Metformin and Canagliflozin Are Equally Renoprotective in Diabetic Kidney Disease but Have No Synergistic Effect
Abstract
1. Introduction
2. Results
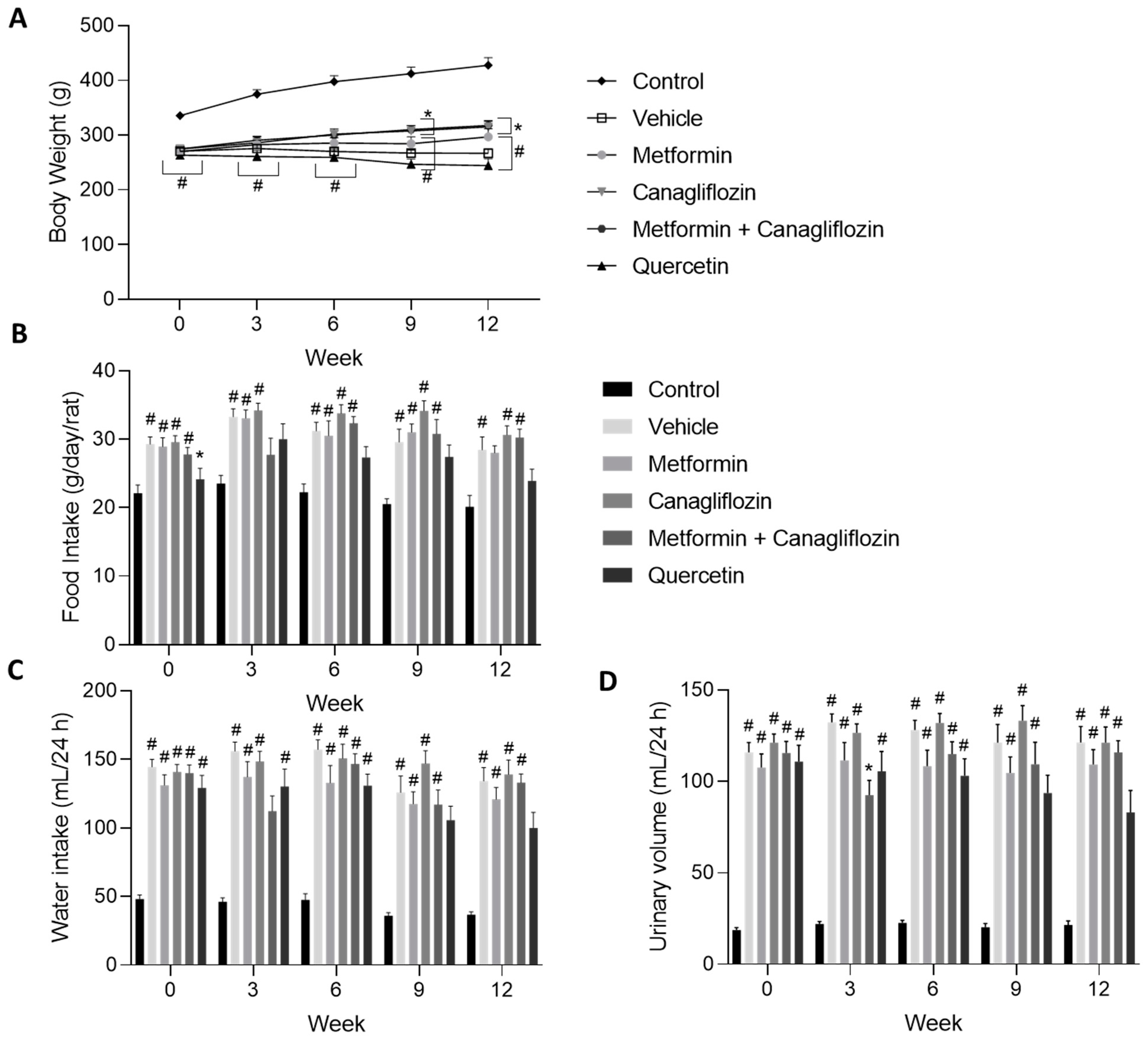
2.1. General Animal Aspects
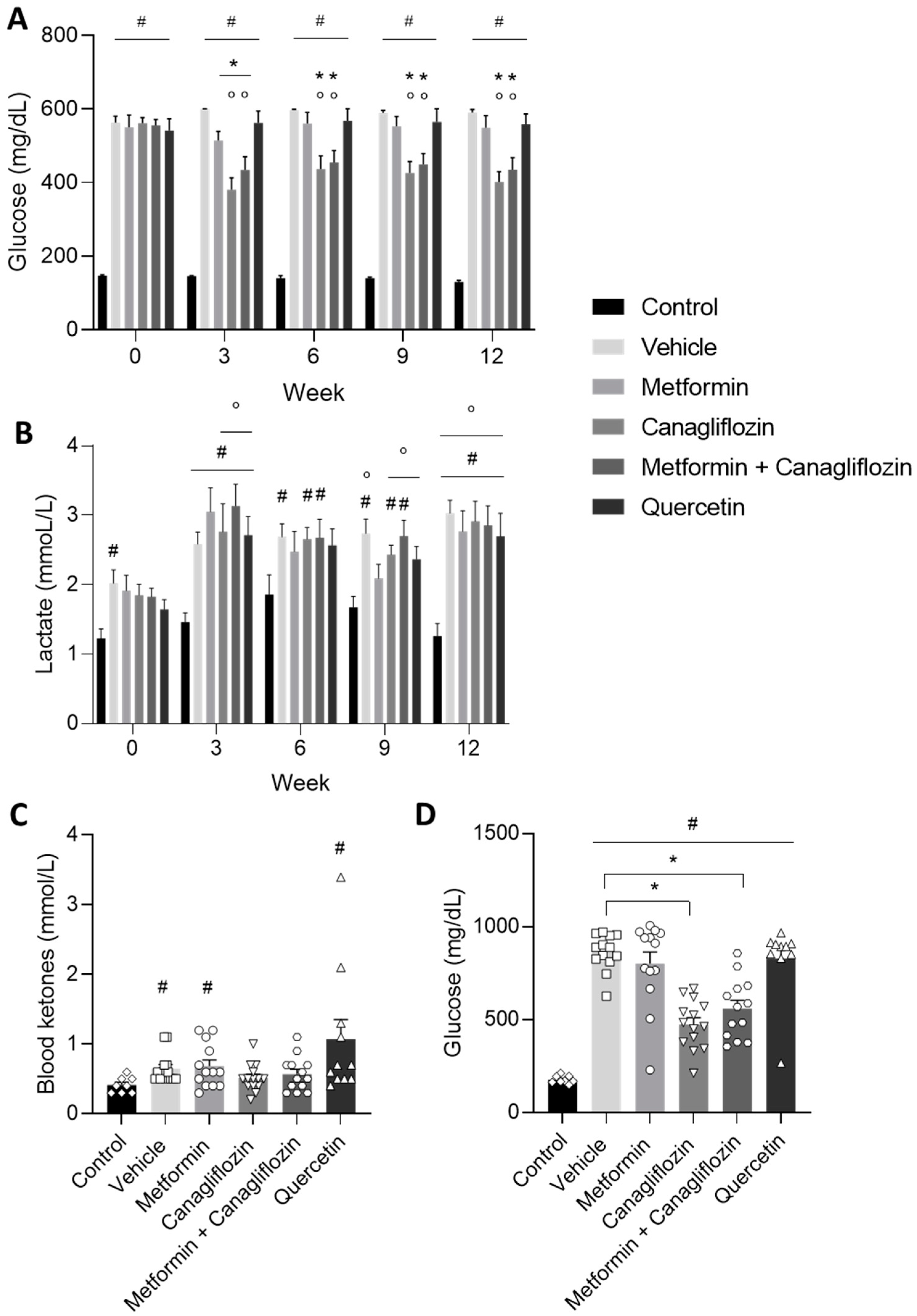
2.2. Diabetes-Related Biochemical Parameters
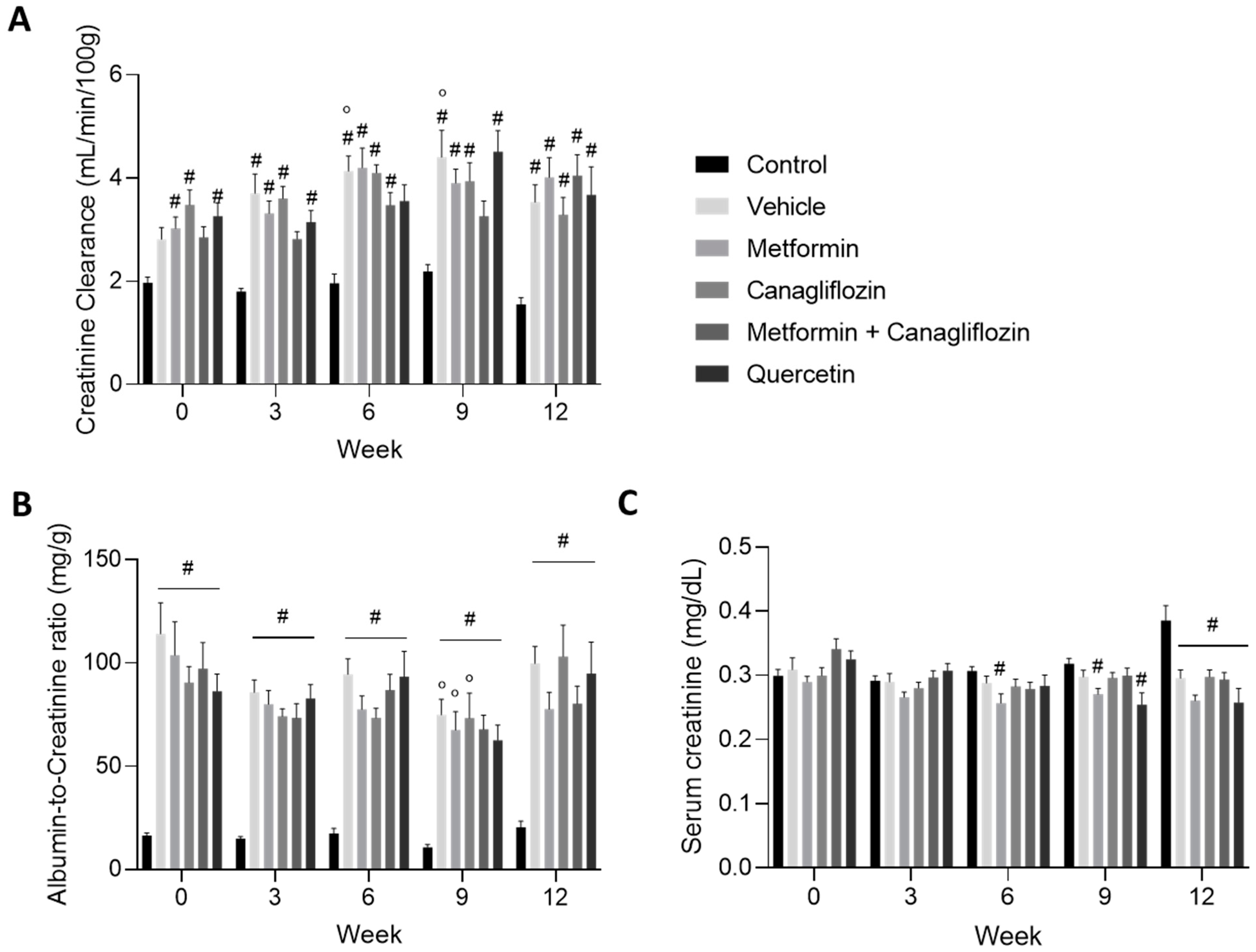
