Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders
Abstract
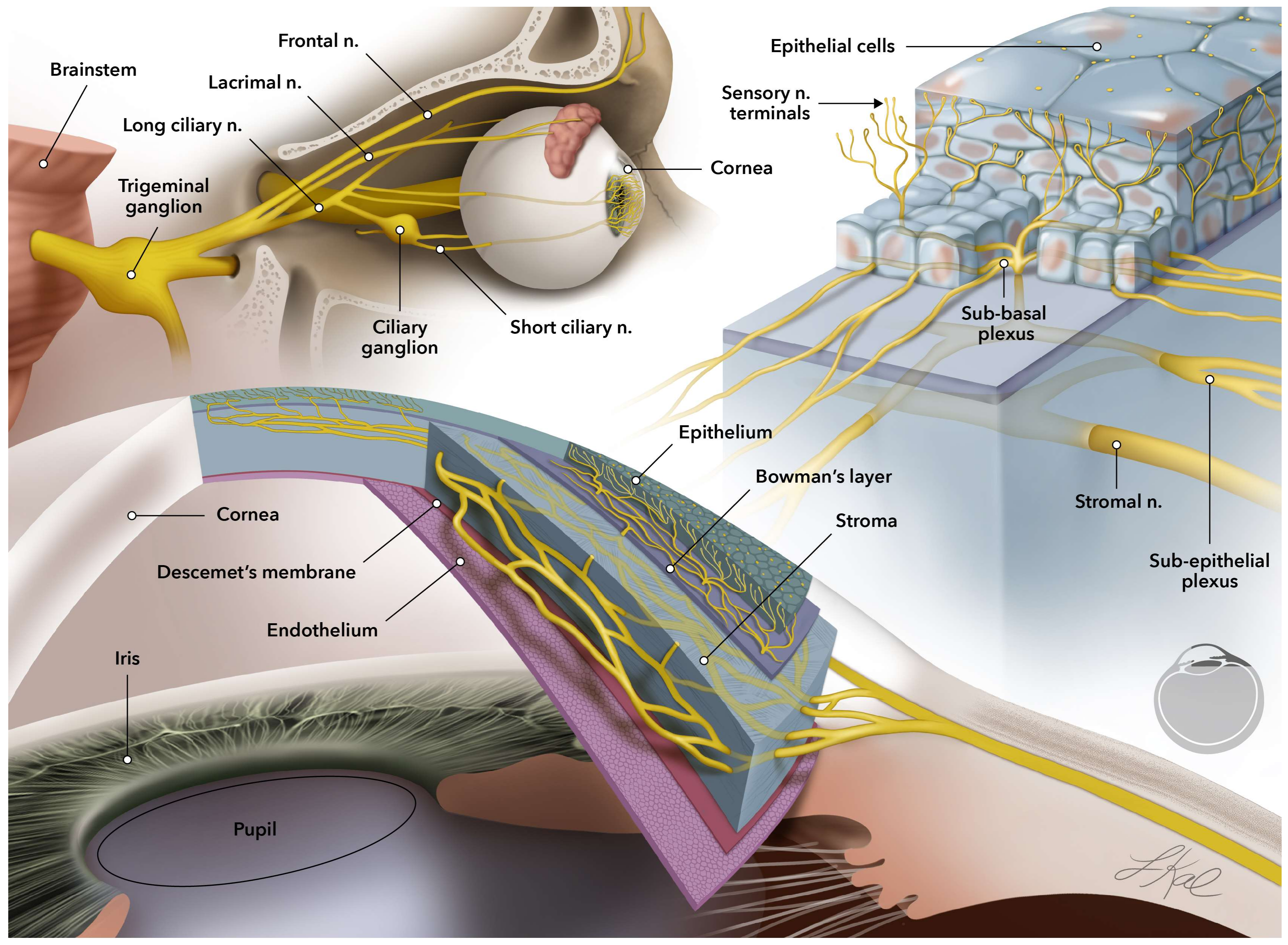
:1. Introduction
2. Current Treatments for Corneal Neurodegenerative Diseases
3. EV Characterization and Extraction Methods
4. Therapeutic Applications of EVs
4.1. Use of EVs for Ocular Neurodegenerative Diseases
4.1.1. Neurotrophic Keratitis
4.1.2. Dry Eye Syndrome (DES)
4.2. Retinal Diseases Causing Nerve Malfunctions
4.2.1. Diabetic Retinopathy (DR)
4.2.2. Retinitis Pigmentosa (RP)
4.2.3. Optic Neuritis and Neuromyelitis Optica (NMO)
4.2.4. Optic Neuropathy
4.2.5. Glaucoma
5. Engineered EVs for Advanced Therapies
6. Future Directions of EV-Based Therapeutics in Ocular Disorders
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gehring, W.J. The evolution of vision. Wiley Interdiscip Rev. Dev. Biol. 2014, 3, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Khosravimelal, S.; Mobaraki, M.; Eftekhari, S.; Ahearne, M.; Seifalian, A.M.; Gholipourmalekabadi, M. Hydrogels as Emerging Materials for Cornea Wound Healing. Small 2021, 17, e2006335. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Chow, J.; Liu, J. Corneal Innervation and Sensation: The Eye and Beyond. Yale J. Biol. Med. 2018, 91, 13–21. [Google Scholar] [PubMed]
- Pham, T.L.; Bazan, H.E. Docosanoid signaling modulates corneal nerve regeneration: Effect on tear secretion, wound healing, and neuropathic pain. J. Lipid Res. 2021, 62, 100033. [Google Scholar] [CrossRef]
- Bouheraoua, N.; Fouquet, S.; Marcos-Almaraz, M.T.; Karagogeos, D.; Laroche, L.; Chédotal, A. Genetic Analysis of the Organization, Development, and Plasticity of Corneal Innervation in Mice. J. Neurosci. 2019, 39, 1150–1168. [Google Scholar] [CrossRef]
- Al-Aqaba, M.A.; Dhillon, V.K.; Mohammed, I.; Said, D.G.; Dua, H.S. Corneal nerves in health and disease. Prog. Retin. Eye Res. 2019, 73, 100762. [Google Scholar] [CrossRef]
- Jalilian, E.; Massoumi, H.; Bigit, B.; Amin, S.; Katz, E.A.; Guaiquil, V.H.; Anwar, K.N.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R. Bone marrow mesenchymal stromal cells in a 3D system produce higher concentration of extracellular vesicles (EVs) with increased complexity and enhanced neuronal growth properties. Stem Cell Res. Ther. 2022, 13, 425. [Google Scholar] [CrossRef]
- Eslani, M.; Putra, I.; Shen, X.; Hamouie, J.; Afsharkhamseh, N.; Besharat, S.; Rosenblatt, M.I.; Dana, R.; Hematti, P.; Djalilian, A.R. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Investig. Opthalmology Vis. Sci. 2017, 58, 5507–5517. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Jabbehdari, S.; Djalilian, A.R. Emerging Approaches for Ocular Surface Regeneration. Curr. Ophthalmol. Rep. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Adak, S.; Magdalene, D.; Deshmukh, S.; Das, D.; Jaganathan, B.G. A Review on Mesenchymal Stem Cells for Treatment of Retinal Diseases. Stem Cell Rev. Rep. 2021, 17, 1154–1173. [Google Scholar] [CrossRef]
- Mahdavi, S.S.; Abdekhodaie, M.J.; Mashayekhan, S.; Baradaran-Rafii, A.; Djalilian, A.R. Bioengineering Approaches for Corneal Regenerative Medicine. Tissue Eng. Regen. Med. 2020, 17, 567–593. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, T.R.; Sears, C.M.; Park, J.K.; Kossler, A.L. Corneal Neurotization and Novel Medical Therapies for Neurotrophic Keratopathy. Curr. Ophthalmol. Rep. 2020, 8, 252–266. [Google Scholar] [CrossRef]
- Jalilian, E.; Putra, I.; Katz, E.; Yazdanpanah, G.; Guaiquil, V.H.; Shen, X.; Anwar, K.; An, S.; Rosenblatt, M.; Djalilian, A.R. Interactions between mesenchymal stem cells (MSCs) and trigeminal ganglion (TGs) improve neurite growth and elongation. Investig. Ophthalmol. Vis. Sci. 2021, 62, 892. [Google Scholar]
- Amin, S.; Jalilian, E.; Katz, E.; Frank, C.; Yazdanpanah, G.; Guaiquil, V.H.; Rosenblatt, M.I.; Djalilian, A.R. The Limbal Niche and Regenerative Strategies. Vision 2021, 5, 43. [Google Scholar] [CrossRef]
