The Role of REV-ERB Receptors in Cancer Pathogenesis
Abstract
:1. Introduction
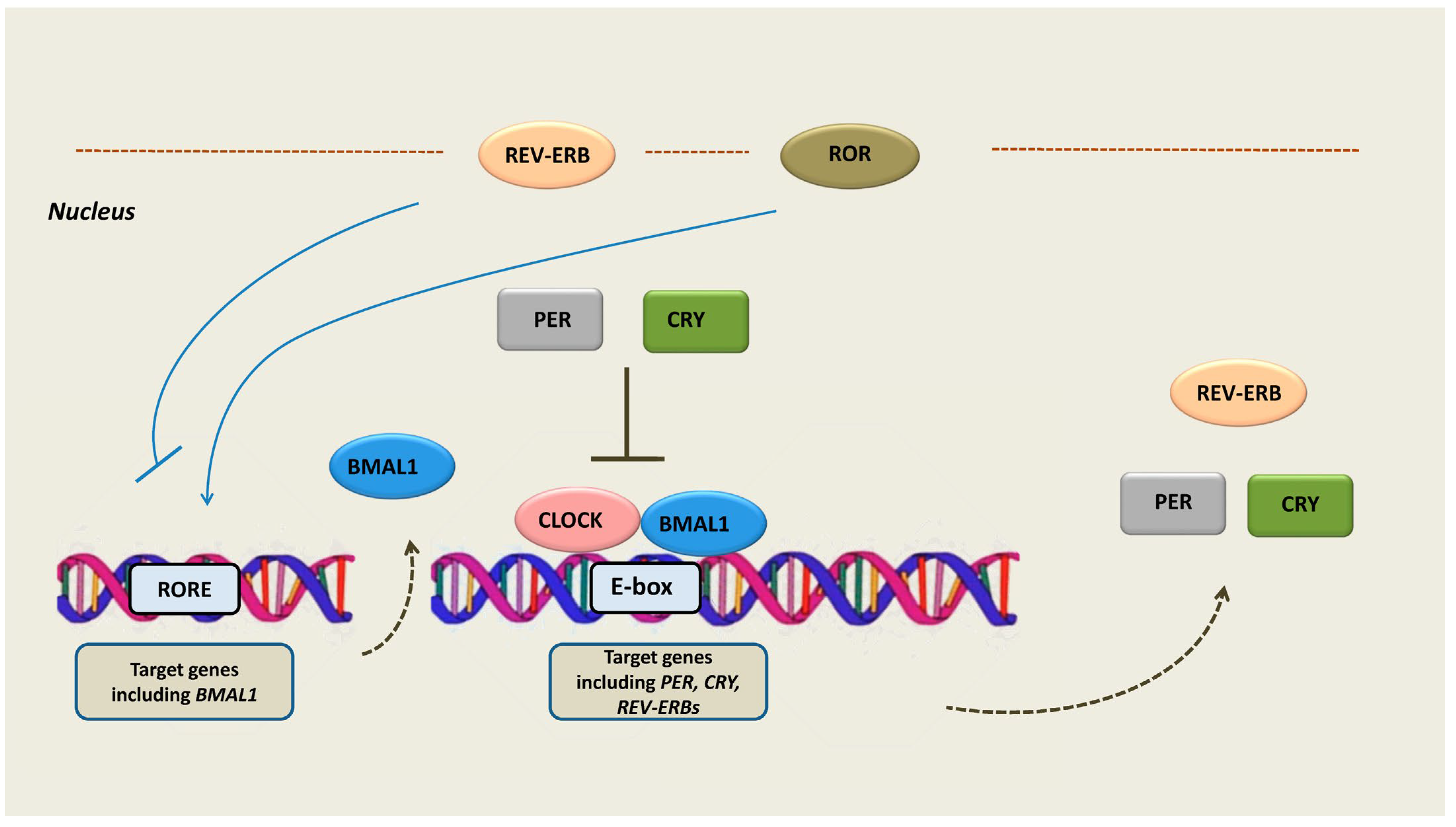
2. Circadian Rhythm
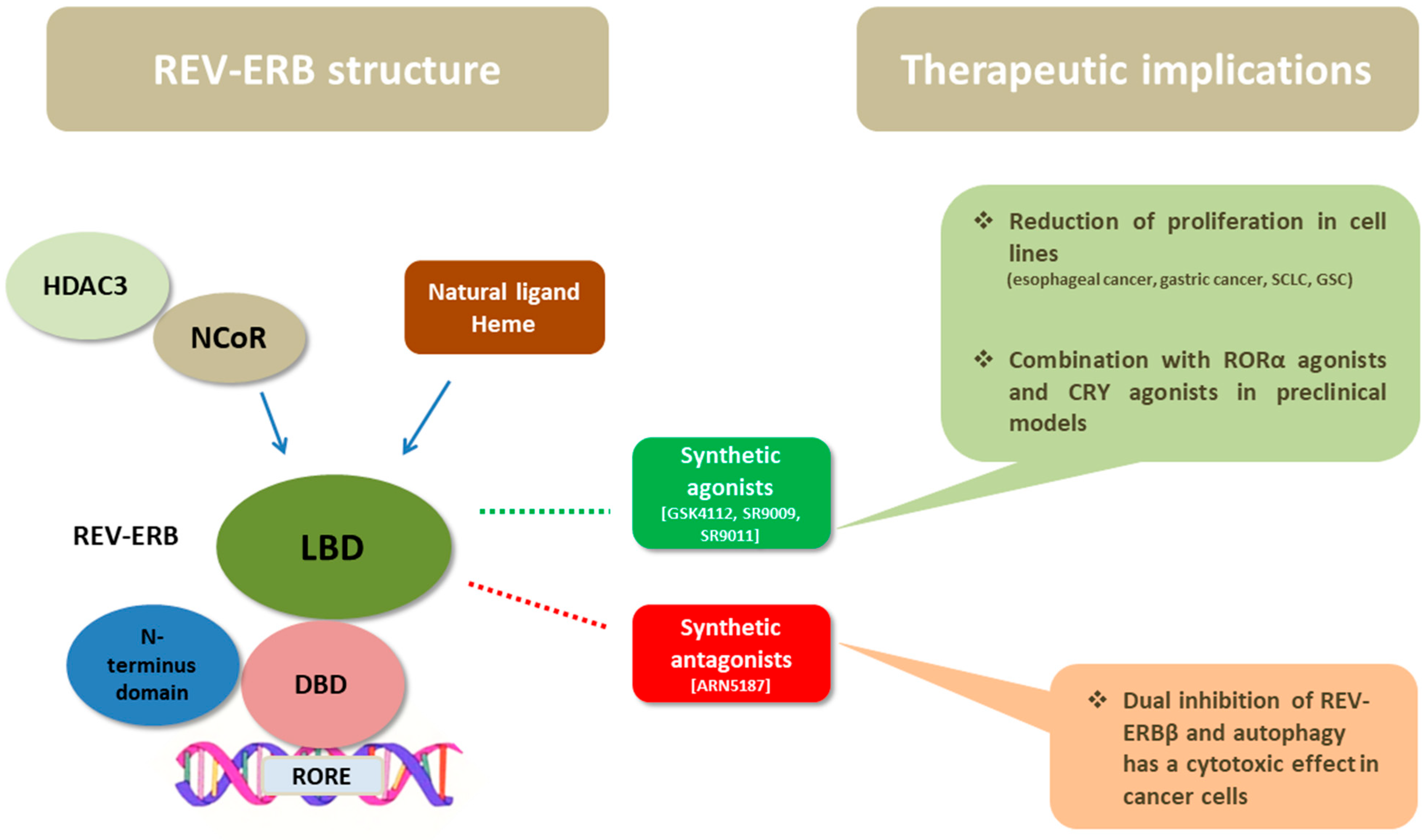
3. The Structure of REV-ERB Receptors
4. Transcriptional Activity and Function of REV-ERBs
5. The Role of REV-ERB Receptors in Cancer Pathogenesis
| Author (Year) | Tumor Type | Main Findings | [Ref.] |
|---|---|---|---|
| Zhang et al. (2022) | NSCLC (adenocarcinoma) | REV-ERBα downregulation; correlation with T,M stage | [42] |
| Verlande et al. (2021) | NSCLC (adenocarcinoma) | REV-ERBα downregulation in liver tissue; involved in lung cancer-associated cachexia | [44] |
| van de Watt et al. (2020) | Esophageal cancer | REV-ERBα downregulation; reduced cancer cell proliferation after REV-ERBa agonist | [45] |
| Cervical cancer | REV-ERBα downregulated in cervical cancer and high-grade squamous intraepithelial lesions | [45] | |
| Wang et al. (2018) | Gastric cancer | REV-ERBα downregulation; correlation with TNM; correlation with poor survival | [46] |
| Wang et al. (2021) | Gastric cancer | REV-ERBα downregulation; correlation with TNM stage, histological grade, CEA | [47] |
| Tao et al. (2019) | Gastric cancer | Knockdown of REV-ERBα leads to increased proliferation; REV-ERBα agonist restores the effect | [48] |
| Sotak et al. (2013) | Colorectal cancer | REV-ERBα downregulation in CRC tissue; temporal shift of circadian rhythm in liver tissue | [49] |
| Huisman et al. (2015) | Colorectal cancer | REV-ERBα downregulation in liver metastases | [51] |
| Wagner et al. (2019) | Glioblastoma | Glioblastoma stem cells exhibit growth dependence on circadian rhythm | [52] |
| Angelousi et al. (2020) | Adrenal benign and malignant tumors | REV-ERBs downregulation in cortisol-secreting adenomas and adrenocortical carcinomas | [53] |
6. Therapeutic Considerations
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burris, T.P. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol. Endocrinol. 2008, 22, 1509–1520. [Google Scholar] [CrossRef]
- Sever, R.; Glass, C.K. Signaling by nuclear receptors. Cold Spring Harb. Perspect. Biol. 2013, 5, a016709. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.H.; Downes, M.; Evans, R.M. Nuclear receptors as modulators of the tumor microenvironment. Cancer Prev. Res. 2012, 5, 3–10. [Google Scholar] [CrossRef]
