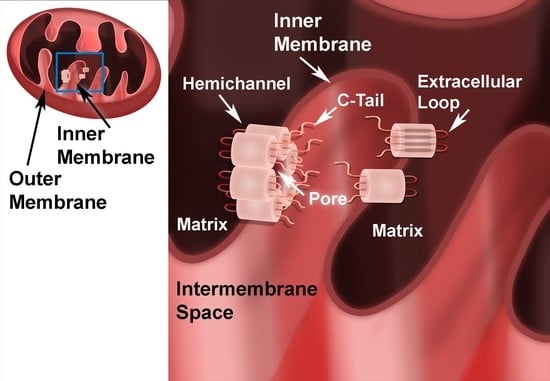
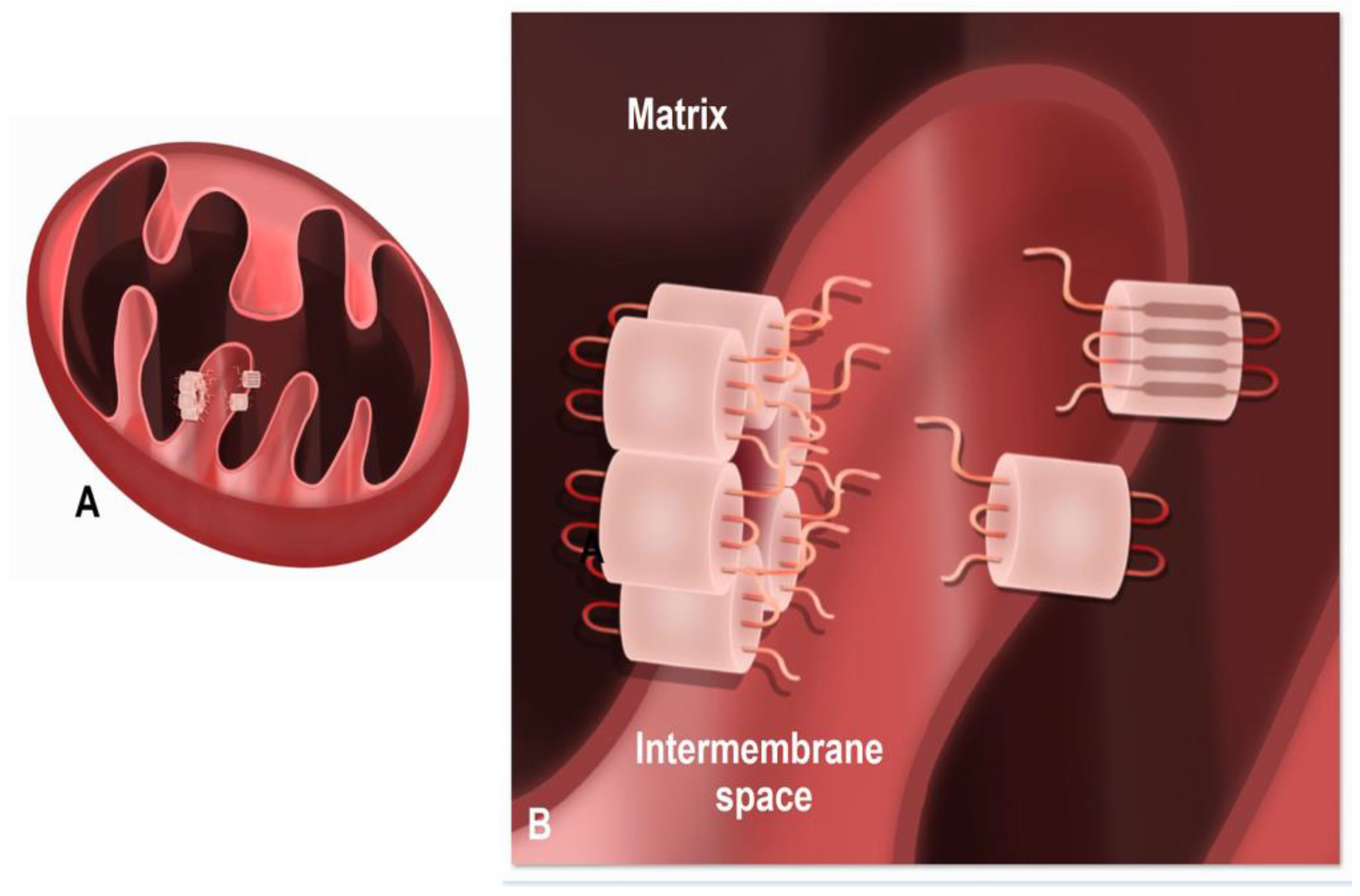
Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure
Abstract
1. Introduction
1.1. Mitochondrial/Gap Junction Plaque Contact Sites
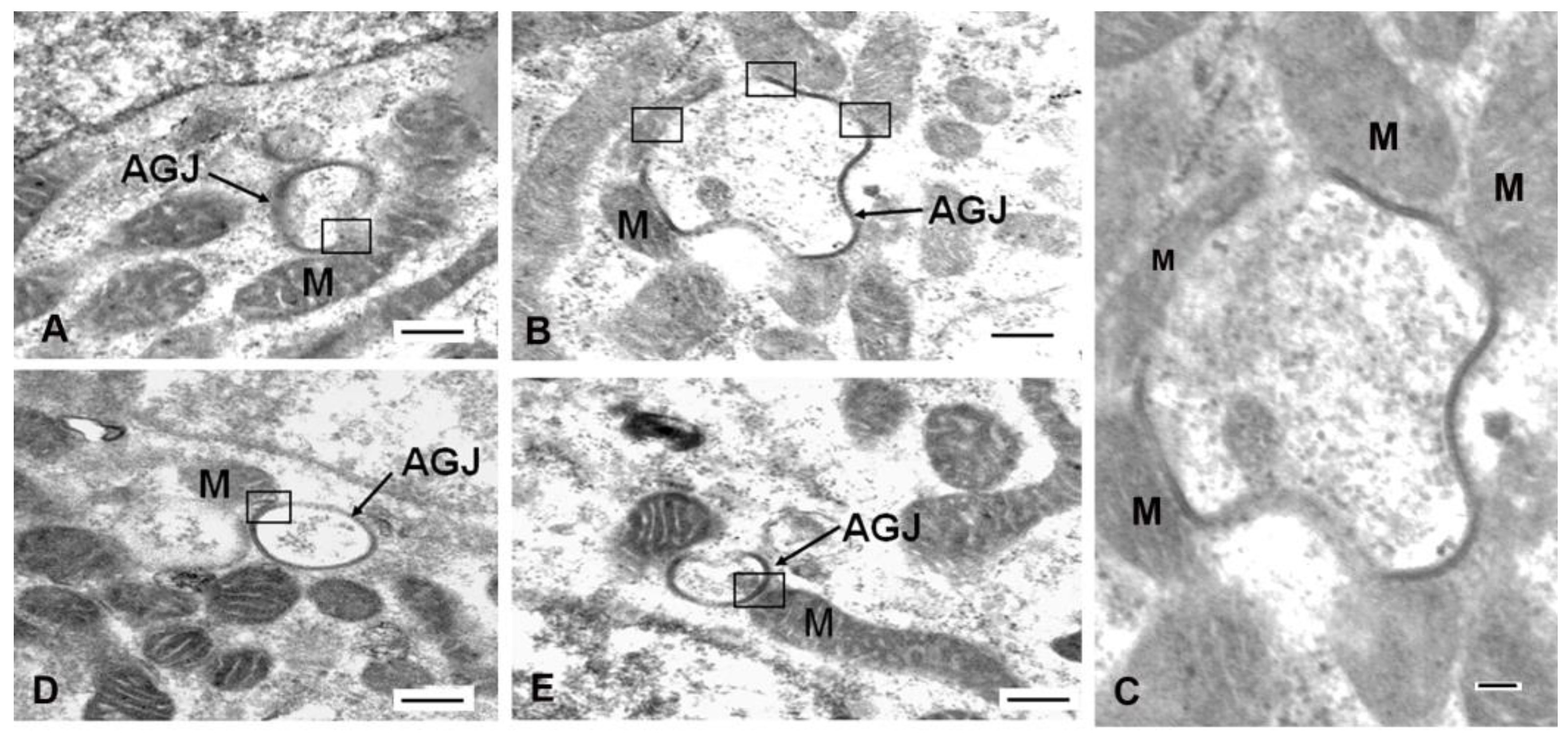
1.2. Mitochondrial/Annular Gap Junction Vesicle Contact Sites
1.3. The Mechanism of Mitochondria/Gap Junction Structure Interactions
1.4. Presence of Connexins within Mitochondria
1.5. Function of Mitochondrial Cx43
1.6. Pathophysiological Significance of Mitochondrial Cx43
2. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prinz, W.A.; Toulmay, A.; Balla, T. The functional universe of membrane contact sites. Nat. Rev. Mol. Cell Biol. 2020, 21, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Brodsky, S.; Kumari, S.; Valiunas, V.; Brink, P.; Kaide, J.; Nasjletti, A.; Goligorsky, M.S. Paradoxical overexpression and translocation of connexin43 in homocysteine-treated endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H2124–H2133. [Google Scholar] [CrossRef] [PubMed]
- Fowler, S.L.; Akins, M.; Zhou, H.; Figeys, D.; Bennett, S.A. The liver connexin32 interactome is a novel plasma membrane-mitochondrial signaling nexus. J. Proteome Res. 2013, 12, 2597–2610. [Google Scholar] [CrossRef] [PubMed]
- Martins-Marques, T.; Ribeiro-Rodrigues, T.; Batista-Almeida, D.; Aasen, T.; Kwak, B.R.; Girao, H. Biological Functions of Connexin43 Beyond Intercellular Communication. Trends Cell Biol. 2019, 29, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Riquelme, M.A.; Hua, R.; Acosta, F.M.; Gu, S.; Jiang, J.X. Connexin 43 hemichannels regulate mitochondrial ATP generation, mobilization, and mitochondrial homeostasis against oxidative stress. eLife 2022, 11, e82206. [Google Scholar] [CrossRef]
- Bell, C.L.; Shakespeare, T.I.; Murray, S.A. Visualization of Annular Gap Junction Vesicle Processing: The Interplay Between Annular Gap Junctions and Mitochondria. Int. J. Mol. Sci. 2018, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Garant, P.R. The demonstration of complex gap junctions between the cells of the enamel organ with lanthanum nitrate. J. Ultrastruct. Res. 1972, 40, 333–348. [Google Scholar] [CrossRef]
- Nunez, E.A. Secretory processes in follicular cells of the bat thyroid. II. The occurrence of organelle-associated intercellular junctions during late hibernation. Am. J. Anat. 1971, 131, 227–239. [Google Scholar] [CrossRef]
- Peracchia, C. Connexin/Innexin Channels in Cytoplasmic Organelles. Are There Intracellular Gap Junctions? A Hypothesis! Int. J. Mol. Sci. 2020, 21, 2163. [Google Scholar] [CrossRef]
- Kumar, N.M.; Gilula, N.B. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J. Cell Biol. 1986, 103, 767–776. [Google Scholar] [CrossRef]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, Connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Goodenough, D.A.; Sosinsky, G.E. Formation of the gap junction intercellular channel requires a 30 degree rotation for interdigitating two apposing connexons. J. Mol. Biol. 1998, 277, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Friend, D.S.; Gilula, N.B. Variations in tight and gap junctions in mammalian tissues. J. Cell Biol. 1972, 53, 758–776. [Google Scholar] [CrossRef]
- Kumar, N.M.; Gilula, N.B. Molecular biology and genetics of gap junction channels. Semin. Cell Biol. 1992, 3, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.H. Assembly of gap junction intercellular communication channels. Biochem. Soc. Trans. 1994, 22, 788–792. [Google Scholar] [CrossRef]