2.3. Renal Function Is Affected by Diabetes Induction
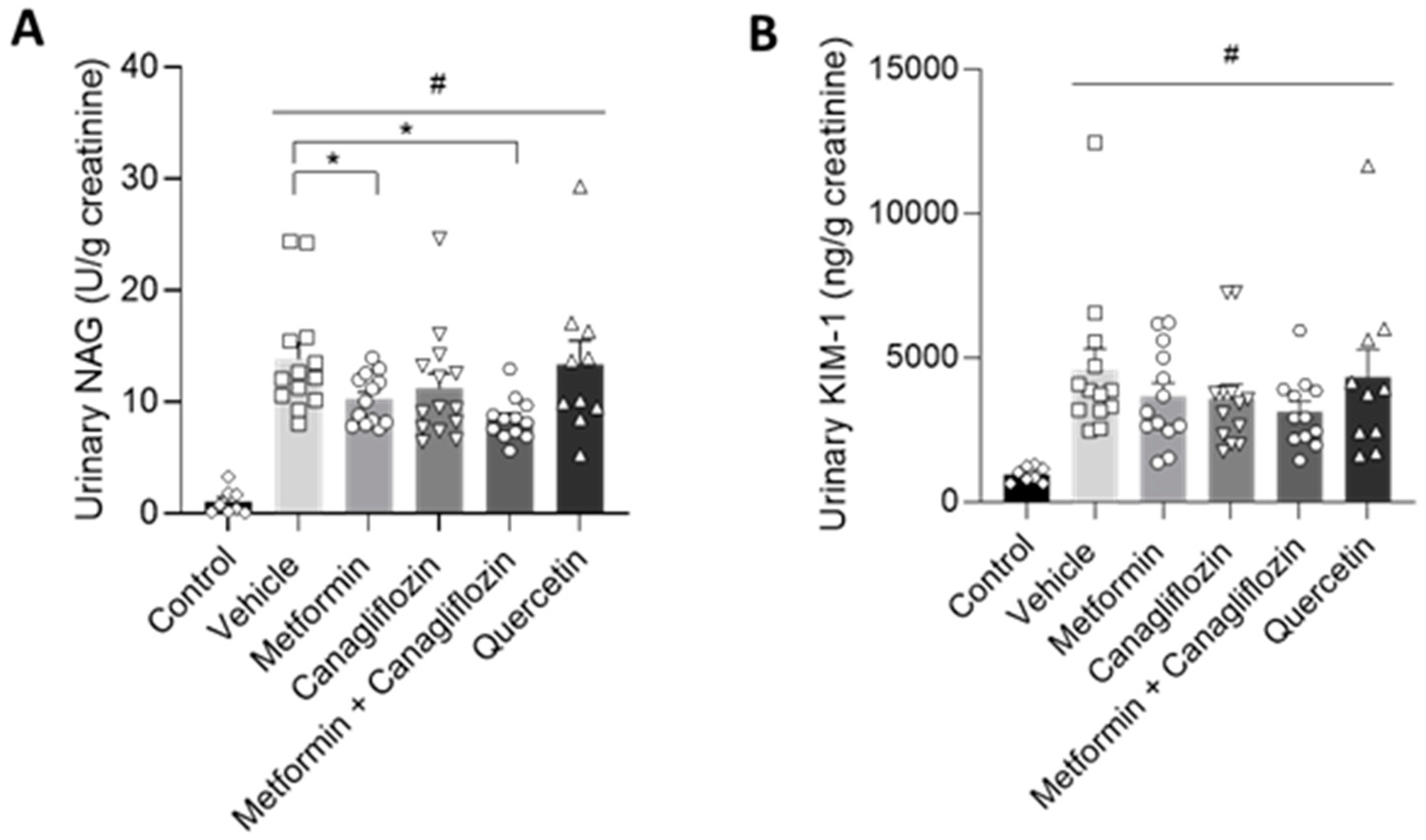
2.4. Metformin Ameliorates Urinary Markers of Kidney Damage
2.5. Blood Pressure
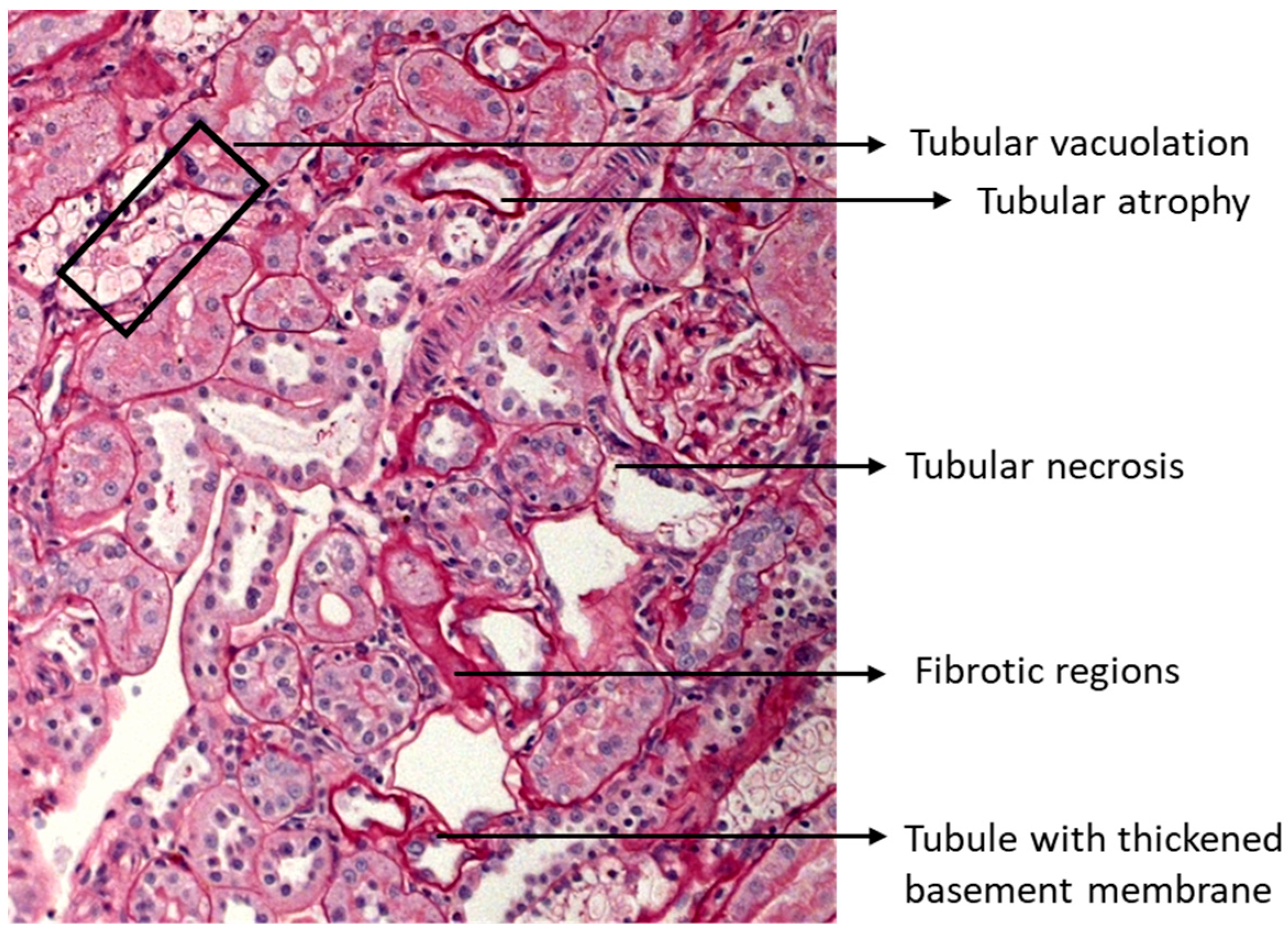
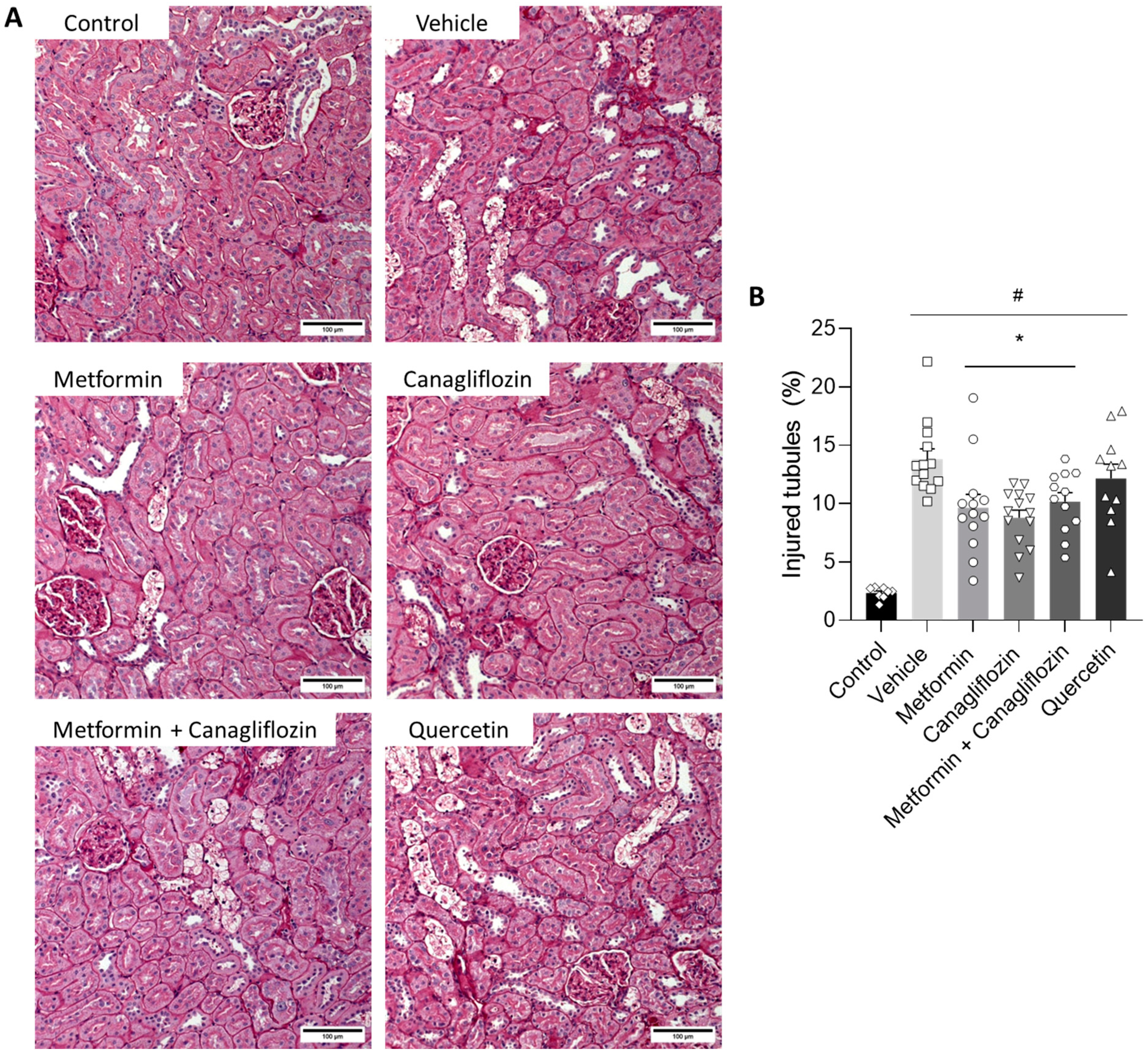
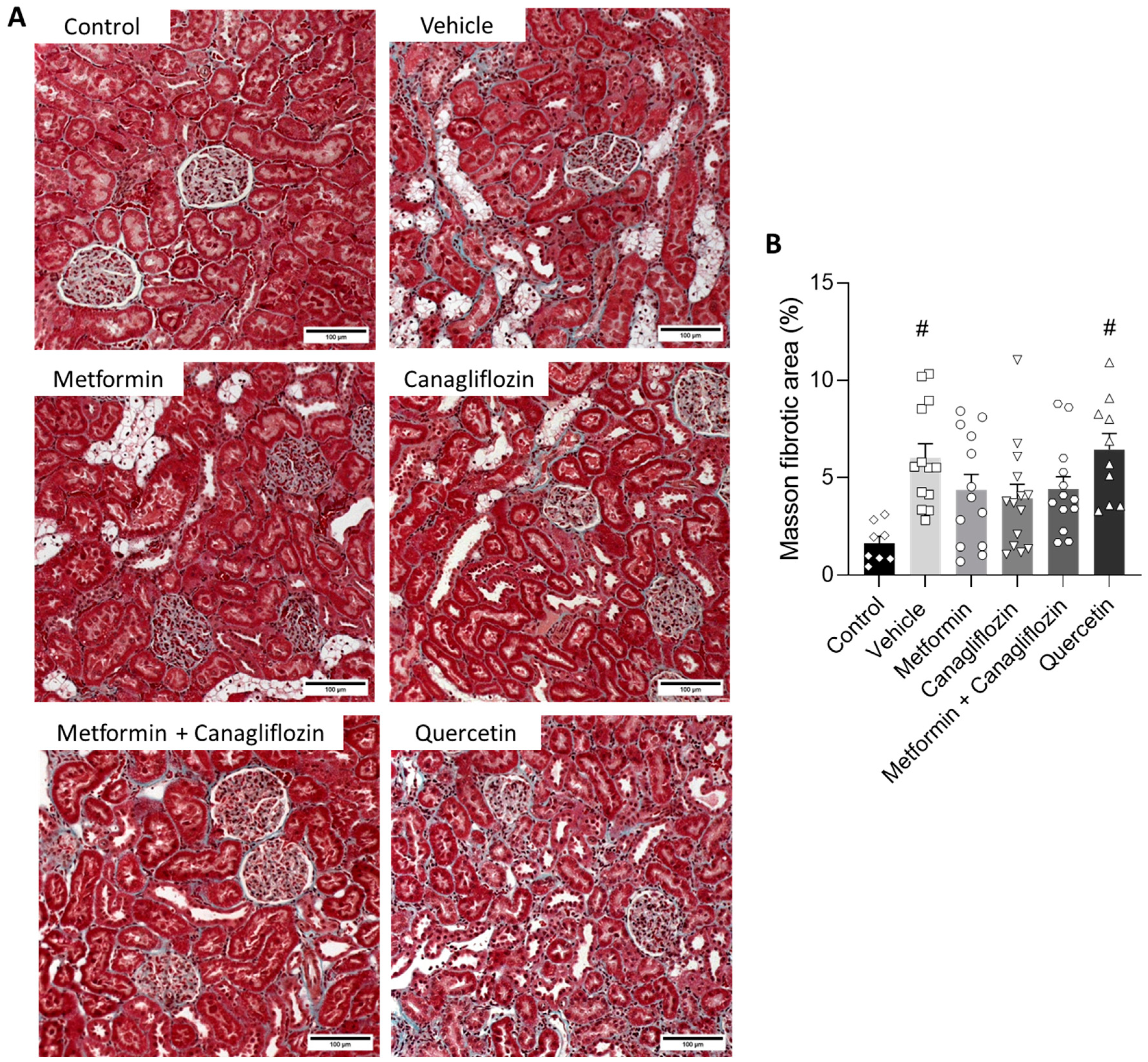
2.6. Metformin and Canagliflozin Both Halt Histological DKD Progression