- Safe, S.; Jin, U.H.; Hedrick, E.; Reeder, A.; Lee, S.O. Minireview: Role of orphan nuclear receptors in cancer and potential as drug targets. Mol. Endocrinol. 2014, 28, 157–172. [Google Scholar] [CrossRef]
- Duez, H.; Staels, B. Rev-erb-alpha: An integrator of circadian rhythms and metabolism. J. Appl. Physiol. 2009, 107, 1972–1980. [Google Scholar] [CrossRef]
- Ikeda, R.; Tsuchiya, Y.; Koike, N.; Umemura, Y.; Inokawa, H.; Ono, R.; Inoue, M.; Sasawaki, Y.; Grieten, T.; Okubo, N.; et al. REV-ERBalpha and REV-ERBbeta function as key factors regulating Mammalian Circadian Output. Sci. Rep. 2019, 9, 10171. [Google Scholar] [CrossRef]
- Lee, Y. Roles of circadian clocks in cancer pathogenesis and treatment. Exp. Mol. Med. 2021, 53, 1529–1538. [Google Scholar] [CrossRef]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Cordina-Duverger, E.; Menegaux, F.; Popa, A.; Rabstein, S.; Harth, V.; Pesch, B.; Bruning, T.; Fritschi, L.; Glass, D.C.; Heyworth, J.S.; et al. Night shift work and breast cancer: A pooled analysis of population-based case-control studies with complete work history. Eur. J. Epidemiol. 2018, 33, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, Z.; Nice, E.; Huang, C.; Zhang, W.; Tang, Y. Circadian rhythms and cancers: The intrinsic links and therapeutic potentials. J. Hematol. Oncol. 2022, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Valafar, B.; Zaravinos, A.; Bonavida, B. Cross Talk between the Circadian Clock Proteins and TP53 in Cancer and Therapeutic Significance. Crit. Rev. Oncog. 2021, 26, 19–36. [Google Scholar] [CrossRef]
- Astiz, M.; Heyde, I.; Oster, H. Mechanisms of Communication in the Mammalian Circadian Timing System. Int. J. Mol. Sci. 2019, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Sollars, P.J.; Pickard, G.E. The Neurobiology of Circadian Rhythms. Psychiatr. Clin. N. Am. 2015, 38, 645–665. [Google Scholar] [CrossRef]
- Roenneberg, T.; Merrow, M. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef]
- Hughes, S.; Jagannath, A.; Hankins, M.W.; Foster, R.G.; Peirson, S.N. Photic regulation of clock systems. Methods Enzymol. 2015, 552, 125–143. [Google Scholar] [CrossRef]