- Jalilian, E.; Bigit, B.; Amin, S.; Massoumi, H.; Katz, E.; Guaiquil, V.H.; Rosenblatt, M.; Djalilian, A.R. Exosomes from 3D cultures of Bone Marrow Mesenchymal Stem Cell (BM-MSCs) have higher neuro-regenerative potential than those generated from 2D conditions. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1209-A0209. [Google Scholar]
- Katz, E.; Guaiquil, V.H.; Ivakhnitskaia, E.; Lara, D.; Anwar, K.; Jalilian, E.; Rosenblatt, M.; Djalilian, A.R. Exosomes as a Novel Multitarget Approach to Promote Growth of Corneal Sensory Neurons. Investig. Ophthalmol. Vis. Sci. 2021, 62, 749. [Google Scholar]
- Mauritz, C.; Grothe, C.; Haastert, K.; Haastert-Talini, K. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. J. Neurosci. Res. 2004, 77, 453–461. [Google Scholar] [CrossRef]
- Kingham, P.J.; Kalbermatten, D.F.; Mahay, D.; Armstrong, S.J.; Wiberg, M.; Terenghi, G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp. Neurol. 2007, 207, 267–274. [Google Scholar] [CrossRef]
- Dezawa, M.; Kanno, H.; Hoshino, M.; Cho, H.; Matsumoto, N.; Itokazu, Y.; Tajima, N.; Yamada, H.; Sawada, H.; Ishikawa, H.; et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J. Clin. Investig. 2004, 113, 1701–1710. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef]
- Osugi, M.; Katagiri, W.; Yoshimi, R.; Inukai, T.; Hibi, H.; Ueda, M. Conditioned Media from Mesenchymal Stem Cells Enhanced Bone Regeneration in Rat Calvarial Bone Defects. Tissue Eng. Part A 2012, 18, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Widgerow, A.D.; Salibian, A.A.; Kohan, E.; Sartiniferreira, T.; Afzel, H.; Tham, T.; Evans, G.R. “Strategic sequences” in adipose-derived stem cell nerve regeneration. Microsurgery 2014, 34, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Toh, W.S.; Lai, R.C.; Zhang, B.; Lim, S.K. MSC exosome works through a protein-based mechanism of action. Biochem. Soc. Trans. 2018, 46, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Liu, Y.; Yang, Y.; Wang, H.; Xu, Y.; Zhang, Z. MSC-Derived Exosomes-Based Therapy for Peripheral Nerve Injury: A Novel Therapeutic Strategy. BioMed Res. Int. 2019, 2019, 6458237. [Google Scholar] [CrossRef]
- Nieuwland, R.; Falcon-Perez, J.M.; Soekmadji, C.; Boilard, E.; Carter, D.; Buzas, E.I. Essentials of extracellular vesicles: Posters on basic and clinical aspects of extracellular vesicles. J. Extracell. Vesicles 2018, 7, 1548234. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Van der Merwe, Y.; Steketee, M.B. Extracellular Vesicles: Biomarkers, Therapeutics, and Vehicles in the Visual System. Curr. Ophthalmol. Rep. 2017, 5, 276–282. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H.; Jin, Y. Isolation and characterization of extracellular vesicles from Broncho-alveolar lavage fluid: A review and comparison of different methods. Respir. Res. 2019, 20, 240. [Google Scholar] [CrossRef]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ma, L.; Gong, M.; Su, G.; Zhu, S.; Zhang, W.; Wang, S.; Li, Z.; Chen, C.; Li, L.; et al. Protein Profiling and Sizing of Extracellular Vesicles from Colorectal Cancer Patients via Flow Cytometry. ACS Nano 2018, 12, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Obeid, S.; Ceroi, A.; Mourey, G.; Saas, P.; Elie-Caille, C.; Boireau, W. Development of a NanoBioAnalytical platform for “on-chip” qualification and quantification of platelet-derived microparticles. Biosens. Bioelectron. 2017, 93, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Mihaly, J.; Deak, R.; Szigyarto, I.C.; Bota, A.; Beke-Somfai, T.; Varga, Z. Characterization of extracellular vesicles by IR spectroscopy: Fast and simple classification based on amide and CH stretching vibrations. Biochim. Biophys. Acta Biomembr. 2017, 1859, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Van der Pol, E.; Hoekstra, A.G.; Sturk, A.; Otto, C.; VAN Leeuwen, T.G.; Nieuwland, R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J. Thromb. Haemost. 2010, 8, 2596–2607. [Google Scholar] [CrossRef]
