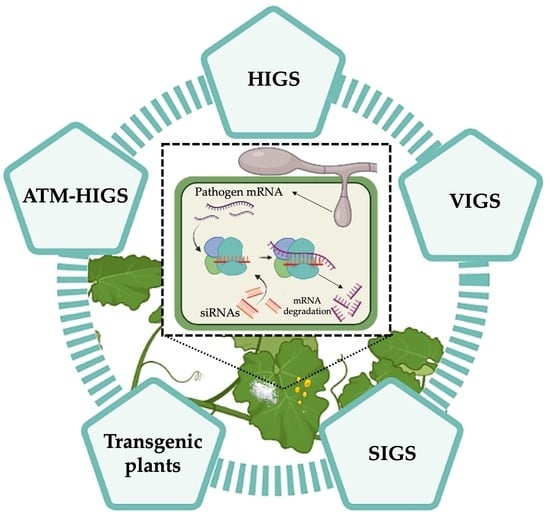
RNAi Technology: A New Path for the Research and Management of Obligate Biotrophic Phytopathogenic Fungi
Abstract
1. Introduction
2. Powdery Mildew and Rust Fungi
3. RNAi Tools for Gene Function Analysis of Obligate Biotrophic Fungi
3.1. Virus-Induced Gene Silencing (VIGS)
3.2. Host Induced Gene Silencing (HIGS)
3.3. Agrobacterium tumefaciens-Mediated Host-Induced Gene Silencing (ATM-HIGS)
3.4. Direct Application of dsRNA
4. Control of Powdery Mildew and Rust Diseases by RNAi Technology
4.1. Transgenic Plants Expressing RNAi Constructs
| Plant Host | Cultivar | Pathogen | Target Gene | Gene Function | Effects | References |
|---|---|---|---|---|---|---|
| H. vulgare | Golden Promise | Blumeria graminis | BgGTF1 | 1,3-β-glucanosyltransferase 1 | Reduced manifestation of powdery mildew symptoms | [53] |
| T. aestivum | Bobwhite | B. graminis f. sp. tritici | SvrPm3a1/f1 | RNase-like effector | Enhanced resistance to powdery mildew | [9] |
| Bgt-Bcg-6 | ||||||
| Bgt-Bcg-7 | ||||||
| Xinong1376 | Puccinia striiformis f. sp. tritici | PsFUZ7 | MAPK kinase | Enhanced resistance to rust | [68] | |
| PKA | Protein kinase A | Enhanced resistance to rust | [70] | |||
| PsCPK1 | Catalytic subunit | |||||
| Fielder | Pst_4 | Effector | Enhanced resistance to rust | [111] | ||
| Pst_5 | ||||||
| Fielder | Puccinia triticina | PtMAPK1 | MAP kinase | Reduction of wheat leaf rust disease symptoms | [111] | |
| PtCYC1 | Cyclophilin |
4.2. Spray-Induced Gene Silencing (SIGS)
| Plant Host | Cultivar | Pathogen | Target Gene | Possible Gene Function | RNA Amount | RNA Application | Effects | References |
|---|---|---|---|---|---|---|---|---|
| C. melo | cv. Rochet | Podosphaera xanthii | PxCNAP1048 | Glycosylation | 5–30 μg/mL | Leaves were spray-inoculated with 104 conidia/mL after dsRNA application | Effective management of PM disease | [29] |
| PxCNAP10905 | Respiration | |||||||
| PxCNAP30520 | ||||||||
| G. max | cv. Enrei | Phakopsora pachyrhizi | ATC | Acetyl-CoA acyltransferase | 20 μg/mL | Leaves were spray-inoculated with 105 uredinia/mL after dsRNA application | Effective management of Asian soybean rust (ASR) disease | [115] |
| RP_S16 | 40S ribosomal protein S16 | |||||||
| GCS_H | Glycine cleavage system H protein | |||||||
| CHS | Chitin synthase | 10 ng/mL | Leaves were drop-inoculated with 105 uredinia/mL and dsRNA simultaneously | Effective management of Asian soybean rust (ASR) disease | [100] | |||
| Syzygium jambos | - | Austropuccinia psidii | β-TUB | β-tubulin | 100 ng/μL | Young, emerging leaves were inoculated with 1 mL of dsRNA solutions | Reduction in fungal growth and in the number of urediniospores | [116] |
| EF1-a | Translation elongation factor 1ɑ | |||||||
| ATC | Acetyl-CoA transferase | |||||||
| CYP450 | Cytochrome P450 | |||||||
| MAPK | Mitogen-activated protein kinase | |||||||
| GCS-H | Glycine cleavage system H | |||||||
| 28S rRNA | 28S ribosomal RNA | |||||||
| HAUS01215 | Haustoria target |
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gheysen, G.; Vanholme, B. RNAi from plants to nematodes. Trends Biotechnol. 2007, 25, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, S.A.; Koonin, E.V. Origins and evolution of eukaryotic RNA interference. Trends Ecol. Evol. 2008, 23, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- D’Ario, M.; Griffiths-Jones, S.; Kim, M. Small RNAs: Big impact on plant development. Trends Plant Sci. 2017, 22, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, J.; Tung, J.; Liu, D.; Zhou, Y.; He, S.; Du, Y.; Baker, B.; Li, F. A role for small RNA in regulating innate immunity during plant growth. PLoS Pathog. 2018, 14, e1006756. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Zhao, J.H.; Guo, H.S. Trans-kingdom RNA silencing in plant–fungal pathogen interactions. Mol. Plant 2018, 11, 235–244. [Google Scholar] [CrossRef]
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA interference mechanisms and applications in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhang, F.; Zhang, Y.; Liang, Y. RNA interference: A natural immune system of plants to counteract biotic stressors. Cells 2019, 8, 38. [Google Scholar] [CrossRef]
- Schaefer, L.K.; Parlange, F.; Buchmann, G.; Jung, E.; Wehrli, A.; Herren, G.; Müller, M.C.; Stehlin, J.; Schmid, R.; Wicker, T.; et al. Cross-kingdom RNAi of pathogen effectors leads to quantitative adult plant resistance in wheat. Front. Plant Sci. 2020, 11, 253. [Google Scholar] [CrossRef]
- Zrachya, A.; Kumar, P.P.; Ramakrishnan, U.; Levy, Y.; Loyter, A.; Arazi, T.; Lapidot, M.; Gafni, Y. Production of siRNA targeted against TYLCV coat protein transcripts leads to silencing of its expression and resistance to the virus. Transgenic Res. 2007, 16, 385–398. [Google Scholar] [CrossRef]