- Schibler, U.; Gotic, I.; Saini, C.; Gos, P.; Curie, T.; Emmenegger, Y.; Sinturel, F.; Gosselin, P.; Gerber, A.; Fleury-Olela, F.; et al. Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals. Cold Spring Harb. Symp. Quant. Biol. 2015, 80, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, A.M.; Turek, F.W. Neurobiology of Circadian Rhythm Regulation. Sleep Med. Clin. 2015, 10, 403–412. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Preitner, N.; Damiola, F.; Lopez-Molina, L.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Lee, C.C. The circadian clock: Pacemaker and tumour suppressor. Nat. Rev. Cancer 2003, 3, 350–361. [Google Scholar] [CrossRef]
- Lazar, M.A.; Jones, K.E.; Chin, W.W. Isolation of a cDNA encoding human Rev-ErbA alpha: Transcription from the noncoding DNA strand of a thyroid hormone receptor gene results in a related protein that does not bind thyroid hormone. DNA Cell Biol. 1990, 9, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Hodin, R.A.; Darling, D.S.; Chin, W.W. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol. Cell. Biol. 1989, 9, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Dumas, B.; Harding, H.P.; Choi, H.S.; Lehmann, K.A.; Chung, M.; Lazar, M.A.; Moore, D.D. A new orphan member of the nuclear hormone receptor superfamily closely related to Rev-Erb. Mol. Endocrinol. 1994, 8, 996–1005. [Google Scholar] [CrossRef]
- Mullican, S.E.; Dispirito, J.R.; Lazar, M.A. The orphan nuclear receptors at their 25-year reunion. J. Mol. Endocrinol. 2013, 51, T115–T140. [Google Scholar] [CrossRef]
- Uriz-Huarte, A.; Date, A.; Ang, H.; Ali, S.; Brady, H.J.M.; Fuchter, M.J. The transcriptional repressor REV-ERB as a novel target for disease. Bioorg. Med. Chem. Lett. 2020, 30, 127395. [Google Scholar] [CrossRef]
- Murray, M.H.; Valfort, A.C.; Koelblen, T.; Ronin, C.; Ciesielski, F.; Chatterjee, A.; Veerakanellore, G.B.; Elgendy, B.; Walker, J.K.; Hegazy, L.; et al. Structural basis of synthetic agonist activation of the nuclear receptor REV-ERB. Nat. Commun. 2022, 13, 7131. [Google Scholar] [CrossRef]
- Woo, E.J.; Jeong, D.G.; Lim, M.Y.; Jun Kim, S.; Kim, K.J.; Yoon, S.M.; Park, B.C.; Ryu, S.E. Structural insight into the constitutive repression function of the nuclear receptor Rev-erbbeta. J. Mol. Biol. 2007, 373, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Everett, L.J.; Lazar, M.A. Nuclear receptor Rev-erbalpha: Up, down, and all around. Trends Endocrinol. Metab. 2014, 25, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, F.; Lin, Y.; Wu, B. Targeting REV-ERBalpha for therapeutic purposes: Promises and challenges. Theranostics 2020, 10, 4168–4182. [Google Scholar] [CrossRef]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef] [PubMed]