- Hercher, D.; Nguyen, M.Q.; Dworak, H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp. Neurol. 2021, 350, 113968. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, D.; Laskin, D.L.; Jin, Y. Functional Evidence of Pulmonary Extracellular Vesicles in Infectious and Noninfectious Lung Inflammation. J. Immunol. 2018, 201, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Groot, M.; Pinilla-Vera, M.; Fredenburgh, L.E.; Jin, Y. Identification of miRNA-rich vesicles in bronchoalveolar lavage fluid: Insights into the function and heterogeneity of extracellular vesicles. J. Control. Release 2019, 294, 43–52. [Google Scholar] [CrossRef]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Lucchetti, D.; Fattorossi, A.; Sgambato, A. Extracellular Vesicles in Oncology: Progress and Pitfalls in the Methods of Isolation and Analysis. Biotechnol. J. 2019, 14, e1700716. [Google Scholar] [CrossRef] [PubMed]
- Revenfeld, A.L.S.; Bæk, R.; Nielsen, M.H.; Stensballe, A.; Varming, K.; Jørgensen, M. Diagnostic and Prognostic Potential of Extracellular Vesicles in Peripheral Blood. Clin. Ther. 2014, 36, 830–846. [Google Scholar] [CrossRef]
- Lee, H.; Zhang, D.; Zhu, Z.; Cruz, C.S.D.; Jin, Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci. Rep. 2016, 6, 35250. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.j.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Chen, C.; Skog, J.; Hsu, C.-H.; Lessard, R.T.; Balaj, L.; Wurdinger, T.; Carter, B.S.; Breakefield, X.O.; Toner, M.; Irimia, D. Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 2010, 10, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Goudie, M.J.; Singha, P.; Hopkins, S.P.; Brisbois, E.J.; Handa, H. Active Release of an Antimicrobial and Antiplatelet Agent from a Nonfouling Surface Modification. ACS Appl. Mater. Interfaces 2019, 11, 4523–4530. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Ji, S.; Wang, X.; Huang, P.; Zhang, C.; Wang, W.; Kong, D. NO prodrug-conjugated, self-assembled, pH-responsive and galactose receptor targeted nanoparticles for co-delivery of nitric oxide and doxorubicin. Nanoscale 2018, 10, 4179–4188. [Google Scholar] [CrossRef] [PubMed]
- Wo, Y.; Brisbois, E.J.; Wu, J.; Li, Z.; Major, T.C.; Mohammed, A.; Wang, X.; Colletta, A.; Bull, J.L.; Matzger, A.J.; et al. Reduction of Thrombosis and Bacterial Infection via Controlled Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated CarboSil Intravascular Catheters. ACS Biomater. Sci. Eng. 2017, 3, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Na, K.-S.; Putra, I.; Lee, H.J.; Hull, S.; Cheng, Y.-C.; Blanco, I.J.; Eslani, M.; Djalilian, A.R.; Myung, D. Corneal Wound Healing Effects of Mesenchymal Stem Cell Secretome Delivered Within a Viscoelastic Gel Carrier. Stem Cells Transl. Med. 2019, 8, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- ISEV2021 Abstract Book. J. Extracell. Vesicles 2021, 10, e12083.
- Soekmadji, C.; Li, B.; Huang, Y.; Wang, H.; An, T.; Liu, C.; Pan, W.; Chen, J.; Cheung, L.; Falcon-Perez, J.M.; et al. The future of Extracellular Vesicles as Theranostics—An ISEV meeting report. J. Extracell. Vesicles 2020, 9, 1809766. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Snitzer, J.D.; Rusnak, S.; Lang, J.K. Therapeutic Potential of Engineered Extracellular Vesicles. AAPS J. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, A.; Liu, A.; Moss, H.E.; Feinstein, D.; Ravindran, S.; Mathew, B.; Roth, S. Emerging concepts in the treatment of optic neuritis: Mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res. Ther. 2021, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Castro, O.W.; Kim, D.-K.; Thomas, A.; Shuai, B.; Attaluri, S.; Upadhya, R.; Gitai, D.; Madhu, L.N.; Prockop, D.J.; et al. Intranasally Administered Human MSC-Derived Extracellular Vesicles Pervasively Incorporate into Neurons and Microglia in both Intact and Status Epilepticus Injured Forebrain. Int. J. Mol. Sci. 2019, 21, 181. [Google Scholar] [CrossRef]