- Evans, W.H.; Ahmad, S.; Diez, J.; George, C.H.; Kendall, J.M.; Martin, P.E. Trafficking pathways leading to the formation of gap junctions. Novartis Found. Symp. 2007, 219, 44–59. [Google Scholar] [CrossRef]
- Ahmad, S.; Martin, P.E.; Evans, W.H. Assembly of gap junction channels: Mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. 2001, 268, 4544–4552. [Google Scholar] [CrossRef]
- Weber, P.A.; Chang, H.-C.; Spaeth, K.E.; Nitsche, J.M.; Nicholson, B.J. The permeability of gap junction channels to probes of different size is dependent on connexin composition and permeant-pore affinities. Biophys. J. 2004, 87, 958–973. [Google Scholar] [CrossRef]
- Goldberg, G.S.; Lampe, P.D.; Nicholson, B.J. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat. Cell Biol. 1999, 1, 457–459. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Ri, Y.; Bennett, M.V.L.; Trexler, E.B.; Verselis, V.K.; Bargiello, T.A. Changes in permeability caused by connexin 32 mutations underlie X-linked Charcot-Marie-Tooth disease. Neuron 1997, 19, 927–938. [Google Scholar] [CrossRef]
- Murray, S.A.; Larsen, W.J.; Trout, J.; Donta, S.T. Gap junction assembly and endocytosis correlated with patterns of growth in a cultured adrenocortical tumor cell (SW-13). Cancer Res. 1981, 41, 4063–4074. [Google Scholar] [PubMed]
- Murray, S.A.; Nickel, B.M.; Gay, V.L. Endocytosis of connexin protein in adrenal cells. Endocr. Res. 2004, 30, 647–654. [Google Scholar] [CrossRef]
- Laird, D.W. The life cycle of a connexin: Gap junction formation, removal, and degradation. J. Bioenerg. Biomembr. 1996, 28, 311–318. [Google Scholar] [CrossRef]
- Jordan, K.; Chodock, R.; Hand, A.R.; Laird, D.W. The origin of annular junctions: A mechanism of gap junction internalization. J. Cell Sci. 2001, 114, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.M.; Fong, J.T.; Kells, R.M.; O’Laughlin, M.C.; Kowal, T.J.; Thevenin, A.F. Degradation of endocytosed gap junctions by autophagosomal and endo-/lysosomal pathways: A perspective. J. Membr. Biol. 2012, 245, 465–476. [Google Scholar] [CrossRef]
- Vanderpuye, O.A.; Bell, C.L.; Murray, S.A. Redistribution of connexin 43 during cell division. Cell Biol. Int. 2016, 40, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Defourny, J.; Audouard, C.; Davy, A.; Thiry, M. Efnb2 haploinsufficiency induces early gap junction plaque disassembly and endocytosis in the cochlea. Brain Res. Bull. 2021, 174, 153–160. [Google Scholar] [CrossRef]
- Kotova, A.; Timonina, K.; Zoidl, G.R. Endocytosis of Connexin 36 is Mediated by Interaction with Caveolin-1. Int. J. Mol. Sci. 2020, 21, 5401. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, X.; Zhao, Y.; Gu, J.; Yuan, Y.; Liu, Z.; Zou, H.; Bian, J. Rat Hepatocytes Mitigate Cadmium Toxicity by Forming Annular Gap Junctions and Degrading Them via Endosome-Lysosome Pathway. Int. J. Mol. Sci. 2022, 23, 15607. [Google Scholar] [CrossRef]
- Falk, M.M.; Bell, C.L.; Andrews, R.M.K.; Murray, S.A. Molecular Mechanisms Regulating the Formation, Trafficking and Processing of Annular Gap Junctions. BMC Cell Biol. Sect. Cell-Cell Contacts 2016, 17 (Suppl. 1), 5–23. [Google Scholar] [CrossRef]