- Bugge, A.; Feng, D.; Everett, L.J.; Briggs, E.R.; Mullican, S.E.; Wang, F.; Jager, J.; Lazar, M.A. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012, 26, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.S.; Sodum, N.; Kaya, Y.; Herzig, K.H. Role of Circadian Transcription Factor Rev-Erb in Metabolism and Tissue Fibrosis. Int. J. Mol. Sci. 2022, 23, 2954. [Google Scholar] [CrossRef] [PubMed]
- Pourcet, B.; Duez, H. Nuclear Receptors and Clock Components in Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 9721. [Google Scholar] [CrossRef]
- Liu, A.C.; Tran, H.G.; Zhang, E.E.; Priest, A.A.; Welsh, D.K.; Kay, S.A. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008, 4, e1000023. [Google Scholar] [CrossRef]
- Duez, H.; Staels, B. The nuclear receptors Rev-erbs and RORs integrate circadian rhythms and metabolism. Diab. Vasc. Dis. Res. 2008, 5, 82–88. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; Di Tacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef]
- Vaissiere, A.; Berger, S.; Harrus, D.; Dacquet, C.; Le Maire, A.; Boutin, J.A.; Ferry, G.; Royer, C.A. Molecular mechanisms of transcriptional control by Rev-erbalpha: An energetic foundation for reconciling structure and binding with biological function. Protein Sci. 2015, 24, 1129–1146. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Feng, D.; Emmett, M.J.; Everett, L.J.; Loro, E.; Briggs, E.R.; Bugge, A.; Hou, C.; Ferrara, C.; Seale, P.; et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 2013, 503, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Coste, H.; Rodriguez, J.C. Orphan nuclear hormone receptor Rev-erbalpha regulates the human apolipoprotein CIII promoter. J. Biol. Chem. 2002, 277, 27120–27129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shu, R.; Liu, X.; Zhang, X.; Sun, D. Downregulation of REV-ERBalpha is associated with the progression of lung adenocarcinoma. Ann. Transl. Med. 2022, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Papagiannakopoulos, T.; Kinouchi, K.; Liu, Y.; Cervantes, M.; Baldi, P.; Jacks, T.; Sassone-Corsi, P. Lung Adenocarcinoma Distally Rewires Hepatic Circadian Homeostasis. Cell 2016, 165, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Verlande, A.; Chun, S.K.; Goodson, M.O.; Fortin, B.M.; Bae, H.; Jang, C.; Masri, S. Glucagon regulates the stability of REV-ERBalpha to modulate hepatic glucose production in a model of lung cancer-associated cachexia. Sci. Adv. 2021, 7, eabf3885. [Google Scholar] [CrossRef] [PubMed]