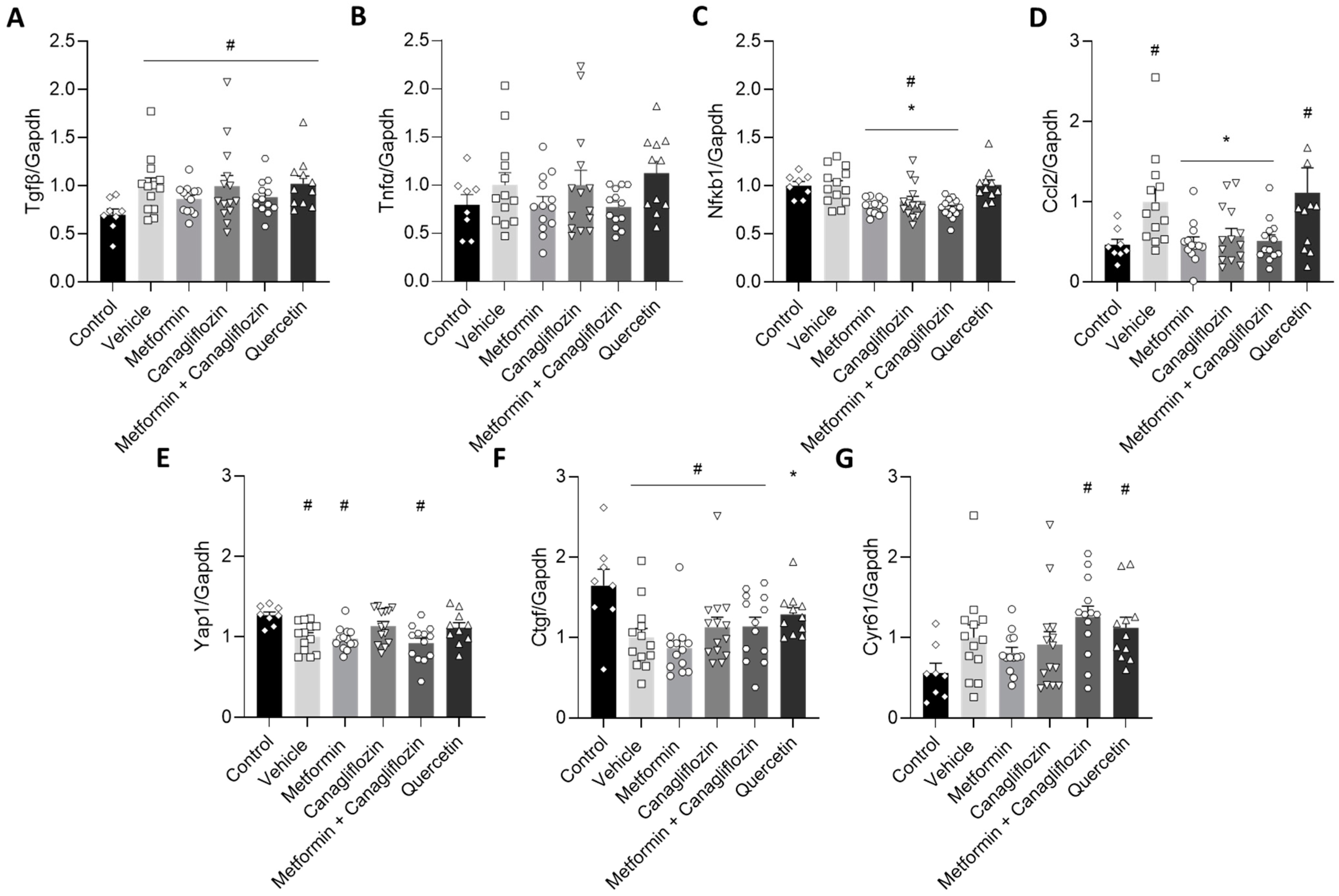
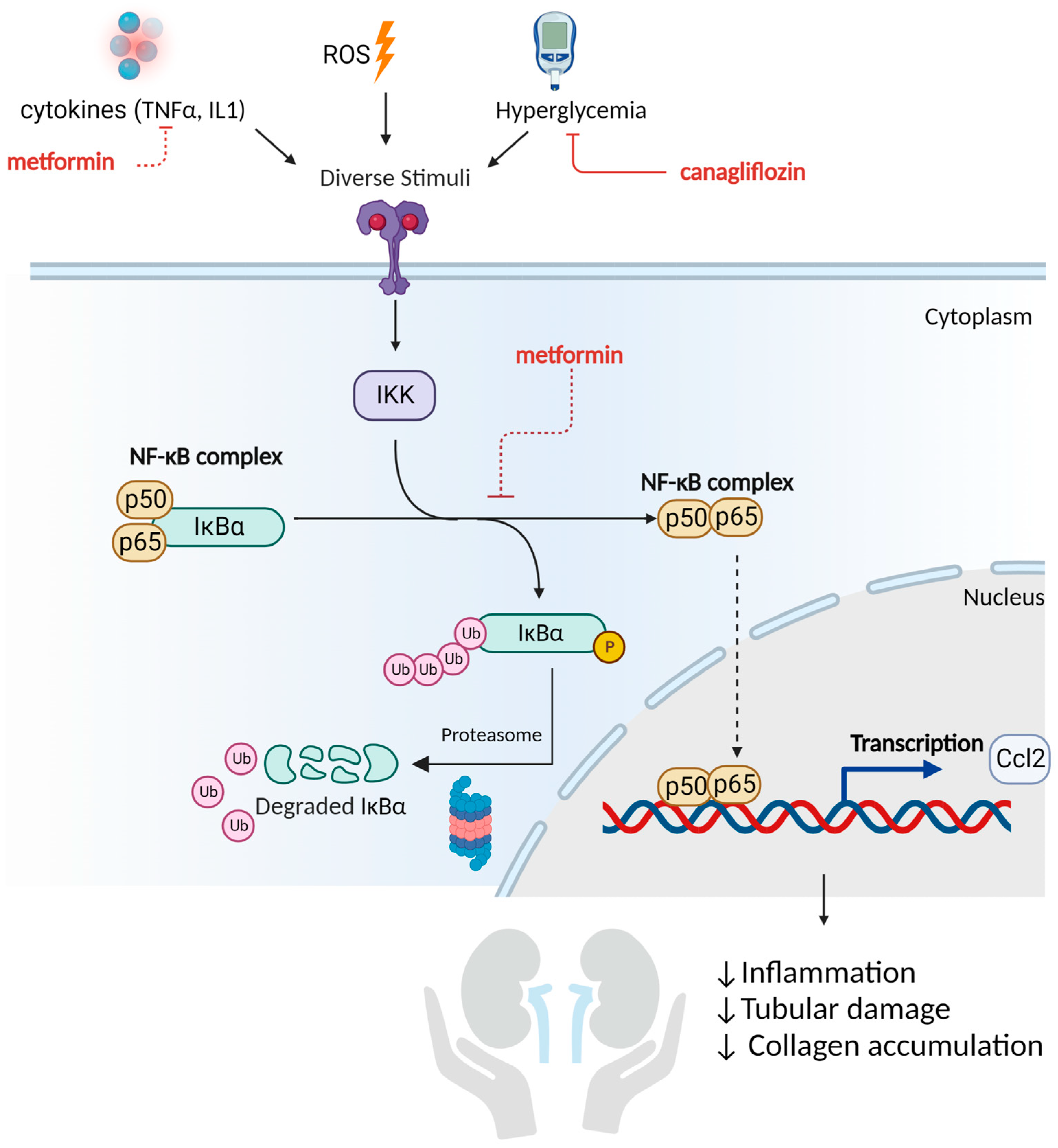
2.7. Metformin and Canagliflozin Inhibit NF-κB Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Drug Preparation
4.2. Animal Experiment
4.3. Biochemical Analyses
4.4. Blood Pressure
4.5. Histology
4.6. Quantitative Real-Time PCR
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic kidney disease: Challenges, progress, and possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Persson, F.; Rossing, P. Diagnosis of diabetic kidney disease: State of the art and future perspective. Kidney Int. Suppl. 2018, 8, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.R.; Brenner, B.M.; Magee, C. Pocket Companion to Brenner and Rector’s the Kidney; W.B. Saunders: Philadephia, PA, USA, 2010; pp. 345–359. [Google Scholar]
- MacIsaac, R.J.; Jerums, G.; Ekinci, E.I. Effects of glycaemic management on diabetic kidney disease. World J. Diabetes 2017, 8, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Jafar, T.H. FDA approval of dapagliflozin for chronic kidney disease: A remarkable achievement? Lancet 2021, 398, 283–2846. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. FDA Approves Treatment for Chronic Kidney Disease [online]. FDA News Release. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-treatment-chronic-kidney-disease (accessed on 30 April 2021).