- Guo, S.; Perets, N.; Betzer, O.; Ben-Shaul, S.; Sheinin, A.; Michaelevski, I.; Popovtzer, R.; Offen, D.; Levenberg, S. Intranasal Delivery of Mesenchymal Stem Cell Derived Exosomes Loaded with Phosphatase and Tensin Homolog siRNA Repairs Complete Spinal Cord Injury. ACS Nano 2019, 13, 10015–10028. [Google Scholar] [CrossRef]
- Peng, H.; Li, Y.; Ji, W.; Zhao, R.; Lu, Z.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Wang, J.; et al. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson’s Disease. ACS Nano 2022, 16, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell–Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Opthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Alonso, M.L.; García-Posadas, L.; Diebold, Y. Extracellular Vesicles from Human Adipose-Derived Mesenchymal Stem Cells: A Review of Common Cargos. Stem Cell Rev. Rep. 2021, 18, 854–901. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.-E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef]
- Agorogiannis, G.I.; Alexaki, V.-I.; Castana, O.; Kymionis, G.D. Topical application of autologous adipose-derived mesenchymal stem cells (MSCs) for persistent sterile corneal epithelial defect. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Chen, X.; Cao, H.; Zheng, L.; Li, Q.; Zhang, K.; Han, Z.; Han, Z.-C.; Guo, Z.; Li, Z.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Corneal Wound Repair. Stem Cells Int. 2019, 2019, 9. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, F.; Jiang, Y.; Wang, Y.; Li, Z.; Shi, X.; Zhu, Y.; Wang, H.; Zhang, Z. Roles of Exosomes in Ocular Diseases. Int. J. Nanomed. 2020, 15, 10519–10538. [Google Scholar] [CrossRef]
- Coulson-Thomas, V.J.; Caterson, B.; Kao, W.W.-Y. Transplantation of human umbilical mesenchymal stem cells cures the corneal defects of mucopolysaccharidosis VII mice. Stem Cells 2013, 31, 2116–2126. [Google Scholar] [CrossRef] [PubMed]
- Samaeekia, R.; Rabiee, B.; Putra, I.; Shen, X.; Park, Y.J.; Hematti, P.; Eslani, M.; Djalilian, A.R. Effect of Human Corneal Mesenchymal Stromal Cell-derived Exosomes on Corneal Epithelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Zheng, Q.-Q.; Shen, J.; Li, Q.-S.; Song, X.-H.; Luo, H.-B.; Hong, C.-Y.; Yao, K. Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin. Med. J. 2018, 131, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H.-F. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef]
- Goerig, M.; Bacon, D.; van Zundert, A. Carl Koller, cocaine, and local anesthesia: Some less known and forgotten facts. Reg. Anesth. Pain Med. 2012, 37, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Jeng, B.H.; Dupps, W.J. Autologous Serum 50% Eyedrops in the Treatment of Persistent Corneal Epithelial Defects. Cornea 2009, 28, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Utine, C.A.; Durmaz, C.E.; Koçak, N. Corneal matrix repair therapy with the regenerating agent in neurotrophic persistent epithelial defects. Int. J. Ophthalmol. 2017, 10, 1935–1939. [Google Scholar] [PubMed]
- Versura, P.; Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Campos, E.C. Neurotrophic keratitis: Current challenges and future prospects. Eye Brain 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Heinz, C.; Eckstein, A.; Steuhl, K.-P.; Meller, D. Amniotic Membrane Transplantation for Reconstruction of Corneal Ulcer in Graves Ophthalmopathy. Cornea 2004, 23, 524–526. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther.-Nucleic. Acids 2020, 21, 512–522. [Google Scholar] [CrossRef]
- Shaheen, B.S.; Bakir, M.; Jain, S. Corneal nerves in health and disease. Surv. Ophthalmol. 2014, 59, 263–285. [Google Scholar] [CrossRef] [PubMed]