- van der Watt, P.J.; Roden, L.C.; Davis, K.T.; Parker, M.I.; Leaner, V.D. Circadian Oscillations Persist in Cervical and Esophageal Cancer Cells Displaying Decreased Expression of Tumor-Suppressing Circadian Clock Genes. Mol. Cancer Res. 2020, 18, 1340–1353. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Wei, X.; Yu, H.; Wang, Z. REV-ERBalpha reduction is associated with clinicopathological features and prognosis in human gastric cancer. Oncol. Lett. 2018, 16, 1499–1506. [Google Scholar] [CrossRef]
- Wang, X.; Jia, R.; Chen, K.; Wang, J.; Jiang, K.; Wang, Z. RORalpha and REV-ERBalpha are Associated With Clinicopathological Parameters and are Independent Biomarkers of Prognosis in Gastric Cancer. Technol. Cancer Res. Treat. 2021, 20, 15330338211039670. [Google Scholar] [CrossRef]
- Tao, L.; Yu, H.; Liang, R.; Jia, R.; Wang, J.; Jiang, K.; Wang, Z. Rev-erbalpha inhibits proliferation by reducing glycolytic flux and pentose phosphate pathway in human gastric cancer cells. Oncogenesis 2019, 8, 57. [Google Scholar] [CrossRef]
- Sotak, M.; Polidarova, L.; Ergang, P.; Sumova, A.; Pacha, J. An association between clock genes and clock-controlled cell cycle genes in murine colorectal tumors. Int. J. Cancer 2013, 132, 1032–1041. [Google Scholar] [CrossRef]
- Huisman, S.A.; Oklejewicz, M.; Ahmadi, A.R.; Tamanini, F.; Ijzermans, J.N.; van der Horst, G.T.; de Bruin, R.W. Colorectal liver metastases with a disrupted circadian rhythm phase shift the peripheral clock in liver and kidney. Int. J. Cancer 2015, 136, 1024–1032. [Google Scholar] [CrossRef]
- Dong, Z.; Zhang, G.; Qu, M.; Gimple, R.C.; Wu, Q.; Qiu, Z.; Prager, B.C.; Wang, X.; Kim, L.J.Y.; Morton, A.R.; et al. Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discov. 2019, 9, 1556–1573. [Google Scholar] [CrossRef] [PubMed]
- Wagner, P.M.; Sosa Alderete, L.G.; Gorne, L.D.; Gaveglio, V.; Salvador, G.; Pasquare, S.; Guido, M.E. Proliferative Glioblastoma Cancer Cells Exhibit Persisting Temporal Control of Metabolism and Display Differential Temporal Drug Susceptibility in Chemotherapy. Mol. Neurobiol. 2019, 56, 1276–1292. [Google Scholar] [CrossRef] [PubMed]