- Puyam, A.; Sharma, S.; Kashyap, P.L. RNA interference- a novel approach for plant disease management. J. Appl. Nat. Sci. 2017, 9, 1612–1618. [Google Scholar] [CrossRef]
- Vaucheret, H.; Vazquez, F.; Crété, P.; Bartel, D.P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004, 18, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Limera, C.; Sabbadini, S.; Sweet, J.B.; Mezzetti, B. New biotechnological tools for the genetic improvement of major woody fruit species. Front. Plant Sci. 2017, 8, 1418. [Google Scholar] [CrossRef] [PubMed]
- Gebremichael, D.E.; Haile, Z.M.; Negrini, F.; Sabbadini, S.; Capriotti, L.; Mezzetti, B.; Baraldi, E. RNA interference strategies for future management of plant pathogenic fungi: Prospects and challenges. Plants 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Rossi, J. RNAi mechanisms and applications. BioTechniques 2008, 44, 613–616. [Google Scholar] [CrossRef]
- Knip, M.; Constantin, M.E.; Thordal-Christensen, H. Trans-kingdom cross-talk: Small RNAs on the move. PLoS Genet. 2014, 10, e1004602. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1573. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Wytinck, N.; Manchur, C.L.; Li, V.H.; Whyard, S.; Belmonte, M.F. dsRNA uptake in plant pests and pathogens: Insights into RNAi-based insect and fungal control technology. Plants 2020, 9, 1780. [Google Scholar] [CrossRef]
- Shih, J.D.; Hunter, C.P. SID-1 is a dsRNA-selective dsRNA-gated channel. RNA 2011, 17, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.; Fujiwara, Y.; Contu, V.R.; Hase, K.; Takahashi, M.; Kikuchi, H.; Kabuta, C.; Wada, K.; Kabuta, T. Lysosomal putative RNA transporter SIDT2 mediates direct uptake of RNA by lysosomes. Autophagy 2016, 12, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Lin, F.-M.; Thomma, B.P.H.J.; Huang, H.-D.; Jin, H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat. Plants 2016, 2, 16151. [Google Scholar] [CrossRef]
- McLoughlin, A.G.; Wytinck, N.; Walker, P.L.; Girard, I.J.; Rashid, K.Y.; de Kievit, T.; Fernando, W.G.D.; Whyard, S.; Belmonte, M.F. Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci. Rep. 2018, 8, 7320. [Google Scholar] [CrossRef] [PubMed]
- Nerva, L.; Sandrini, M.; Gambino, G.; Chitarra, W. Double-stranded RNAs (dsRNAs) as a sustainable tool against gray mold (Botrytis cinerea) in grapevine: Effectiveness of different application methods in an open-air environment. Biomolecules 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Werner, B.T.; Gaffar, F.Y.; Schuemann, J.; Biedenkopf, D.; Koch, A.M. RNA-spray-mediated silencing of Fusarium graminearum AGO and DCL genes improve barley disease resistance. Front. Plant Sci. 2020, 11, 476. [Google Scholar] [CrossRef]
- Marcianò, D.; Ricciardi, V.; Marone Fassolo, E.; Passera, A.; Bianco, P.A.; Failla, O.; Casati, P.; Maddalena, G.; De Lorenzis, G.; Toffolatti, S.L. RNAi of a putative grapevine susceptibility gene as a possible downy mildew control strategy. Front. Plant Sci. 2021, 12, 667319. [Google Scholar] [CrossRef]
- Ruiz-Jiménez, L.; Polonio, Á.; Vielba-Fernández, A.; Pérez-García, A.; Fernández-Ortuño, D. Gene mining for conserved, non-annotated proteins of Podosphaera xanthii identifies novel target candidates for controlling powdery mildews by spray-induced gene silencing. J Fungi 2021, 7, 735. [Google Scholar] [CrossRef]
- Fitzgerald, A.; van Kan, J.A.L.; Plummer, K.M. Simultaneous silencing of multiple genes in the apple scab fungus, Venturia inaequalis, by expression of RNA with chimeric inverted repeats. Fungal Genet. Biol. 2004, 41, 963–971. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.D.; Jin, H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection: Effectors of biotrophic fungi. Cell. Microbiol. 2011, 13, 1849–1857. [Google Scholar] [CrossRef]
- Tang, C.; Xu, Q.; Zhao, M.; Wang, X.; Kang, Z. Understanding the lifestyles and pathogenicity mechanisms of obligate biotrophic fungi in wheat: The emerging genomics era. Crop. J. 2018, 6, 60–67. [Google Scholar] [CrossRef]
- Braun, U. The current systematics and taxonomy of the powdery mildews (Erysiphales): An overview. Mycoscience 2011, 52, 210–212. [Google Scholar] [CrossRef]
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide resistance in powdery mildew fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; De Vicente, A.; Torés, J.A. The powdery mildew fungus Podosphaera fusca (synonym Podosphaera xanthii), a constant threat to cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef]