- Tidball, A.M.; Niu, W.; Ma, Q.; Takla, T.N.; Walker, J.C.; Margolis, J.L.; Mojica-Perez, S.P.; Sudyk, R.; Moore, S.J.; Chopra, R.; et al. Self-organizing Single-Rosette Brain Organoids from Human Pluripotent Stem Cells. Biorxiv 2022, 2022, 482350. [Google Scholar] [CrossRef]
- Zhou, T.; He, C.; Lai, P.; Yang, Z.; Liu, Y.; Xu, H.; Lin, X.; Ni, B.; Ju, R.; Yi, W.; et al. miR-204–containing exosomes ameliorate GVHD-associated dry eye disease. Sci. Adv. 2022, 8, eabj9617. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.L.; Roach, J.M. Treatment of Dry Eye Disease. Consult. Pharm 2016, 31, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.; Teng, L.; Chen, C.; Lo, T.; Hung, W.; Chen, H.; Hsueh, P.; Tsai, J. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Lasers Surg. Med. 2009, 41, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Baiula, M.; Spampinato, S. Experimental Pharmacotherapy for Dry Eye Disease: A Review. J. Exp. Pharmacol. 2021, 13, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, P.; Xu, J.; Liu, Y.; Li, H.; Wang, L.; Di, G. hADSCs derived extracellular vesicles inhibit NLRP3inflammasome activation and dry eye. Sci. Rep. 2020, 10, 14521. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Di Lauro, S.; García-Gutierrez, M.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019, 185, 107671. [Google Scholar] [CrossRef]
- Nuzzi, R.; Caselgrandi, P.; Vercelli, A. Effect of Mesenchymal Stem Cell-Derived Exosomes on Retinal Injury: A Review of Current Findings. Stem Cells Int. 2020, 2020, 8883616. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.-R.; Zhang, X.-M. Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J. Stem Cells 2020, 12, 178–187. [Google Scholar] [CrossRef]
- Yu, B.; Shao, H.; Su, C.; Jiang, Y.; Chen, X.; Bai, L.; Zhang, Y.; Li, Q.; Zhang, X.; Li, X. Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci. Rep. 2016, 6, srep34562. [Google Scholar] [CrossRef]
- Sun, F.; Xu, W.; Qian, H.J.A.J.O.T.R. The emerging role of extracellular vesicles in retinal diseases. Am. J. Transl. Res. 2021, 13, 13227. [Google Scholar] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, 93751. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic Retinopathy: Pathophysiology and Treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Maisto, R.; Trotta, M.C.; Petrillo, F.; Izzo, S.; Cuomo, G.; Alfano, R.; Hermenean, A.; Barcia, J.M.; Galdiero, M.; Platania, C.B.M.; et al. Corrigendum: Resolvin D1 Modulates the Intracellular VEGF-Related miRNAs of Retinal Photoreceptors Challenged With High Glucose. Front. Pharmacol. 2020, 11, 871. [Google Scholar] [CrossRef]
- Huang, C.; Fisher, K.P.; Hammer, S.S.; Navitskaya, S.; Blanchard, G.J.; Busik, J.V. Plasma Exosomes Contribute to Microvascular Damage in Diabetic Retinopathy by Activating the Classical Complement Pathway. Diabetes 2018, 67, 1639–1649. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Kong, Y. Exosomes Derived from Mesenchymal Stem Cells Modulate miR-126 to Ameliorate Hyperglycemia-Induced Retinal Inflammation Via Targeting HMGB1. Investig. Opthalmol. Vis. Sci. 2019, 60, 294–303. [Google Scholar] [CrossRef]
- Safwat, A.; Sabry, D.; Ragiae, A.; Amer, E.; Mahmoud, R.; Shamardan, R. Adipose mesenchymal stem cells–derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark. 2018, 7, 1849454418807827. [Google Scholar] [CrossRef]
- Sanghani, A.; Andriesei, P.; Kafetzis, K.N.; Tagalakis, A.D.; Yu-Wai-Man, C. Advances in exosome therapies in ophthalmology–From bench to clinical trial. Acta Ophthalmol. 2022, 100, 243–252. [Google Scholar] [CrossRef]