- Nickel, B.M.; DeFranco, B.H.; Gay, V.L.; Murray, S.A. Clathrin and Cx43 gap junction plaque endoexocytosis. Biochem. Biophys. Res. Commun. 2008, 374, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.N.; Kwon, H.J.; Im, S.W.; Son, Y.H.; Akindehin, S.; Jung, Y.S.; Lee, S.J.; Rhyu, I.J.; Kim, I.Y.; Seong, J.K.; et al. Connexin 43 is required for the maintenance of mitochondrial integrity in brown adipose tissue. Sci. Rep. 2017, 7, 7159. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Dodoni, G.; Rodriguez-Sinovas, A.; Cabestrero, A.; Ruiz-Meana, M.; Gres, P.; Konietzka, I.; Lopez-Iglesias, C.; Garcia-Dorado, D.; Di Lisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Hegde, R.S.; Zavodszky, E. Recognition and Degradation of Mislocalized Proteins in Health and Disease. Cold Spring Harb. Perspect. Biol. 2019, 11, a033902. [Google Scholar] [CrossRef]
- Dang, X.; Doble, B.W.; Kardami, E. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol. Cell. Biochem. 2003, 242, 35–38. [Google Scholar] [CrossRef]
- Rodriguez-Sinovas, A.; Cabestrero, A.; López, D.; Torre, I.; Morente, M.; Abellán, A.; Miró, E.; Ruiz-Meana, M.; García-Dorado, D. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Prog. Biophys. Mol. Biol. 2007, 94, 219–232. [Google Scholar] [CrossRef]
- Guo, R.; Si, R.; Scott, B.T.; Makino, A. Mitochondrial connexin40 regulates mitochondrial calcium uptake in coronary endothelial cells. Am. J. Physiol. Cell Physiol. 2017, 312, C398–C406. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Ruiz-Meana, M.; Agullo, E.; Stahlhofen, S.; Rodriguez-Sinovas, A.; Cabestrero, A.; Jorge, I.; Torre, I.; Vazquez, J.; Boengler, K.; et al. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc. Res. 2009, 83, 747–756. [Google Scholar] [CrossRef]
- Azarashvili, T.; Baburina, Y.; Grachev, D.; Krestinina, O.; Evtodienko, Y.; Stricker, R.; Reiser, G. Calcium-induced Permeability Transition in Rat Brain Mitochondria Is Promoted by Carbenoxolone through Targeting Connexin43. Am. J. Physiol. Cell Physiol. 2011, 300, C707–C720. [Google Scholar] [CrossRef]
- Kozoriz, M.G.; Church, J.; Ozog, M.A.; Naus, C.C.; Krebs, C. Temporary sequestration of potassium by mitochondria in astrocytes. J. Biol. Chem. 2010, 285, 31107–31119. [Google Scholar] [CrossRef]
- Trudeau, K.; Muto, T.; Roy, S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Investig. Opthalmology Vis. Sci. 2012, 53, 6675–6681. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sinovas, A.; Ruiz-Meana, M.; Denuc, A.; Garcia-Dorado, D. Mitochondrial Cx43, an important component of cardiac preconditioning. Biochim. Biophys. Acta Biomembr. 2018, 1860, 174–181. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Y.; Zheng, B.; Wang, Y.; Miao, C.; Su, Y.; Li, K.; Wang, X.; He, X.; Wu, X.; et al. Bilirubin Improves Gap Junction to Alleviate Doxorubicin-Induced Cardiotoxicity by Regulating AMPK-Axl-SOCS3-Cx43 Axis. Front. Pharmacol. 2022, 13, 828890. [Google Scholar] [CrossRef]
- Leybaert, L.; De Smet, M.A.; Lissoni, A.; Allewaert, R.; Roderick, H.L.; Bultynck, G.; Delmar, M.; Sipido, K.R.; Witschas, K. Connexin hemichannels as candidate targets for cardioprotective and anti-arrhythmic treatments. J. Clin. Investig. 2023, 133, e168117. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Sinovas, A.; Sánchez, J.A.; Valls-Lacalle, L.; Consegal, M.; Ferreira-González, I. Connexins in the Heart: Regulation, Function and Involvement in Cardiac Disease. Int. J. Mol. Sci. 2021, 22, 4413. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Konietzka, I.; Buechert, A.; Heinen, Y.; Garcia-Dorado, D.; Heusch, G.; Schulz, R. Loss of ischemic preconditioning’s cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1764–H1769. [Google Scholar] [CrossRef]