- Eichmann, R.; Hückelhoven, R. Accommodation of powdery mildew fungi in intact plant cells. J. Plant Physiol. 2008, 165, 5–18. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef]
- Heffer, V.; Powelson, M.L.; Johnson, K.B.; Shishkoff, N. Identification of powdery mildew fungi anno 2006. Plant Heath Instr. 2006. [Google Scholar] [CrossRef]
- Sidhu, G.S. Genetics of plant pathogenic fungi. In Advances in Plant Pathology; Ingram, D.S., Williams, P.H., Eds.; Academic Press: Cambridge, MA, USA, 1988; Volume 6. [Google Scholar]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph: Grapevine powdery mildew. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Saharan, G.S.; Mehta, N.K.; Meena, P.D. Infection, pathogenesis and disease cycle. In Powdery Mildew Disease of Crucifers: Biology, Ecology and Disease Management; Springer: Singapore, 2019; pp. 95–130. [Google Scholar]
- Jarvis, W.R.; Gubler, W.D.; Grove, G.G. Epidemiology of Powdery Mildews in Agricultural Pathosystems; Bélanger, R.R., Bushnell, W.R., Dik, A.J., Carver, T.L.W., Eds.; APS Press: St. Paul, MN, USA, 2002. [Google Scholar]
- Helfer, S. Rust fungi and global change. New Phytol. 2014, 201, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Aime, M.; Toome, M.; McLaughlin, D. Pucciniomycotina. Systematics and Evolution. In The Mycota; Springer: Berlin/Heidelberg, Germany, 2014; Volume 7A. [Google Scholar]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, C.; Gonçalves dos Santos, K.C.; Germain, H.; Hecker, A.; Duplessis, S. Advances in understanding obligate biotrophy in rust fungi. New Phytol. 2019, 222, 1190–1206. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, S.; Petre, B.; Frey, P.; Hecker, A.; Rouhier, N.; Duplessis, S. The poplar-poplar rust interaction: Insights from genomics and transcriptomics. J. Pathog. 2011, 2011, 716041. [Google Scholar] [CrossRef]
- Sohn, K.H.; Lei, R.; Nemri, A.; Jones, J.D. The downy mildew effector proteins ATR1 and ATR13 promote disease susceptibility in Arabidopsis thaliana. Plant Cell 2008, 19, 4077–4090. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; de Wit, P.J. Fungal effector proteins. Annu. Rev. Phytopathol. 2009, 47, 233–263. [Google Scholar] [CrossRef]
- Polonio, A.; Pérez-García, A.; Martínez-Cruz, J.; Fernández-Ortuño, D.; de Vicente, A. The haustorium of phytopathogenic fungi: A short overview of a specialized cell of obligate biotrophic plant parasites. In Progress in Botany; Cánovas, F.M., Lüttge, U., Risueño, M.C., Pretzsch, H., Eds.; Springer Nature Switzerland AG: Basel, Switzerland, 2020; Volume 82, ISBN 978-3-030-68619-2. [Google Scholar]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef]
- Pliego, C.; Nowara, D.; Bonciani, G.; Gheorghe, D.M.; Xu, R.; Surana, P.; Whigham, E.; Nettleton, D.; Bogdanove, A.J.; Wise, R.P.; et al. Host-induced gene silencing in barley powdery mildew reveals a class of ribonuclease-like effectors. Mol. Plant Microbe Interact. 2013, 26, 633–642. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.; Romero, D.; de la Torre, F.N.; Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. The functional characterization of Podosphaera xanthii candidate effector genes reveals novel target functions for fungal pathogenicity. Mol. Plant Microbe Interact. 2018, 31, 914–931. [Google Scholar] [CrossRef]
- Jiang, D.; Zhu, W.; Wang, Y.; Sun, C.; Zhang, K.-Q.; Yang, J. Molecular tools for functional genomics in filamentous fungi: Recent advances and new strategies. Biotech. Adv. 2013, 31, 1562–1574. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.; Romero, D.; Vicente, A.; Pérez-García, A. Transformation of the cucurbit powdery mildew pathogen Podosphaera xanthii by Agrobacterium tumefaciens. New Phytol. 2017, 213, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Lange, M. VIGS—Genomics goes functional. Trends Plant Sci. 2010, 15, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.H.; Keller, Y.; Bouvier, F.; Clary, D.; Camara, B. Functional integration of non-native carotenoids into chloroplasts by viral-derived expression of capsanthin–capsorubin synthase in Nicotiana benthamiana. Plant J. 1998, 14, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.O.; Lim, H.-S.; Bragg, J.; Ganesan, U.; Lee, M.Y. Hordeivirus replication, movement, and pathogenesis. Annu. Rev. Phytopathol. 2009, 47, 385–422. [Google Scholar] [CrossRef]
- Lee, W.-S.; Hammond-Kosack, K.E.; Kanyuka, K. Barley stripe mosaic virus—Mediated tools for investigating gene function in cereal plants and their pathogens: Virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiol. 2012, 160, 582–590. [Google Scholar] [CrossRef]