- Paukku, K.; Silvennoinen, O. STATs as critical mediators of signal transduction and transcription: Lessons learned from STAT5. Cytokine Growth Factor Rev. 2004, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jin, L.; Cui, Y.; Nie, A.; Xie, N.; Liang, G. Bone marrow mesenchymal stem cells-induced exosomal microRNA-486-3p protects against diabetic retinopathy through TLR4/NF-κB axis repression. J. Endocrinol. Investig. 2021, 44, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Strauss, R.W.; Lu, L.; Hafiz, G.; Wolfson, Y.; Shah, S.M.; Sophie, R.; Mir, T.A.; Scholl, H.P. Is there Excess Oxidative Stress and Damage in Eyes of Patients with Retinitis Pigmentosa? Mary Ann Liebert, Inc.: New Rochelle, NY, USA, 2015. [Google Scholar]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Nebbioso, M. Antioxidant and Biological Properties of Mesenchymal Cells Used for Therapy in Retinitis Pigmentosa. Antioxidants 2020, 9, 983. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, T.; Yang, Z.; Sun, X.; Huang, Z.; Deng, X.; He, C.; Liu, X.J.I.O. Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Attenuate Neuroinflammation and Promote Survival of Photoreceptor in Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3108. [Google Scholar]
- Mangunsong, C.O.; Wirohadidjojo, Y.W.; Djaja, M.S.; Sartika, C.R.; Wijaya, A.; Putera, B.W.; Sasongko, M.B. Development, Multifocalelectroretinography Result before and after Peribulbar Injection of Allogeneic Umbilical Cord–Mesenchymal Stem Cell Secretome for Late-Stage Retinitis Pigmentosa. Indian J. Public Health Res. Dev. 2021, 12, 322–329. [Google Scholar]
- Wilhelm, H.; Schabet, M. The Diagnosis and Treatment of Optic Neuritis. Dtsch. Ärzteblatt Int. 2015, 112, 616–626. [Google Scholar] [CrossRef]
- Ahmed, Z.; Douglas, M.R.; John, G.; Berry, M.; Logan, A. AMIGO3 is an NgR1/p75 co-receptor signalling axon growth inhibition in the acute phase of adult central nervous system injury. PLoS ONE 2013, 8, e61878. [Google Scholar] [CrossRef]
- Ahmed, Z.; Fulton, D.; Douglas, M.R. Opicinumab: Is it a potential treatment for multiple sclerosis? Ann. Transl. Med. 2020, 8, 892. [Google Scholar] [CrossRef]
- Marangon, D.; Boda, E.; Parolisi, R.; Negri, C.; Giorgi, C.; Montarolo, F.; Perga, S.; Bertolotto, A.; Buffo, A.; Abbracchio, M.P.; et al. In vivo silencing of miR-125a-3p promotes myelin repair in models of white matter demyelination. Glia 2020, 68, 2001–2014. [Google Scholar] [CrossRef]
- Tripathi, A.; Volsko, C.; Garcia, J.P.; Agirre, E.; Allan, K.C.; Tesar, P.J.; Trapp, B.D.; Castelo-Branco, G.; Sim, F.J.; Dutta, R.; et al. Faculty Opinions recommendation of Oligodendrocyte Intrinsic miR-27a Controls Myelination and Remyelination. Cell Rep. 2019, 29, 904–919. [Google Scholar] [CrossRef]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of Brain Inflammatory Diseases by Delivering Exosome Encapsulated Anti-inflammatory Drugs from the Nasal Region to the Brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Management of ischemic optic neuropathies. Indian J. Ophthalmol. 2011, 59, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S. Controversies on neuroprotection therapy in non-arteritic anterior ischaemic optic neuropathy. Br. J. Ophthalmol. 2019, 104, 153–156. [Google Scholar] [CrossRef]
- Rath, E.Z.; Hazan, Z.; Adamsky, K.; Solomon, A.; Segal, Z.I.; Levin, L.A. Randomized controlled phase 2a study of RPh201 in previous nonarteritic anterior ischemic optic neuropathy. J. Neuro Ophthalmol. Off. J. N. Am. Neuro Ophthalmol. Soc. 2019, 39, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Ravichandran, S. Corneal graft rejection after COVID-19 vaccination. Indian J. Ophthalmol. 2021, 69, 1953–1954. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2016, 6, 1273–1285. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, S.; Lee, C.; Kumar, A.; Arjunan, P.; Li, Y.; Zhang, F.; Li, X. An Optic Nerve Crush Injury Murine Model to Study Retinal Ganglion Cell Survival. JoVE 2011, 50, e2685. [Google Scholar]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef]
- Schuster, A.K.; Erb, C.; Hoffmann, E.M.; Dietlein, T.; Pfeiffer, N. The Diagnosis and Treatment of Glaucoma. Deutsches Ärzteblatt Int. 2020, 117, 225–234. [Google Scholar] [CrossRef]
- Kolko, M. Present and New Treatment Strategies in the Management of Glaucoma. Open Ophthalmol. J. 2015, 9, 89–100. [Google Scholar]