- Pecoraro, M.; Pinto, A.; Popolo, A. Inhibition of Connexin 43 translocation on mitochondria accelerates CoCl2-induced apoptotic response in a chemical model of hypoxia. Toxicol. Vitr. 2018, 47, 120–128. [Google Scholar] [CrossRef]
- Peracchia, C. Low resistance junctions in crayfish: I. Two arrays of globules in junctional membranes. J. Cell Biol. 1973, 57, 54–65. [Google Scholar] [CrossRef]
- Forbes, M.S.; Sperelakis, N. Association between mitochondria and gap junctions in mammalian myocardial cells. Tissue Cell 1982, 14, 25–37. [Google Scholar] [CrossRef]
- de Oca Balderas, P.M. Mitochondria-plasma membrane interactions and communication. J. Biol. Chem. 2021, 297, 101164. [Google Scholar] [CrossRef]
- Peracchia, C.; Robertson, J.D. Increase in osmiophilia of axonal membranes of crayfish as a result of electrical stimulation, asphyxia, or treatment with reducing agents. J. Cell Biol. 1971, 51, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Csordás, G.; Renken, C.; Várnai, P.; Walter, L.; Weaver, D.; Buttle, K.F.; Balla, T.; Mannella, C.A.; Hajnόczky, G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 2006, 174, 915–921. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.R.; Webster, B.M.; Mastronarde, D.N.; Verhey, K.J.; Voeltz, G.K. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 2010, 190, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Leybaert, L.; Ruiz-Meana, M.; Schulz, R. Connexin 43 in Mitochondria: What Do We Really Know about Its Function? Front. Physiol. 2022, 13, 928934. [Google Scholar] [CrossRef]
- Rodriguez-Sinovas, A.; Sánchez, J.A.; González-Loyola, A.; Barba, I.; Morente, M.; Aguilar, R.; Agulló, E.; Miró-Casas, E.; Esquerda, N.; Ruiz-Meana, M.; et al. Effects of substitution of Cx43 by Cx32 on myocardial energy metabolism, tolerance to ischaemia and preconditioning protection. J. Physiol. 2010, 588, 1139–1151. [Google Scholar] [CrossRef]
- Boengler, K.; Ruiz-Meana, M.; Gent, S.; Ungefug, E.; Soetkamp, D.; Miro-Casas, E.; Cabestrero, A.; Fernandez-Sanz, C.; Semenzato, M.; Di Lisa, F.; et al. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J. Cell. Mol. Med. 2012, 16, 1649–1655. [Google Scholar] [CrossRef]
- Fry, G.N.; Devine, C.E.; Burnstock, G. Freeze-fracture studies of nexuses between smooth muscle cells. Close relationship to sarcoplasmic reticulum. J. Cell Biol. 1977, 72, 26–34. [Google Scholar] [CrossRef]
- Peracchia, C.; Peracchia, L.L. Gap junction dynamics: Reversible effects of hydrogen ions. J. Cell Biol. 1980, 87, 719–727. [Google Scholar] [CrossRef]
- Peracchia, C.; Peracchia, L.L. Bridges linking gap junction particles extracellularly: A freeze-etching rotary-shadowing study of split junctions. Eur. J. Cell Biol. 1985, 36, 286–293. [Google Scholar]