- Yin, C.; Jurgenson, J.E.; Hulbert, S.H. Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol. Plant Microbe Interact. 2011, 24, 554–561. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, J.; Voegele, R.T.; Zhang, J.; Duan, Y.; Luo, H.; Kang, Z. Functional characterization of calcineurin homologs PsCNA1/PsCNB1 in Puccinia striiformis f. sp. tritici using a host-induced RNAi system. PLoS ONE 2012, 7, e49262. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Yao, J.; Voegele, R.T.; Zhang, Y.; Wang, W.; Huang, L.; Kang, Z. Characterization of protein kinase PsSRPKL, a novel pathogenicity factor in the wheat stripe rust fungus: Kinases and rust pathogenicity. Environ. Microbiol. 2015, 17, 2601–2617. [Google Scholar] [CrossRef]
- Tang, C.; Wei, J.; Han, Q.; Liu, R.; Duan, X.; Fu, Y.; Huang, X.; Wang, X.; Kang, Z. PsANT, the adenine nucleotide translocase of Puccinia striiformis, promotes cell death and fungal growth. Sci. Rep. 2015, 5, 11241. [Google Scholar] [CrossRef]
- Liu, J.; Guan, T.; Zheng, P.; Chen, L.; Yang, Y.; Huai, B.; Li, D.; Chang, Q.; Huang, L.; Kang, Z. An extracellular Zn-Only superoxide dismutase from Puccinia striiformis confers enhanced resistance to host-derived oxidative stress. Environ. Microbiol. 2016, 18, 4118–4135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yao, J.; Zhang, Y.; Li, S.; Kang, Z. Characterization of a Ran gene from Puccinia striiformis f. sp. tritici involved in fungal growth and anti-cell death. Sci. Rep. 2016, 6, 35248. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Qi, T.; Yang, Q.; He, F.; Tan, C.; Ma, W.; Voegele, R.T.; Kang, Z.; Guo, J. Host-induced gene silencing of the MAPKK gene PsFUZ7 confers stable resistance to wheat stripe rust. Plant Physiol. 2017, 175, 1853–1863. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Chu, X.; Sun, Q.; Tan, C.; Yang, Q.; Jiao, M.; Guo, J.; Kang, Z. The transcription factor PstSTE12 is required for virulence of Puccinia striiformis f. sp. tritici. Mol. Plant Pathol. 2018, 19, 961–974. [Google Scholar] [CrossRef]
- Qi, T.; Zhu, X.; Tan, C.; Liu, P.; Guo, J.; Kang, Z.; Guo, J. Host-induced gene Silencing of an important pathogenicity factor PsCPK1 in Puccinia striiformis f. sp. tritici enhances resistance of wheat to stripe rust. Plant Biotechnol. J. 2018, 16, 797–807. [Google Scholar] [CrossRef]
- Zhu, X.; Jiao, M.; Guo, J.; Liu, P.; Tan, C.; Yang, Q.; Zhang, Y.; Thomas Voegele, R.; Kang, Z.; Guo, J. A novel MADS-box transcription factor PstMCM1-1 is responsible for full virulence of Puccinia striiformis f. sp. tritici. Environ. Microbiol. 2018, 20, 1452–1463. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, J.; He, F.; Zhang, Y.; Tan, C.; Yang, Q.; Huang, C.; Kang, Z.; Guo, J. Silencing PsKPP4, a MAP kinase kinase kinase gene, reduces pathogenicity of the stripe rust fungus. Mol. Plant Pathol. 2018, 19, 2590–2602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, J.; Ji, S.; Chen, Z.; Xu, J.; Tang, C.; Chen, S.; Kang, Z.; Wang, X. Candidate effector Pst_8713 impairs the plant immunity and contributes to virulence of Puccinia striiformis f. sp. tritici. Front. Plant Sci. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Qi, T.; Guo, J.; Liu, P.; He, F.; Wan, C.; Islam, M.A.; Tyler, B.M.; Kang, Z.; Guo, J. Stripe rust effector PstGSRE1 disrupts nuclear localization of ROS-promoting transcription factor TaLOL2 to defeat ROS-induced defense in wheat. Mol. Plant 2019, 12, 1624–1638. [Google Scholar] [CrossRef]
- Panwar, V.; McCallum, B.; Bakkeren, G. Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. 2013, 73, 521–532. [Google Scholar] [CrossRef]
- Yin, C.; Park, J.-J.; Gang, D.R.; Hulbert, S.H. Characterization of a tryptophan 2-monooxygenase gene from Puccinia graminis f. sp. tritici involved in auxin biosynthesis and rust pathogenicity. Mol. Plant Microbe Interact. 2014, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Downey, S.I.; Klages-Mundt, N.L.; Ramachandran, S.; Chen, X.; Szabo, L.J.; Pumphrey, M.; Hulbert, S.H. Identification of promising host-induced silencing targets among genes preferentially transcribed in haustoria of Puccinia. BMC Genomics 2015, 16, 579. [Google Scholar] [CrossRef] [PubMed]
- Margo, R. Genome improvement for rust disease resistance in wheat. In Genome Engineering for Crop Improvement; Sarmah, B.K., Borah, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-63371-4. [Google Scholar]
- Govindarajulu, M.; Epstein, L.; Wroblewski, T.; Michelmore, R.W. Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol. J. 2015, 13, 875–883. [Google Scholar] [CrossRef]