- Angelousi, A.; Nasiri-Ansari, N.; Karapanagioti, A.; Kyriakopoulos, G.; Aggeli, C.; Zografos, G.; Choreftaki, T.; Parianos, C.; Kounadi, T.; Alexandraki, K.; et al. Expression of clock-related genes in benign and malignant adrenal tumors. Endocrine 2020, 68, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Hering, Y.; Berthier, A.; Duez, H.; Lefebvre, P.; Deprez, B.; Gribbon, P.; Wolf, M.; Reinshagen, J.; Halley, F.; Hannemann, J.; et al. Development and implementation of a cell-based assay to discover agonists of the nuclear receptor REV-ERBalpha. J. Biol. Methods 2018, 5, e94. [Google Scholar] [CrossRef]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Grant, D.; Yin, L.; Collins, J.L.; Parks, D.J.; Orband-Miller, L.A.; Wisely, G.B.; Joshi, S.; Lazar, M.A.; Willson, T.M.; Zuercher, W.J. GSK4112, a small molecule chemical probe for the cell biology of the nuclear heme receptor Rev-erbalpha. ACS Chem. Biol. 2010, 5, 925–932. [Google Scholar] [CrossRef]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.J.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553, 351–355. [Google Scholar] [CrossRef] [PubMed]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, W.; Ye, W.; Wang, H.; Zhang, Q.; Shen, J.; Hong, Q.; Li, X.; Wen, G.; Wei, T.; et al. SR9009 induces a REV-ERB dependent anti-small-cell lung cancer effect through inhibition of autophagy. Theranostics 2020, 10, 4466–4480. [Google Scholar] [CrossRef]
- Wang, Y.; Kojetin, D.; Burris, T.P. Anti-proliferative actions of a synthetic REV-ERBalpha/beta agonist in breast cancer cells. Biochem. Pharmacol. 2015, 96, 315–322. [Google Scholar] [CrossRef]
- Ercolani, L.; Ferrari, A.; De Mei, C.; Parodi, C.; Wade, M.; Grimaldi, B. Circadian clock: Time for novel anticancer strategies? Pharmacol. Res. 2015, 100, 288–295. [Google Scholar] [CrossRef]
- De Mei, C.; Ercolani, L.; Parodi, C.; Veronesi, M.; Lo Vecchio, C.; Bottegoni, G.; Torrente, E.; Scarpelli, R.; Marotta, R.; Ruffili, R.; et al. Dual inhibition of REV-ERBbeta and autophagy as a novel pharmacological approach to induce cytotoxicity in cancer cells. Oncogene 2015, 34, 2597–2608. [Google Scholar] [CrossRef]