- Peracchia, C. Low resistance junctions in crayfish: II. Structural details and further evidence for intercellular channels by freeze-fracture and negative staining. J. Cell Biol. 1973, 57, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Emdad, L.; Uzzaman, M.; Takagishi, Y.; Honjo, H.; Uchida, T.; Severs, N.J.; Kodama, I.; Murata, Y. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: Prevention by angiotensin II type 1 receptor blockade. J. Mol. Cell. Cardiol. 2001, 33, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.M.; Baker, S.M.; Gumpert, A.M.; Segretain, D.; Buckheit, R.W., 3rd. Gap junction turnover is achieved by the internalization of small endocytic double-membrane vesicles. Mol. Biol. Cell 2009, 20, 3342–3352. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Ysselstein, D.; Krainc, D. Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 2018, 554, 382–386. [Google Scholar] [CrossRef]
- Albertini, D.F.; Anderson, E. Structural modifications of lutein cell gap junctions during pregnancy in the rat and the mouse. Anat. Rec. 1975, 181, 171–194. [Google Scholar] [CrossRef]
- Connell, C.J.; Connell, G.M. The interstitial tissue of the testis. In The Testis; Johnson, A.D., Gomes, W.R., Eds.; Academic Press: New York, NY, USA, 1977; pp. 333–369. [Google Scholar]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef]
- Rowland, A.A.; Voeltz, G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Norris, R.P. Transfer of mitochondria and endosomes between cells by gap junction internalization. Traffic 2021, 22, 174–179. [Google Scholar] [CrossRef]
- Norris, R.P.; Terasaki, M. Gap junction internalization and processing in vivo: A 3D immuno-electron microscopy study. J. Cell Sci. 2021, 134, jcs252726. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front. Oncol. 2021, 11, 672781. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014, 3, 24641. [Google Scholar] [CrossRef]
- Soares, A.R.; Martins-Marques, T.; Ribeiro-Rodrigues, T.; Ferreira, J.V.; Catarino, S.; Pinho, M.J.; Zuzarte, M.; Anjo, S.I.; Manadas, B.; Sluijter, J.P.G.; et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci. Rep. 2015, 5, 13243–13256, Erratum in Sci. Rep. 2015, 5, 14888. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Src Regulation of Cx43 Phosphorylation and Gap Junction Turnover. Biomolecules 2020, 10, 1596. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.; Unger, V.M.; Mitra, A.K. Three-dimensional structure of membrane proteins determined by two-dimensional crystallization, electron cryomicroscopy, and image analysis. Methods Enzym. 1999, 294, 135–180. [Google Scholar]
- Beyer, E.C.; Lipkind, G.M.; Kyle, J.W.; Berthoud, V.M. Structural organization of intercellular channels II. Amino terminal domain of the connexins: Sequence, functional roles, and structure. Biochim. Biophys. Acta 2012, 1818, 1823–1830. [Google Scholar] [CrossRef]
- Kopanic, J.L.; Sorgen, P.L. Chemical shift assignments of the connexin45 carboxyl terminal domain: Monomer and dimer conformations. Biomol. NMR Assign. 2013, 7, 293–297. [Google Scholar] [CrossRef]
- Bouvier, D.; Spagnol, G.; Chenavas, S.; Kieken, F.; Vitrac, H.; Brownell, S.; Kellezi, A.; Forge, V.; Sorgen, P.L. Characterization of the structure and intermolecular interactions between the connexin40 and connexin43 carboxyl-terminal and cytoplasmic loop domains. J. Biol. Chem. 2009, 284, 34257–34271. [Google Scholar] [CrossRef] [PubMed]
- Sorgen, P.L.; Duffy, H.S.; Sahoo, P.; Coombs, W.; Delmar, M.; Spray, D.C. Structural changes in the carboxyl terminus of the gap junction protein connexin43 indicates signaling between binding domains for c-Src and zonula occludens-1. J. Biol. Chem. 2004, 279, 54695–54701. [Google Scholar] [CrossRef] [PubMed]