- Zhang, W.J.; Pedersen, C.; Kwaaitaal, M.; Gregersen, P.L.; Mørch, S.M.; Hanisch, S.; Kristensen, A.; Fuglsang, A.T.; Collinge, D.B.; Thordal-Christensen, H. Interaction of barley powdery mildew effector candidate CSEP0055 with the defence protein PR17c. Mol. Plant Pathol. 2012, 13, 1110–1119. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Pedersen, C.; Schultz-Larsen, T.; Kwaaitaal, M.; Jørgensen, H.J.; Thordal-Christensen, H. The barley powdery mildew candidate secreted effector protein CSEP0105 inhibits the chaperone activity of a small heat shock protein. Plant Physiol. 2015, 168, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Jin, C.; Pei, H.; Zhao, L.; Li, X.; Li, J.; Huang, W.; Fan, R.; Liu, W.; Shen, Q.H. The powdery mildew effector CSEP0027 interacts with barley catalase to regulate host immunity. Front. Plant Sci. 2021, 12, 733237. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, G.B.; Pedersen, C.; Thordal-Christensen, H. Identification of eight effector candidate genes involved in early aggressiveness of the barley powdery mildew fungus. Plant Pathol. 2016, 65, 953–958. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Pedersen, C.; Thordal-Christensen, H. The barley powdery mildew effector candidates CSEP0081 and CSEP0254 promote fungal infection success. PLoS ONE 2016, 11, e157586. [Google Scholar] [CrossRef]
- Li, X.; Jin, C.; Yuan, H.; Huang, W.; Liu, F.; Fan, R.; Xie, J.; Shen, Q.H. The barley powdery mildew effectors CSEP0139 and CSEP0182 suppress cell death and promote B. graminis fungal virulence in plants. Phytopathol. Res. 2021, 3, 7. [Google Scholar] [CrossRef]
- Sanford, J.C. Biolistic plant transformation. Physiol. Plant 1990, 79, 206–209. [Google Scholar] [CrossRef]
- Harwood, W.A. Advances and remaining challenges in the transformation of barley and wheat. J. Exp. Bot. 2012, 63, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.K.; Carrington, J.C. Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol. 2001, 126, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.G.; Wang, C.H.; Guo, H.S. Application of RNA silencing to plant disease resistance. Silence 2012, 3, 5. [Google Scholar] [CrossRef]
- Bertazzon, N.; Raiola, A.; Castiglioni, C.; Gardiman, M.; Angelini, E.; Borgo, M.; Ferrari, S. Transient silencing of the grapevine gene VvPGIP1 by agroinfiltration with a construct for RNA interference. Plant Cell Rep. 2012, 31, 133–143. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.; Romero, D.; Hierrezuelo, J.; Thon, M.; de Vicente, A.; Pérez-García, A. Effectors with chitinase activity (EWCAs), a family of conserved, secreted fungal chitinases that suppress chitin-triggered immunity. Plant Cell 2021, 33, 1319–1340. [Google Scholar] [CrossRef]
- Polonio, Á.; Fernández-Ortuño, D.; Vicente, A.; Pérez-García, A. A haustorial-expressed lytic polysaccharide monooxygenase from the cucurbit powdery mildew pathogen Podosphaera xanthii contributes to the suppression of chitin-triggered immunity. Mol. Plant. Pathol. 2021, 22, 580–601. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.M.; Polonio, Á.; Zanni, R.; Romero, D.; Gálvez, J.; Fernández-Ortuño, D.; Pérez-García, A. chitin deacetylase, a novel target for the design of agricultural fungicides. J. Fungi 2021, 7, 1009. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for gene regulation and plant resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Mumbanza, F.M.; Kiggundu, A.; Tusiime, G.; Tushemereirwe, W.K.; Niblett, C.; Bailey, A. In vitro antifungal activity of synthetic dsRNA molecules against two pathogens of banana, Fusarium oxysporum f. sp. cubense and Mycosphaerella fijiensis. Pest. Manag. Sci. 2013, 69, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Weiberg, A.; Dellota, E.; Yamane, D.; Jin, H. Botrytis small RNA Bc -siR37 suppresses plant defense genes by cross-kingdom RNAi. RNA Biol. 2017, 14, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Aminedi, R.; Saxena, D.; Gupta, A.; Banerjee, P.; Jain, D.; Chandran, D. Effector mining from the Erysiphe pisi haustorial transcriptome identifies novel candidates involved in pea powdery mildew pathogenesis. Mol. Plant Pathol. 2019, 20, 1506–1522. [Google Scholar] [CrossRef]
- Saito, H.; Sakata, N.; Ishiga, T.; Ishiga, Y. Efficacy of RNA-spray-induced silencing of Phakopsora pachyrhizi chitin synthase genes to control soybean rust. J. Gen. Plant Pathol. 2022, 88, 203–206. [Google Scholar] [CrossRef]
- Cai, Q.; He, B.; Kogel, K.-H.; Jin, H. Cross-kingdom RNA trafficking and environmental RNAi—Nature’s blueprint for modern crop protection strategies. Curr. Opin. Microbiol. 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.-H. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase–encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef]