- Sulli, G.; Manoogian, E.N.C.; Taub, P.R.; Panda, S. Training the Circadian Clock, Clocking the Drugs, and Drugging the Clock to Prevent, Manage, and Treat Chronic Diseases. Trends Pharmacol. Sci. 2018, 39, 812–827. [Google Scholar] [CrossRef]
- Dierickx, P.; Emmett, M.J.; Jiang, C.; Uehara, K.; Liu, M.; Adlanmerini, M.; Lazar, M.A. SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 12147–12152. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, J.; Zheng, X.; Tan, P.; Xiong, X.; Yi, X.; Yang, Y.; Wang, Y.; Liao, D.; Li, H.; et al. SR9009 inhibits lethal prostate cancer subtype 1 by regulating the LXRalpha/FOXM1 pathway independently of REV-ERBs. Cell Death Dis. 2022, 13, 949. [Google Scholar] [CrossRef]
- Innominato, P.F.; Roche, V.P.; Palesh, O.G.; Ulusakarya, A.; Spiegel, D.; Levi, F.A. The circadian timing system in clinical oncology. Ann. Med. 2014, 46, 191–207. [Google Scholar] [CrossRef]
- Amidi, A.; Wu, L.M. Circadian disruption and cancer- and treatment-related symptoms. Front. Oncol. 2022, 12, 1009064. [Google Scholar] [CrossRef] [PubMed]
- Hrushesky, W.J. Circadian timing of cancer chemotherapy. Science 1985, 228, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Zidani, R.; Misset, J.L. Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 1997, 350, 681–686. [Google Scholar] [CrossRef]
- Ballesta, A.; Innominato, P.F.; Dallmann, R.; Rand, D.A.; Levi, F.A. Systems Chronotherapeutics. Pharmacol. Rev. 2017, 69, 161–199. [Google Scholar] [CrossRef]
- Anafi, R.C.; Francey, L.J.; Hogenesch, J.B.; Kim, J. CYCLOPS reveals human transcriptional rhythms in health and disease. Proc. Natl. Acad. Sci. USA 2017, 114, 5312–5317. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomatou, G.; Karachaliou, A.; Veloudiou, O.-Z.; Karvela, A.; Syrigos, N.; Kotteas, E. The Role of REV-ERB Receptors in Cancer Pathogenesis. Int. J. Mol. Sci. 2023, 24, 8980. https://doi.org/10.3390/ijms24108980
Gomatou G, Karachaliou A, Veloudiou O-Z, Karvela A, Syrigos N, Kotteas E. The Role of REV-ERB Receptors in Cancer Pathogenesis. International Journal of Molecular Sciences. 2023; 24(10):8980. https://doi.org/10.3390/ijms24108980
Chicago/Turabian StyleGomatou, Georgia, Anastasia Karachaliou, Orsalia-Zoi Veloudiou, Alexandra Karvela, Nikolaos Syrigos, and Elias Kotteas. 2023. "The Role of REV-ERB Receptors in Cancer Pathogenesis" International Journal of Molecular Sciences 24, no. 10: 8980. https://doi.org/10.3390/ijms24108980