- Nickel, B.; Boller, M.; Schneider, K.; Shakespeare, T.; Gay, V.; Murray, S.A. Visualizing the effect of dynamin inhibition on annular gap vesicle formation and fission. J. Cell Sci. 2013, 126, 2607–2616. [Google Scholar] [CrossRef]
- Ginzberg, R.D.; Gilula, N.B. Modulation of cell junctions during differentiation of the chicken otocyst sensory epithelium. Dev. Biol. 1979, 68, 110–129. [Google Scholar] [CrossRef]
- Ping, H.A.; Kraft, L.M.; Chen, W.; Nilles, A.E.; Lackner, L.L. Num1 anchors mitochondria to the plasma membrane via two domains with different lipid binding specificities. J. Cell Biol. 2016, 213, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Lackner, L.L.; Ping, H.; Graef, M.; Murley, A.; Nunnari, J. Endoplasmic reticulum-associated mitochondria-cortex tether functions in the distribution and inheritance of mitochondria. Proc. Natl. Acad. Sci. USA 2013, 110, E458–E467. [Google Scholar] [CrossRef] [PubMed]
- Gago-Fuentes, R.; Fernández-Puente, P.; Megias, D.; Carpintero-Fernández, P.; Mateos, J.; Acea, B.; Fonseca, E.; Blanco, F.J.; Mayan, M.D. Proteomic Analysis of Connexin 43 Reveals Novel Interactors Related to Osteoarthritis. Mol. Cell. Proteom. 2015, 14, 1831–1845. [Google Scholar] [CrossRef] [PubMed]
- Kraft, L.M.; Lackner, L.L. Mitochondria-driven assembly of a cortical anchor for mitochondria and dynein. J. Cell Biol. 2017, 216, 3061–3071. [Google Scholar] [CrossRef] [PubMed]
- Slivko-Koltchik, G.A.; Kuznetsov, V.P.; Panchin, Y.V. Are there gap junctions without connexins or pannexins? BMC Evol. Biol. 2019, 19, 46. [Google Scholar] [CrossRef]
- Le Vasseur, M.; Chen, V.C.; Huang, K.; Vogl, W.A.; Naus, C.C. Pannexin 2 Localizes at ER-Mitochondria Contact Sites. Cancers 2019, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Chen, L.; Li, L. Pannexin-2, a novel mitochondrial-associated membrane protein, may become the new strategy to treat and prevent neurological disorders. Acta Biochim. Biophys. Sin. 2020, 152, 1178–1180. [Google Scholar] [CrossRef]
- Wang, J.D.; Shao, Y.; Liu, D.; Liu, N.Y.; Zhu, D.Y. Rictor/mTORC2 involves mitochondrial function in ES cells derived cardiomyocytes via mitochondrial Connexin43. Acta Pharmacol. Sin. 2021, 42, 1790–1797. [Google Scholar] [CrossRef]
- Ruiz-Meana, M.; Rodriguez-Sinovas, A.; Cabestrero, A.; Boengler, K.; Heusch, G.; Garcia-Dorado, D. Mitochondrial connexin43 as a new player in the pathophysiology of myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2008, 77, 325–333. [Google Scholar] [CrossRef]
- Rodriguez-Sinovas, A.; Boengler, K.; Cabestrero, A.; Gres, P.; Morente, M.; Ruiz-Meana, M.; Konietzka, I.; Miro, E.; Totzeck, A.; Heusch, G.; et al. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ. Res. 2006, 99, 93–101. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Anjo, S.I.; Pereira, P.; Manadas, B.; Girão, H. Interacting Network of the Gap Junction (GJ) Protein Connexin43 (Cx43) is Modulated by Ischemia and Reperfusion in the Heart. Mol. Cell. Proteom. 2015, 14, 3040–3055. [Google Scholar] [CrossRef] [PubMed]
- Gilula, N.B. Topology of gap junction protein and channel function. Ciba Found. Symp. 2007, 125, 128–139. [Google Scholar] [CrossRef]
- Wang, M.; Smith, K.; Yu, Q.; Miller, C.; Singh, K.; Sen, C.K. Mitochondrial Connexin 43 in Sex-dependent Myocardial Responses and Estrogen-Mediated Cardiac Protection following Acute Ischemia/reperfusion Injury. Basic Res. Cardiol. 2019, 115, 1. [Google Scholar] [CrossRef]