- Liu, Y.; Bailey, J.C.; Helwa, I.; Dismuke, W.M.; Cai, J.; Drewry, M.; Brilliant, M.H.; Budenz, D.L.; Christen, W.G.; Chasman, D.I.; et al. A Common Variant in MIR182 Is Associated With Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Investig. Opthalmol. Vis. Sci. 2016, 57, 4528–4535. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell–Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Opthalmology Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Cullather, E.; Nakaya, N.; Niu, Y.; Kole, C.; Ahmed, Z.; Tomarev, S. Viral delivery of multiple miRNAs promotes retinal ganglion cell survival and functional preservation after optic nerve crush injury. Exp. Eye Res. 2020, 197, 108071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Zheng, J.; Wang, X.-Z.; Wang, X.; Liu, W.-J.; Gao, J.-L. Mesenchymal stem cell-derived exosomes protect trabecular meshwork from oxidative stress. Sci. Rep. 2021, 11, 14863. [Google Scholar] [CrossRef] [PubMed]
- Meldolesi, J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Fang, H.; Kusuma, G.D.; Schwab, R.; Barabadi, M.; Chan, S.T.; McDonald, H.; Leong, C.M.; Wallace, E.M.; Greening, D.W. Impact of chemically defined culture media formulations on extracellular vesicle production by amniotic epithelial cells. Proteomics 2021, 21, e2000080. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Bryniarski, K. Perspectives in Manipulating EVs for Therapeutic Applications: Focus on Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 4623. [Google Scholar] [CrossRef]
- Piffoux, M.; Silva, A.K.A.; Wilhelm, C.; Gazeau, F.; Tareste, D. Modification of Extracellular Vesicles by Fusion with Liposomes for the Design of Personalized Biogenic Drug Delivery Systems. ACS Nano 2018, 12, 6830–6842. [Google Scholar] [CrossRef]
- Tang, T.-T.; Wang, B.; Lv, L.-L.; Liu, B.-C. Extracellular vesicle-based Nanotherapeutics: Emerging frontiers in anti-inflammatory therapy. Theranostics 2020, 10, 8111–8129. [Google Scholar] [CrossRef]
- Gong, M.; Wang, M.; Xu, J.; Yu, B.; Wang, Y.-G.; Liu, M.; Ashraf, M.; Xu, M. Nano-Sized Extracellular Vesicles Secreted from GATA-4 Modified Mesenchymal Stem Cells Promote Angiogenesis by Delivering Let-7 miRNAs. Cells 2022, 11, 1573. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, D.; Sun, L.; Duke, L.C.; Patel, N.; Surapaneni, S.K.; Singh, M.; Meckes, D.G. Epstein-Barr Virus LMP1 Promotes Syntenin-1- and Hrs-Induced Extracellular Vesicle Formation for Its Own Secretion to Increase Cell Proliferation and Migration. mBio 2020, 11, e00589-20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Heon, M.; Zhao, Z.; He, M. Microfluidic engineering of exosomes: Editing cellular messages for precision therapeutics. Lab Chip 2018, 18, 1690–1703. [Google Scholar] [CrossRef]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Hao, M.; Yeo, S.K.; Turner, K.; Harold, A.; Yang, Y.; Zhang, X.; Guan, J.-L. Autophagy Blockade Limits HER2+ Breast Cancer Tumorigenesis by Perturbing HER2 Trafficking and Promoting Release Via Small Extracellular Vesicles. Dev. Cell 2021, 56, 341–355.e5. [Google Scholar] [CrossRef]
- Schiffelers, R.; Kooijmans, S.; Vader, P.; Dommelen, V.; Van Solinge, W. Exosome mimetics: A novel class of drug delivery systems. Int. J. Nanomed. 2012, 7, 1525–1541. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Teng, Y.; Samykutty, A.; Mu, J.; Deng, Z.; Zhang, L.; Cao, P.; Rong, Y.; Yan, J.; Miller, D.; et al. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef]
- Zhao, Z.; McGill, J.; Gamero-Kubota, P.; He, M. Microfluidic on-demand engineering of exosomes towards cancer immunotherapy. Lab Chip 2019, 19, 1877–1886. [Google Scholar] [CrossRef]
- Armstrong, J.P.K.; Holme, M.N.; Stevens, M.M. Re-Engineering Extracellular Vesicles as Smart Nanoscale Therapeutics. ACS Nano 2017, 11, 69–83. [Google Scholar] [CrossRef]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef] [PubMed]
