussling, M. Sarcomere Length–Tension Relationship of Rat Cardiac Myocytes at Lengths Greater than Optimum. J. Mol. Cell. Cardiol. 2000, 32, 247–259. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lookin, O.; Balakin, A.; Protsenko, Y. Differences in Effects of Length-Dependent Regulation of Force and Ca2+ Transient in the Myocardial Trabeculae of the Rat Right Atrium and Ventricle. Int. J. Mol. Sci. 2023, 24, 8960. https://doi.org/10.3390/ijms24108960
Lookin O, Balakin A, Protsenko Y. Differences in Effects of Length-Dependent Regulation of Force and Ca2+ Transient in the Myocardial Trabeculae of the Rat Right Atrium and Ventricle. International Journal of Molecular Sciences. 2023; 24(10):8960. https://doi.org/10.3390/ijms24108960
Chicago/Turabian StyleLookin, Oleg, Alexander Balakin, and Yuri Protsenko. 2023. "Differences in Effects of Length-Dependent Regulation of Force and Ca2+ Transient in the Myocardial Trabeculae of the Rat Right Atrium and Ventricle" International Journal of Molecular Sciences 24, no. 10: 8960. https://doi.org/10.3390/ijms24108960