- Boengler, K.; Stahlhofen, S.; van de Sand, A.; Gres, P.; Ruiz-Meana, M.; Garcia-Dorado, D.; Heusch, G.; Schulz, R. Presence of connexin 43 in subsarcolemmal, but not in interfibrillar cardiomyocyte mitochondria. Basic Res. Cardiol. 2009, 104, 141–147. [Google Scholar] [CrossRef]
- Goubaeva, F.; Mikami, M.; Giardina, S.; Ding, B.; Abe, J.; Yang, J. Cardiac mitochondrial connexin 43 regulates apoptosis. Biochem. Biophys. Res. Commun. 2007, 352, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gadicherla, A.K.; Wang, N.; Bulic, M.; Agullo-Pascual, E.; Lissoni, A.; De Smet, M.; Delmar, M.; Bultynck, G.; Krysko, D.V.; Camara, A.; et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res. Cardiol. 2017, 112, 27. [Google Scholar] [CrossRef]
- Wang, N.; De Bock, M.; Antoons, G.; Gadicherla, A.K.; Bol, M.; Decrock, E.; Evans, W.H.; Sipido, K.R.; Bukauskas, F.F.; Leybaert, L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res. Cardiol. 2012, 107, 304. [Google Scholar] [CrossRef]
- Boengler, K.; Ungefug, E.; Heusch, G.; Leybaert, L.; Schulz, R. Connexin 43 impacts on mitochondrial potassium uptake. Front. Pharmacol. 2013, 4, 73. [Google Scholar] [CrossRef]
- Waza, A.A.; Bhat, S.A.; Hussain, M.U.; Ganai, B.A. Connexin 43 and ATP-sensitive potassium channels crosstalk: A missing link in hypoxia/ischemia stress. Cell Tissue Res. 2018, 371, 213–222. [Google Scholar] [CrossRef]
- Waza, A.A.; Andrabi, K.; Hussain, M.U. Protein kinase C (PKC) mediated interaction between conexin43 (Cx43) and K+ (ATP) channel subunit (Kir6.1) in cardiomyocyte mitochondria: Implications in cytoprotection against hypoxia induced cell apoptosis. Cell. Signal. 2014, 26, 1909–1917. [Google Scholar] [CrossRef]
- Jones, S.A.; Lancaster, M.K.; Boyett, M.R. Ageing-related changes of connexins and conduction within the sinoatrial node. J. Physiol. 2004, 560, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Kong, W.; Zhang, Q.; Beyer, E.C.; Walcott, G.; Fast, V.G.; Ai, X. c-Jun N-terminal kinase activation contributes to reduced connexin43 and development of atrial arrhythmias. Cardiovasc. Res. 2013, 97, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Bonda, T.A.; Szynaka, B.; Sokoowska, M.; Dziemidowicz, M.; Winnicka, M.M.; Chyczewski, L.; Kamiński, K.A. Remodeling of the intercalated disc related to aging in the mouse heart. J. Cardiol. 2016, 68, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ichinose, S.; Sunamori, M. Age-related changes in gap junctional protein of the rat heart. Exp. Clin. Cardiol. 2004, 9, 130–132. [Google Scholar]
- Benova, T.; Viczenczova, C.; Radosinska, J.; Bacova, B.; Knezl, V.; Dosenko, V.; Weismann, P.; Zeman, M.; Navarova, J.; Tribulova, N. Melatonin attenuates hypertension-related proarrhythmic myocardial maladaptation of connexin-43 and propensity of the heart to lethal arrhythmias. Can. J. Physiol. Pharmacol. 2013, 91, 633–639. [Google Scholar] [CrossRef]
- Gao, R.; Zhang, A.; Jia, Q.; Dang, Z.; Tian, T.; Zhang, J.; Cao, N.; Tang, X.; Ma, K.; Li, L.; et al. The promoting role of Cx43 on the proliferation and migration of arterial smooth muscle cells for angiotensin II-dependent hypertension. Pulm. Pharmacol. Ther. 2021, 70, 102072. [Google Scholar] [CrossRef]