- Kanninen, K.M.; Bister, N.; Koistinaho, J.; Malm, T. Exosomes as new diagnostic tools in CNS diseases. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2016, 1862, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.C.; Liu, J. Epigenetics and Signaling Pathways in Glaucoma. BioMed Res. Int. 2017, 2017, 12. [Google Scholar] [CrossRef]
- Ludwig, N.; Whiteside, T.L.; Reichert, T.E. Challenges in Exosome Isolation and Analysis in Health and Disease. Int. J. Mol. Sci. 2019, 20, 4684. [Google Scholar] [CrossRef]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-based immunotherapy: A promising approach for cancer treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef] [PubMed]



| Isolation Method | Principal Mechanism | Equipment | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Differential centrifugation | Centrifugal force with ultrahigh-speed rotation | Centrifuge, ultracentrifuge, high-speed rotors, and rotary tubes | Straight forward, no additional chemical substance or reaction, high capacity, low processing cost, and high reproducibility | Expensive machinery and specialized equipment. Medium to high processing time (120–600 min) | [43,44] |
| Density gradient ultracentrifugation | Buoyant density in chemical solutions | Centrifuge, ultracentrifuge, high-speed rotors, and rotary tubes | Accurate particle separation, high capacity, minimum contamination chance | Expensive machinery and specialized equipment. High processing time (250 min–2 d) | [39,40,41] |
| Size exclusion chromatography | Separation of biomolecules based on their hydrodynamic radios | Porous beads | Prevention of EV aggregation and preserving their integrity. Low to medium processing time (1 mL/min) | Prospective contaminants of the target size and size overlap confusion. Low capacity | [45] |
| Commercial kit precipitation | Decreasing the solubility of EVs by polymer coating | Superhydrophobic polymers (e.g., PEG) and centrifugation | Simplicity, no need for further chemicals, and fast process (30–60 min) | Unspecified isolation, high polymer contamination, expensive, and low capacity | [46] |
| Immunoprecipitation | Antibody–tetraspanin bounding | Antibody-coated microbeads | Highly specified isolation, medium processing speed (240 min), high purity | Altered surface properties of EVs, difficult separation from beads, loss of EVs, expensive, and low capacity | [47] |
| Ultrafiltration | Filtration of assorted sizes | Porous membranes, and centrifugation | Highly specified sizes, simplicity, and fast processing (130 min) | Membrane plugging, unwanted contaminants, risk of low yield | [48] |
| Microfluidic | Entrapment of EVs by immunoaffinity or within porous structures | Microfluidic device | Highly specified isolation, simplicity, and fast processing for small sample size | Expensive machinery, small capacity, low yield | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massoumi, H.; Amin, S.; Soleimani, M.; Momenaei, B.; Ashraf, M.J.; Guaiquil, V.H.; Hematti, P.; Rosenblatt, M.I.; Djalilian, A.R.; Jalilian, E. Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders. Int. J. Mol. Sci. 2023, 24, 9006. https://doi.org/10.3390/ijms24109006
Massoumi H, Amin S, Soleimani M, Momenaei B, Ashraf MJ, Guaiquil VH, Hematti P, Rosenblatt MI, Djalilian AR, Jalilian E. Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders. International Journal of Molecular Sciences. 2023; 24(10):9006. https://doi.org/10.3390/ijms24109006
Chicago/Turabian StyleMassoumi, Hamed, Sohil Amin, Mohammad Soleimani, Bita Momenaei, Mohammad Javad Ashraf, Victor H. Guaiquil, Peiman Hematti, Mark I. Rosenblatt, Ali R. Djalilian, and Elmira Jalilian. 2023. "Extracellular-Vesicle-Based Therapeutics in Neuro-Ophthalmic Disorders" International Journal of Molecular Sciences 24, no. 10: 9006. https://doi.org/10.3390/ijms24109006