- Cheng, S.; Xie, X.; Xu, Y.; Zhang, C.; Wang, X.; Zhang, J.; Wang, Y. Genetic transformation of a fruit-specific, highly expressed stilbene synthase gene from chinese wild Vitis Quinquangularis. Planta 2016, 243, 1041–1053. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Li, Y.; Fan, J.; Xiong, H.; Xu, F.X.; Shi, J.; Shi, Y.; Zhao, J.Q.; Wang, Y.F.; Cao, X.L.; et al. Host-induced gene silencing of MoAP1 confers broad-spectrum resistance to Magnaporthe oryzae. Front. Plant Sci. 2019, 10, 433. [Google Scholar] [CrossRef]
- Both, M.; Eckert, S.E.; Csukai, M.; Müller, E.; Dimopoulos, G.; Spanu, P.D. Transcript profiles of Blumeria graminis development during infection reveal a cluster of genes that are potential virulence determinants. Mol. Plant Microbe Interact. 2005, 18, 125–133. [Google Scholar] [CrossRef]
- Pedersen, C.; van Themaat, E.V.L.; McGuffin, L.J.; Abbott, J.C.; Burgis, T.A.; Barton, G.; Bindschedler, L.V.; Lu, X.; Maekawa, T.; Wessling, R.; et al. Structure and evolution of barley powdery mildew effector candidates. BMC Genomics 2012, 13, 694. [Google Scholar] [CrossRef]
- Bourras, S.; Praz, C.R.; Spanu, P.D.; Keller, B. Cereal powdery mildew effectors: A complex toolbox for an obligate pathogen. Curr. Opin. Microbiol. 2018, 46, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Fuller, K.K.; Rhodes, J.C. Protein kinase A and fungal virulence: A sinister side to a conserved nutrient sensing pathway. Virulence 2012, 3, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhou, X.; Gu, X.; Cao, S.; Wang, C.; Xu, J.-R. The cAMP-PKA pathway regulates growth, sexual and asexual differentiation, and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Intract. 2014, 27, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Song, X.S.; Li, H.P.; Cao, L.H.; Sun, K.; Qiu, X.L.; Xu, Y.B.; Yang, P.; Huang, T.; Zhang, J.B.; et al. Host-induced gene silencing of an essential chitin synthase gene confers durable resistance to Fusarium head blight and seedling blight in wheat. Plant Biotechnol. J. 2015, 13, 1335–1345. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, T.; Zhang, X.; Tang, C.; Zhuang, R.; Zhao, H.; Xu, Q.; Cheng, Y.; Wang, J.; Duplessis, S.; et al. Two stripe rust effectors impair wheat resistance by suppressing import of host Fe–S protein into chloroplasts. Plant Physiol. 2021, 187, 2530–2543. [Google Scholar] [CrossRef]
- Panwar, V.; Jordan, M.; McCallum, B.; Bakkeren, G. Host-induced silencing of essential genes in Puccinia triticina through transgenic expression of RNAi sequences reduces severity of leaf rust infection in wheat. Plant Biotechnol. J. 2018, 16, 1013–1023. [Google Scholar] [CrossRef]
- Islam, M.T.; Sherif, S.M. RNAi-Based Biofungicides as a promising next-generation strategy for controlling devastating gray mold diseases. Int. J. Mol. Sci. 2020, 21, 2072. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Hu, D.; Chen, Z.; Zhang, C.; Ganiger, M. Reduction of Phakopsora pachyrhizi infection on soybean through host- and spray-induced gene silencing. Mol. Plant Pathol. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Degnan, R.M.; McTaggart, A.R.; Shuey, L.S.; Pame, L.J.S.; Smith, G.R.; Gardiner, D.M.; Nock, V.; Soffe, R.; Sale, S.; Garrill, A.; et al. Exogenous double-stranded RNA inhibits the infection physiology of rust fungi to reduce symptoms in planta. Mol. Plant Pathol. 2023, 24, 191–207. [Google Scholar] [CrossRef]
- Wang, M.; Jin, H. Spray-induced gene silencing: A powerful innovative strategy for crop protection. Trends Microbiol. 2017, 25, 4–6. [Google Scholar] [CrossRef]
- Taning, C.N.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Mitter, N. How nanocarriers delivering cargos in plants can change the GMO landscape. Nat. Nanotechnol. 2019, 14, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.G.; Fletcher, S.J.; Manzie, N.; Robinson, K.E.; Li, P.; Lu, E.; Brosnan, C.A.; Xu, Z.P.; Mitter, N. Foliar application of clay-delivered RNA interference for whitefly control. Nat. Plants 2022, 8, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Mosa, M.A.; Youssef, K. Topical delivery of host induced RNAi silencing by layered double hydroxide nanosheets: An efficient tool to decipher pathogenicity gene function of Fusarium crown and root rot in tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Niño-Sánchez, J.; Sambasivam, P.T.; Sawyer, A.; Hamby, R.; Chen, A.; Czislowski, E.; Li, P.; Manzie, N.; Gardiner, D.M.; Ford, R.; et al. BioClayTM prolongs RNA interference-mediated crop protection against Botrytis cinerea. J. Integr. Plant Biol. 2022, 64, 2187–2198. [Google Scholar] [CrossRef]
- Delgado-Martín, J.; Delgado-Olidén, A.; Velasco, L. Carbon dots boost dsRNA delivery in plants and increase local and systemic siRNA production. Int. J. Mol. Sci. 2022, 23, 5338. [Google Scholar] [CrossRef]
