Circadian-Coupled Genes Expression and Regulation in HIV-Associated Chronic Obstructive Pulmonary Disease (COPD) and Lung Comorbidities
Abstract
:1. Introduction
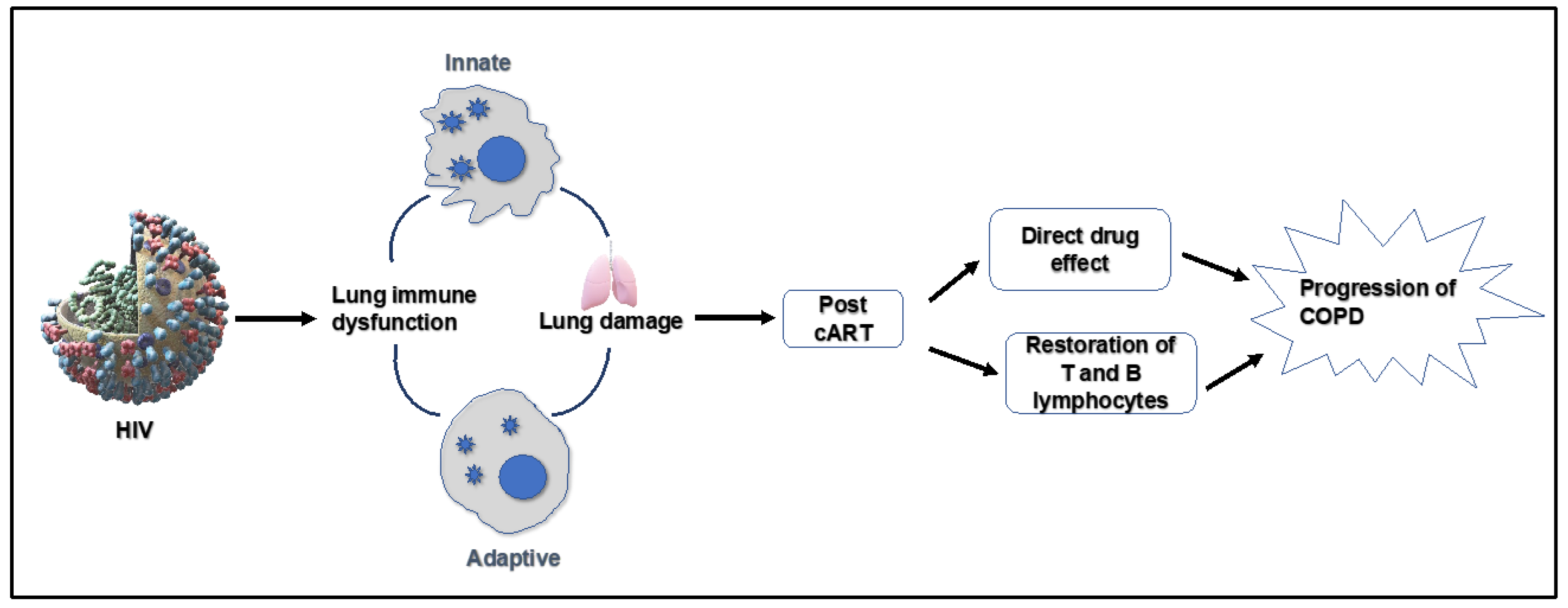
2. Spectrum of HIV-Mediated Lungs Complications
2.1. Chronic Obstructive Pulmonary Disease (COPD)
2.2. Asthma
2.3. Pulmonary Hypertension
2.4. Lung Cancer
3. Molecular Clock Gene Involvement in Pulmonary Complications
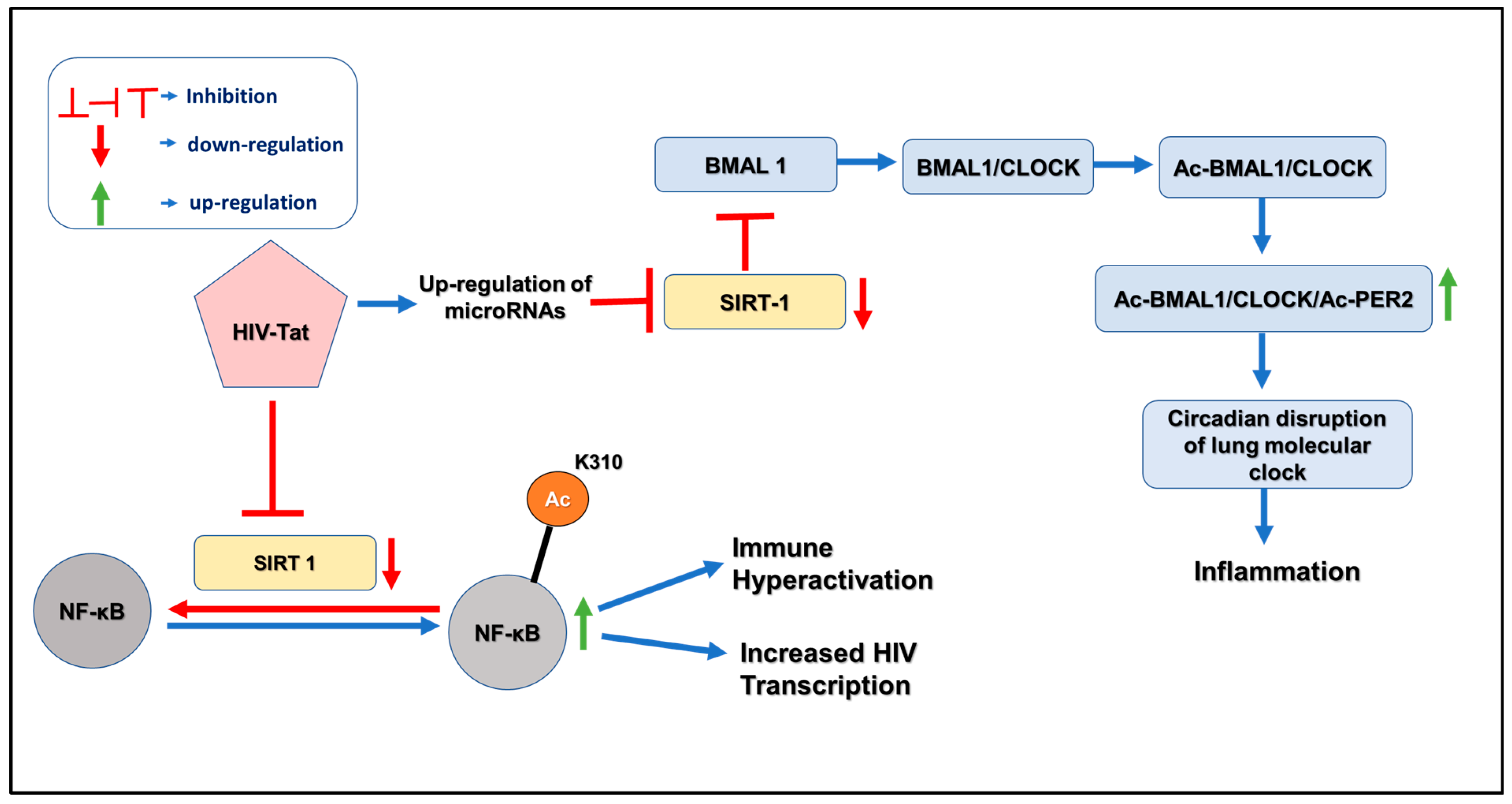
4. Pathogenesis of HIV and Circadian Disruption
5. Aberrant microRNAome Mediated Dysregulation of Clock Genes in HIV-Mediated COPD
6. Discussion
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, S.W.; McKetchnie, S.M.; Batchelder, A.W.; Justice, A.; Safren, S.A.; O’Cleirigh, C. Chronic pain and substance use disorders among older sexual minority men living with HIV: Implications for HIV disease management across the HIV care continuum. AIDS Care 2022, 35, 614–623. [Google Scholar] [CrossRef] [PubMed]
- CDC. Undertanding the HIV Care Continuum. 2019; (Factsheet). Available online: https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf (accessed on 23 April 2023).