- Kruger, D.; Valentine, V. Canagliflozin for the Treatment of Diabetic Kidney Disease and Implications for Clinical Practice: A Narrative Review. Diabetes Ther. 2020, 11, 1237–12508. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, S.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The Long-term Effects of Metformin on Patients With Type 2 Diabetic Kidney Disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Kawanami, D.; Takashi, Y.; Tanabe, M. Significance of metformin use in diabetic kidney disease. Int. J. Mol. Sci. 2020, 21, 4239. [Google Scholar] [CrossRef]
- Ravindran, S.; Kuruvilla, V.; Wilbur, K.; Munusamy, S. Nephroprotective Effects of Metformin in Diabetic Nephropathy. J. Cell. Physiol. 2017, 232, 731–742. [Google Scholar] [CrossRef]
- Corremans, R.; Neven, E.; Maudsley, S.; Leysen, H.; De Broe, M.E.; D’Haese, P.C.; Vervaet, B.A.; Verhulst, A. Progression of established non-diabetic chronic kidney disease is halted by metformin treatment in rats. Kidney Int. 2022, 101, 929–944. [Google Scholar] [CrossRef]
- Neven, E.; Vervaet, B.; Brand, K.; Gottwald-Hostalek, U.; Opdebeeck, B.; De Maré, A.; Verhulst, A.; Lalau, J.D.; Kamel, S.; De Broe, M.E.; et al. Metformin prevents the development of severe chronic kidney disease and its associated mineral and bone disorder. Kidney Int. 2018, 94, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; He, L.; Hao, M.; Li, Y.; Li, X.; Liu, Y.; Jiang, H.; Xu, L.; Li, C.; Wu, W.; et al. YAP mediates the interaction between the Hippo and PI3K/Akt pathways in mesangial cell proliferation in diabetic nephropathy. Acta Diabetol. 2021, 58, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Harris, R.C. Interaction of the EGF Receptor and the Hippo Pathway in the Diabetic Kidney. J. Am. Soc. Nephrol. 2016, 27, 1689–1700. [Google Scholar] [CrossRef] [PubMed]
- Kanda, E.; Nangaku, M. Are SGLT2 inhibitors a targeted treatment for diabetic kidney disease? Kidney Int. 2019, 96, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Qu, C.; Xiao, X.; Zhang, W.; Jiang, Y.; Wu, Z.; Song, D.; Peng, X.; Ma, X.; Zhao, Y. Flavonoids on diabetic nephropathy: Advances and therapeutic opportunities. Chin. Med. 2021, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, C.; Qian, X.; Chen, Y.; Wang, L.; Yang, H.; Li, X.; Li, Y.; Yin, X.; Lu, Q. Quercetin inhibited mesangial cell proliferation of early diabetic nephropathy through the Hippo pathway. Pharmacol. Res. 2019, 146, 104320. [Google Scholar]
- Corremans, R.; D’Haese, P.C.; Vervaet, B.A.; Verhulst, A. L-NAME Administration Enhances Diabetic Kidney Disease Development in an STZ/NAD Rat Model. Int. J. Mol. Sci. 2021, 22, 127672. [Google Scholar] [CrossRef]
- Sheira, G.; Noreldin, N.; Tamer, A.; Saad, M. Urinary biomarker N-acetyl-β-D-glucosaminidase can predict severity of renal damage in diabetic nephropathy. J. Diabetes Metab. Disord. 2015, 14, 4. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Yang, H.; Song, Y.; Liang, Y.N.; Li, R. Quercetin Treatment Improves Renal Function and Protects the Kidney in a Rat Model of Adenine-Induced Chronic Kidney Disease. Med. Sci. Monit. 2018, 24, 4760–4766. [Google Scholar] [CrossRef]
- Lai, P.-B.; Zhang, L.; Yang, L.-Y. Quercetin Ameliorates Diabetic Nephropathy by Reducing the Expressions of Transforming Growth Factor-β1 and Connective Tissue Growth Factor in Streptozotocin-Induced Diabetic Rats. Ren. Fail. 2012, 34, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Popiolek-Kalisz, J.; Fornal, E. The Effects of Quercetin Supplementation on Blood Pressure—Meta-Analysis. Curr. Probl. Cardiol. 2022, 47, 101350. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.J.; Symons, J.D.; Jalili, T. Quercetin: A Treatment for Hypertension?—A Review of Efficacy and Mechanisms. Pharmaceuticals 2010, 3, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Scialla, J.J. Role of Acid-Base Homeostasis in Diabetic Kidney Disease. Curr. Diabetes Rep. 2017, 17, 28. [Google Scholar] [CrossRef]
- Omozee, E.B.; Okaka, E.I.; Edo, A.E.; Obika, L.F. Urinary N-acetyl-beta-d-glucosaminidase levels in diabetic adults. J. Lab. Physicians 2019, 11, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hahr, A.J.; Molitch, M.E. Management of diabetes mellitus in patients with chronic kidney disease. Clin. Diabetes Endocrinol. 2015, 1, 2. [Google Scholar] [CrossRef]
- Hosohata, K. Biomarkers of high salt intake. Adv. Clin. Chem. 2021, 104, 71–106. [Google Scholar]
- Tarrant, J. Emerging Translatable Safety Biomarkers. In Comprehensive Medicinal Chemistry III; Elsevier: Cambridge, MA, USA, 2018; pp. 255–284. [Google Scholar]