- Kostov, K.; Andonova-Lilova, B.; Smagghe, G. Inhibitory activity of carbon quantum dots against Phytophthora infestans and fungal plant pathogens and their effect on dsRNA-induced gene silencing. Biotechnol. Biotechnol. Equip. 2022, 36, 949–959. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic nanoparticles penetrate leaves and deliver nutrients to agricultural crops. Sci. Rep. 2018, 8, 7589. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Demirer, G.S.; González-Grandío, E.; Fan, C.; Landry, M.P. Engineering DNA nanostructures for siRNA delivery in plants. Nat. Protoc. 2020, 15, 3064–3087. [Google Scholar] [CrossRef] [PubMed]





| Plant Host | Pathogen | Target Gene | Possible Gene Function | Application | Phenotype | References |
|---|---|---|---|---|---|---|
| Hordeum vulgare | Blumeria graminis f. sp. hordei | GTF1 | Cell wall elongation and virulence factor | Virus inoculation by rubbing of barley first leaves | Reduction in haustorium formation | [53] |
| GTF2 | ||||||
| Triticum aestivum | Puccinia striiformis f. sp. tritici | PSTha12J12 | Predicted secreted protein | Virus inoculation by rubbing wheat leaves | Reduction in the expression patternsof the fungal genes | [62] |
| PSTha5A23 | ||||||
| PSTha12H2 | ||||||
| PSTha2A5 | ||||||
| PSTha9F18 | ||||||
| PSTha5A1 | Predicted to code for a chitinase protein | |||||
| PSTha12O3 | Homologous to Uromycesfabae hexose trans-porters | |||||
| PsCNA1 | Calcineurin A-like protein (CNA1) | Slower elongation of fungal hyphae and reduction of the production of uredospore | [63] | |||
| PsCNB1 | Calcineurin B-like protein (CNB1) | |||||
| PsSRPKL | Protein kinase | Reduction of fungal growth and increases of ROS accumulation in host cells | [64] | |||
| PsANT | Adenine nucleotide translocase | Attenuated the growth and development of virulent Pst at the early infection stage | [65] | |||
| PsSOD1 | Zn-only superoxide dismutase | Reduction of the virulence-associated with ROS accumulation | [66] | |||
| PsRan | Small GTP-binding protein | Reduction of the number of haustoria and the length of infection hyphae | [67] | |||
| PsFUZ7 | MAPK kinase | Reduction of initial haustorium formation and elongation of secondary hyphae | [68] | |||
| PstSTE12 | Transcription factor | Reduction in the growth and spread of hyphae in Pst and weakened the virulence of Pst on wheat | [69] | |||
| T. aestivum | P. striiformis f. sp. tritici | PsCPK1 | PKA catalytic subunit | Virus inoculation by rubbing wheat leaves | Reduction in the length of infection hyphae and disease phenotype | [70] |
| PstMCM1-1 | MADX-box transcription factor | Reduction of hyphal extension and haustorium formation | [71] | |||
| PsKPP4 | MAPK kinase | Reduction of haustorium number | [72] | |||
| Pst_8713 | Suppresses host defenses and contributes to the pathogenicity of Pst | Reduction of haustorium number | [73] | |||
| PstGSRE1 | Effector to defeat ROS-associated plant defense by modulating the subcellular compartment of a host immune regulator | Reduction in sporulation and in the fungi biomass | [74] | |||
| Puccinia triticina | PtCYC1 | Cyclophilin | Reduction in fungal growth and disease symptoms | [75] | ||
| PtMAPK1 | MAP kinase | |||||
| PtCNB | Calcineurin regulatory subunit | |||||
| Puccinia graminis f. sp. tritici | Pgt-IaaM | Tryptophan mono-oxygenase | Reduction in fungal growth and in the size of uredinia | [76] | ||
| PGTG_01136 | Predicted glycolytic enzyme | Reduction in fungal growth and in the size of uredinia | [77] | |||
| PGTG_01215 | Probably involved in cellular carbohydrate or sugar metabolism | |||||
| PGTG_03478 | ||||||
| PGTG_14350 | Hypothetical secreted protein with homology to periplasmic components of prokaryotic transport systems | |||||
| PGTG_10731 | Hypothetical proteins | |||||
| PGTG_12890 | ||||||
| PGTG_01304 | Protein involved in thiazole biosynthesis | |||||
| PGTG_16914 | Amino acid permease | |||||
| PGTG_03590 | Secreted protein | |||||
| Pgt-IaaM | Tryptophan 2-monooxygenase enzyme |
| Plant Host | Pathogen | Target Gene | Possible Gene Function | Application | Phenotype | References |
|---|---|---|---|---|---|---|
| H. vulgare | Blumeria graminis f. sp. hordei | Avra10 | Virulence effector | Microprojectile bombardment | Reduction in haustorium formation | [53] |
| BEC1054 | Ribonuclease-like protein | Reduction in haustorium formation | [54] | |||
| BEC1011 | ||||||
| BEC1019 | Metalloprotease | |||||
| BEC1005 | Endo β1-3 glucanase | |||||
| CSEP0055 | Effector involved in secondary penetration events | Reduction in haustorium formation | [80] | |||