- Bacova, B.S.; Viczenczova, C.; Andelova, K.; Sykora, M.; Chaudagar, K.; Barancik, M.; Adamcova, M.; Knezl, V.; Benova, T.; Weismann, P.; et al. Antiarrhythmic Effects of Melatonin and Omega-3 Are Linked with Protection of Myocardial Cx43 Topology and Suppression of Fibrosis in Catecholamine Stressed Normotensive and Hypertensive Rats. Antioxidants 2020, 9, 546. [Google Scholar] [CrossRef]
- Viczenczova, C.; Kura, B.; Chaudagar, K.K.; Bacova, B.S.; Egan Benova, T.; Barancik, M.; Knezl, V.; Ravingerova, T.; Tribulova, N.; Slezak, J. Myocardial connexin-43 is upregulated in response to acute cardiac injury in rats. Can. J. Physiol. Pharmacol. 2017, 95, 911–919. [Google Scholar] [CrossRef]
- Formigli, L.; Ibba-Manneschi, L.; Perna, A.M.; Pacini, A.; Polidori, L.; Nediani, C.; Modesti, P.A.; Nosi, D.; Tani, A.; Celli, A.; et al. Altered Cx43 expression during myocardial adaptation to acute and chronic volume overloading. Histol. Histopathol. 2003, 18, 359–369. [Google Scholar] [CrossRef]
- Wei, X.; Chang, A.C.H.; Chang, H.; Xu, S.; Xue, Y.; Zhang, Y.; Lei, M.; Chang, A.C.Y.; Zhang, Q. Hypoglycemia-Exacerbated Mitochondrial Connexin 43 Accumulation Aggravates Cardiac Dysfunction in Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 800185. [Google Scholar] [CrossRef]
- Sankaramoorthy, A.; Roy, S. High Glucose-Induced Apoptosis Is Linked to Mitochondrial Connexin 43 Level in RRECs: Implications for Diabetic Retinopathy. Cells 2021, 10, 3102. [Google Scholar] [CrossRef] [PubMed]
- Görbe, A.; Varga, Z.; Kupai, K.; Bencsik, P.; Kocsis, G.F.; Csont, T.; Boengler, K.; Schulz, R.; Ferdinandy, P. Cholesterol diet leads to attenuation of ischemic preconditioning-induced cardiac protection: The role of connexin 43. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1907–H1913. [Google Scholar] [CrossRef] [PubMed]
- Papuć, E.; Rejdak, K. The role of myelin damage in Alzheimer’s disease pathology. Arch. Med. Sci. 2020, 16, 345–351. [Google Scholar] [CrossRef]
- Xiao, S.; Shimura, D.; Baum, R.; Hernandez, D.M.; Agvanian, S.; Nagaoka, Y.; Katsumata, M.; Lampe, P.D.; Kleber, A.G.; Hong, T.; et al. Auxiliary trafficking subunit GJA1-20k protects connexin-43 from degradation and limits ventricular arrhythmias. J. Clin. Investig. 2020, 130, 4858–4870. [Google Scholar] [CrossRef] [PubMed]
- Shimura, D.; Shaw, R.M. GJA1-20k and Mitochondrial Dynamics. Front. Physiol. 2022, 13, 867358. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cetin-Ferra, S.; Francis, S.C.; Cooper, A.T.; Neikirk, K.; Marshall, A.G.; Hinton, A., Jr.; Murray, S.A. Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure. Int. J. Mol. Sci. 2023, 24, 9036. https://doi.org/10.3390/ijms24109036
Cetin-Ferra S, Francis SC, Cooper AT, Neikirk K, Marshall AG, Hinton A Jr., Murray SA. Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure. International Journal of Molecular Sciences. 2023; 24(10):9036. https://doi.org/10.3390/ijms24109036
Chicago/Turabian StyleCetin-Ferra, Selma, Sharon C. Francis, Anthonya T. Cooper, Kit Neikirk, Andrea G. Marshall, Antentor Hinton, Jr., and Sandra A. Murray. 2023. "Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure" International Journal of Molecular Sciences 24, no. 10: 9036. https://doi.org/10.3390/ijms24109036
APA StyleCetin-Ferra, S., Francis, S. C., Cooper, A. T., Neikirk, K., Marshall, A. G., Hinton, A., Jr., & Murray, S. A. (2023). Mitochondrial Connexins and Mitochondrial Contact Sites with Gap Junction Structure. International Journal of Molecular Sciences, 24(10), 9036. https://doi.org/10.3390/ijms24109036