- Sabbisetti, V.S.; Waikar, S.S.; Antoine, D.J.; Smiles, A.; Wang, C.; Ravisankar, A.; Ito, K.; Sharma, S.; Ramadesikan, S.; Lee, M.; et al. Blood Kidney Injury Molecule-1 Is a Biomarker of Acute and Chronic Kidney Injury and Predicts Progression to ESRD in Type I Diabetes. J. Am. Soc. Nephrol. 2015, 25, 2177–2186. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol. Dial. Transplant. 2009, 24, 3265–3268. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.; Wang, Z.; Xu, Q. The extract of Polygala fallax Hemsl. slows the progression of diabetic nephropathy by targeting TLR4 anti-inflammation and MMP-2/9-mediated anti-fibrosis in vitro. Phytomedicine 2022, 104, 154251. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Cui, D.; Guo, Y.; Wang, M.; Wang, Z.; Huang, Z.; Yang, W.; Chen, F.; Chen, X. A novel polysaccharide obtained from Siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the TLR4-NF-κB pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-κβ: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Kayshyap, S.; Osman, M.; Ferguson, C.M.; Nath, M.C.; Roy, B.; Lien, K.R.; Nath, K.A.; Garovic, V.D.; Lerman, L.O.; Grande, J.P. Ccl2 deficiency protect against chronic renal injury in murine renovascular hypertension. Sci. Rep. 2018, 8, 8598. [Google Scholar] [CrossRef]
- Menne, J.; Eulberg, D.; Beyer, D.; Baumann, M.; Saudek, F.; Valkusz, Z.; Więcek, A.; Haller, H. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dial. Transplant. 2017, 32, 307–315. [Google Scholar] [CrossRef]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin Inhibits Cytokine-Induced Nuclear Factor kappaB Activation Via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertension 2006, 47, 1183–1188. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, X.Y.; Han, J.Y.; Yang, M.; Lv, C.; Shao, Y.; Wang, Y.L.; Kang, J.Y.; Wang, Q.Y. Metformin regulates inflammation and fibrosis in diabetic kidney disease through TNC/TLR4/NF-κB/miR-155-5p inflammatory loop. World J. Diabetes 2021, 12, 19–46. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Ali, B.H.; Al Suleimani, Y.; Adham, S.A.; Ali, H.; Manoj, P.; Ashique, M.; Nemmar, A. The Effect of Metformin in Diabetic and Non-Diabetic Rats with Experimentally-Induced Chronic Kidney Disease. Biomolecules 2021, 11, 814. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Yu, X.; Wu, Y.; Sui, D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 2017, 14, 383–390. [Google Scholar] [CrossRef]
- Sun, T.; Liu, J.; Xie, C.; Yang, J.; Zhao, L.; Yang, J. Metformin attenuates diabetic renal injury via the AMPK-autophagy axis. Exp. Ther. Med. 2021, 21, 578–57838. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Gerasimova, M.; Rose, M.A.; Masuda, T.; Satriano, J.; Mayoux, E.; Koepsell, H.; Thomson, S.C.; Rieg, T. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am. J. Physiol.-Ren. Physiol. 2014, 306, F194–F204. [Google Scholar] [CrossRef] [PubMed]
- Lavalle-González, F.J.; Januszewicz, A.; Davidson, J.; Tong, C.; Qiu, R.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomised trial. Diabetologia 2013, 56, 2582–2592. [Google Scholar] [CrossRef]
- Di Vincenzo, A.; Bettini, S.; Russo, L.; Mazzocut, S.; Mauer, M.; Fioretto, P. Renal structure in type 2 diabetes: Facts and misconceptions. J. Nephrol. 2020, 33, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Fioretto, P.; Caramori, M.L.; Mauer, M. The kidney in diabetes: Dynamic pathways of injury and repair. The Camillo Golgi Lecture 2007. Diabetologia 2008, 51, 1347–1355. [Google Scholar] [CrossRef] [PubMed]

| Blood Pressure | Systolic Blood Pressure (mm Hg) | Diastolic Blood Pressure (mm Hg) | ||||
|---|---|---|---|---|---|---|
| Week | 0 | 6 | 12 | 0 | 6 | 12 |
| Control | 134 ± 7 | 134 ± 11 | 140 ± 7 | 93 ± 8 | 95 ± 4 | 100 ± 9 |
| Vehicle | 172 ± 7 # | 165 ± 7 # | 175 ± 5 # | 128 ± 8 # | 125 ± 7 # | 128 ± 5 # |
| Metformin | 170 ± 6 # | 175 ± 7 # | 168 ± 10 # | 128 ± 6 # | 130 ± 7 # | 131 ± 8 # |
| Canagliflozin | 166 ± 7 # | 184 ± 6 #° | 186 ± 7 #° | 124 ± 6 # | 143 ± 6 #° | 138 ± 7 # |
| Metformin + Canagliflozin | 170 ± 7 # | 177 ± 6 # | 183 ± 8 # | 119 ± 12 | 134 ± 6 # | 141 ± 8 # |
| Quercetin | 166 ± 8 # | 171 ± 7 # | 149 ± 8 * | 128 ± 8 # | 125 ± 8 # | 104 ± 6 °* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corremans, R.; Vervaet, B.A.; Dams, G.; D’Haese, P.C.; Verhulst, A. Metformin and Canagliflozin Are Equally Renoprotective in Diabetic Kidney Disease but Have No Synergistic Effect. Int. J. Mol. Sci. 2023, 24, 9043. https://doi.org/10.3390/ijms24109043
Corremans R, Vervaet BA, Dams G, D’Haese PC, Verhulst A. Metformin and Canagliflozin Are Equally Renoprotective in Diabetic Kidney Disease but Have No Synergistic Effect. International Journal of Molecular Sciences. 2023; 24(10):9043. https://doi.org/10.3390/ijms24109043
Chicago/Turabian StyleCorremans, Raphaëlle, Benjamin A. Vervaet, Geert Dams, Patrick C. D’Haese, and Anja Verhulst. 2023. "Metformin and Canagliflozin Are Equally Renoprotective in Diabetic Kidney Disease but Have No Synergistic Effect" International Journal of Molecular Sciences 24, no. 10: 9043. https://doi.org/10.3390/ijms24109043
APA StyleCorremans, R., Vervaet, B. A., Dams, G., D’Haese, P. C., & Verhulst, A. (2023). Metformin and Canagliflozin Are Equally Renoprotective in Diabetic Kidney Disease but Have No Synergistic Effect. International Journal of Molecular Sciences, 24(10), 9043. https://doi.org/10.3390/ijms24109043