| CSEP0105 | Effector proteins | Reduction in haustorium formation | [81] | |||
| CSEP0162 | ||||||
| CSEP0027 | Interacts with barley HvCAT1 to regulate the host immunity to promote fungal virulence | Reduction in haustoria formation | [82] | |||
| CSEP0007 | Possibly involved in penetration and/or establishment of primary haustoria | Reduction in haustoria formation | [83] | |||
| CSEP0025 | ||||||
| CSEP0128 | ||||||
| CSEP0247 | ||||||
| CSEP0345 | ||||||
| CSEP0420 | ||||||
| CSEP0422 | ||||||
| CSEP0081 | Candidate Secreted Effector Proteins | Microprojectile bombardment | Reduction in fungal growth and in haustorium formation | [84] | ||
| CSEP0254 | ||||||
| CSEP0139 | Suppressed cell death triggered by BAX and NtMEK2DD | Reduction in haustoria formation | [85] | |||
| CSEP0182 |
| Plant Host | Pathogen | Target Gene | Possible Gene Function | Application | Phenotype | References |
|---|---|---|---|---|---|---|
| T. aestivum | Puccinia triticina Puccinia graminis and Puccinia striiformis | PtCYC1 | Cyclophilin | Agroinfiltration through the abaxial surface of wheat seedling leaves | Reduction in fungal growth and sporulation | [75] |
| PtMAPK1 | MAP kinase | |||||
| PtCNB | Calcineurin regulatory subunit | |||||
| Cucumis melo | Podosphaera xanthii | PEC007 | Candidate effector | Agroinfiltration of melon cotyledons | Reduction of fungal growth and increasing of the production of hydrogen peroxide by host cells | [55] |
| PEC009 | ||||||
| PEC034 | ||||||
| PEC032 | α-Mannosidase | |||||
| PEC019 | Phospholipid-binding protein | |||||
| PEC054 | Cellulose-binding protein | |||||
| PEC1666 | Chitinase activity | Reduction of fungal growth and increasing of the production of hydrogen peroxide by host cells | [92] | |||
| PEC1961 | ||||||
| PEC2158 | ||||||
| PEC5191 | ||||||
| PHEC27213 | Lytic polysaccharide mono-oxygenase (LPMO) prevents the activation of chitin-triggered immunity | Reduction of fungal growth and increasing production of hydrogen peroxide by host cells | [93] | |||
| PxCDA | chitin deacetylase | Reduction of fungal growth and increasing production of hydrogen peroxide by host cells | [94] |
| Plant Host | Pathogen | Target Gene | Possible Gene Function | Application | Phenotype | References |
|---|---|---|---|---|---|---|
| Pisum sativum | Erysiphe pisi | EpCSEP001 | Virulence factors | Second leaves of pea plants were infiltrated with 100 parts per million (ppm) EpCSEP/CSP-dsRNA | Reduction in disease symptoms | [99] |
| EpCSEP009 | ||||||
| EpCSP083 | ||||||
| C. melo | Podosphaera xanthii | PxCNAP1048 | Presumably involved in glycosylation | Melon cotyledons were infiltrated with dsRNA solutions of the different target genes in concentrations between 100 and 1000 ng ml−1 | Reduction in fungal growth and disease symptoms | [29] |
| PxCNAP10905 | Presumably involved in respiration | |||||
| PxCNAP30520 | ||||||
| PxCNAP8878 | ||||||
| PxCNAP9066 | ||||||
| PxCNAP948 | Presumably involved in efflux transport | |||||
| PxTUB2 | Involved in β-tubulin synthesis | |||||
| PxCYP51 | Involved in ergosterol synthesis | |||||
| Glycine max | Phakopsora pachyrhizi | CHS | Involved in chitin synthases | Soybean plants were infiltrated with 10 ng ml−1 of dsCHS | Reduction in fungal growth and in the number of urediniospores | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padilla-Roji, I.; Ruiz-Jiménez, L.; Bakhat, N.; Vielba-Fernández, A.; Pérez-García, A.; Fernández-Ortuño, D. RNAi Technology: A New Path for the Research and Management of Obligate Biotrophic Phytopathogenic Fungi. Int. J. Mol. Sci. 2023, 24, 9082. https://doi.org/10.3390/ijms24109082
Padilla-Roji I, Ruiz-Jiménez L, Bakhat N, Vielba-Fernández A, Pérez-García A, Fernández-Ortuño D. RNAi Technology: A New Path for the Research and Management of Obligate Biotrophic Phytopathogenic Fungi. International Journal of Molecular Sciences. 2023; 24(10):9082. https://doi.org/10.3390/ijms24109082
Chicago/Turabian StylePadilla-Roji, Isabel, Laura Ruiz-Jiménez, Nisrine Bakhat, Alejandra Vielba-Fernández, Alejandro Pérez-García, and Dolores Fernández-Ortuño. 2023. "RNAi Technology: A New Path for the Research and Management of Obligate Biotrophic Phytopathogenic Fungi" International Journal of Molecular Sciences 24, no. 10: 9082. https://doi.org/10.3390/ijms24109082
APA StylePadilla-Roji, I., Ruiz-Jiménez, L., Bakhat, N., Vielba-Fernández, A., Pérez-García, A., & Fernández-Ortuño, D. (2023). RNAi Technology: A New Path for the Research and Management of Obligate Biotrophic Phytopathogenic Fungi. International Journal of Molecular Sciences, 24(10), 9082. https://doi.org/10.3390/ijms24109082