- Bigna, J.J.; Kenne, A.M.; Asangbeh, S.L.; Sibetcheu, A.T. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e193–e202. [Google Scholar] [CrossRef]
- Morris, A.; George, M.P.; Crothers, K.; Huang, L.; Lucht, L.; Kessinger, C.; Kleerup, E.C. HIV and chronic obstructive pulmonary disease: Is it worse and why? Proc. Am. Thorac. Soc. 2011, 8, 320–325. [Google Scholar] [CrossRef]
- Lambert, A.A.; Kirk, G.D.; Astemborski, J.; Mehta, S.H.; Wise, R.A.; Drummond, M.B. HIV infection is associated with increased risk for acute exacerbation of COPD. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 69, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Comas, M.; Gordon, C.J.; Oliver, B.G.; Stow, N.W.; King, G.; Sharma, P.; Ammit, A.J.; Grunstein, R.R.; Phillips, C.L. A circadian based inflammatory response–implications for respiratory disease and treatment. Sleep Sci. Pract. 2017, 1, 18. [Google Scholar] [CrossRef]
- Hunter, F.K.; Butler, T.D.; Gibbs, J.E. Circadian rhythms in immunity and host-parasite interactions. Parasite Immunol. 2022, 44, e12904. [Google Scholar] [CrossRef] [PubMed]
- Sundar, I.K.; Yao, H.; Sellix, M.T.; Rahman, I. Circadian molecular clock in lung pathophysiology. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 309, L1056–L1075. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, Z.; Hou, W.; Li, Z.; Cheng, S.; Green, L.; Wang, Y.; Wen, X.; Cai, L.; Clauss, M. HIV T at protein affects circadian rhythmicity by interfering with the circadian system. HIV Med. 2014, 15, 565–570. [Google Scholar] [CrossRef]
- Swoyer, J.; Rhame, F.; Hrushesky, W.; Sackett-Lundeen, L.; Sothern, R.; Gale, H.; Haus, E. Circadian rhythm alteration in HIV infected subjects. Prog. Clin. Biol. Res. 1990, 341, 437–449. [Google Scholar]
- Duncan, M.J.; Bruce-Keller, A.J.; Conner, C.; Knapp, P.E.; Xu, R.; Nath, A.; Hauser, K.F. Effects of chronic expression of the HIV-induced protein, transactivator of transcription, on circadian activity rhythms in mice, with or without morphine. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R1680–R1687. [Google Scholar] [CrossRef]
- Butler, T.D.; Mohammed Ali, A.; Gibbs, J.E.; McLaughlin, J.T. Chronotype in patients with immune-mediated inflammatory disease: A systematic review. J. Biol. Rhythm. 2023, 38, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Rijo-Ferreira, F.; Takahashi, J.S. Circadian Rhythms in Infectious Diseases and Symbiosis. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–44. [Google Scholar]
- Belden, W.J.; Dunlap, J.C. SIRT1 is a circadian deacetylase for core clock components. Cell 2008, 134, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Clark, J., III; Sampair, C.S.; Kofuji, P.; Nath, A.; Ding, J.M. HIV protein, transactivator of transcription, alters circadian rhythms through the light entrainment pathway. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 289, R656–R662. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Naranbhai, V.; Stern, J.; Roche, M.; Dantanarayana, A.; Ruian, K.; Tennakoon, S.; Solomon, A.; Rebecca, H.; Hartogensis, W. Variation in cell associated unspliced HIV RNA on antiretroviral therapy is associated with the circadian regulator BMAL-1. AIDS 2018, 32, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- van Eeden, S.F.; Hogg, J.C. Immune-modulation in chronic obstructive pulmonary disease: Current concepts and future strategies. Respiration 2020, 99, 550–565. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Panzica, L.; Kalathil, S.G.; Thanavala, Y. Immune dysfunction in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2015, 12 (Suppl. S2), S169–S175. [Google Scholar] [CrossRef] [PubMed]
- Kayongo, A.; Robertson, N.M.; Siddharthan, T.; Ntayi, M.L.; Ndawula, J.C.; Sande, O.J.; Bagaya, B.S.; Kirenga, B.; Mayanja-Kizza, H.; Joloba, M.L. Airway microbiome-immune crosstalk in chronic obstructive pulmonary disease. Front. Immunol. 2023, 13, 1085551. [Google Scholar] [CrossRef]
- Cribbs, S.K.; Crothers, K.; Morris, A. Pathogenesis of HIV-related lung disease: Immunity, infection, and inflammation. Physiol. Rev. 2020, 100, 603–632. [Google Scholar] [CrossRef]
- Alexandrova, Y.; Costiniuk, C.T.; Jenabian, M.-A. Pulmonary immune dysregulation and viral persistence during HIV infection. Front. Immunol. 2022, 12, 5637. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Parira, T.; Dutta, R.; Agudelo, M.; Morris, A.; Nair, M.; Unwalla, H. HIV infects bronchial epithelium and suppresses components of the mucociliary clearance apparatus. PLoS ONE 2017, 12, e0169161. [Google Scholar] [CrossRef]
- Fan, X.; Murray, S.C.; Staitieh, B.S.; Spearman, P.; Guidot, D.M. HIV impairs alveolar macrophage function via MicroRNA-144-induced suppression of Nrf2. Am. J. Med. Sci. 2021, 361, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Charles, T.P.; Shellito, J.E. Human immunodeficiency virus infection and host defense in the lungs. In Seminars in Respiratory and Critical Care Medicine; Thieme Medical Publishers: New York, NY, USA, 2016; pp. 147–156. [Google Scholar]
- Antoniou, T.; Yao, Z.; Raboud, J.; Gershon, A.S. Incidence of chronic obstructive pulmonary disease in people with HIV in Ontario, 1996–2015: A retrospective population-based cohort study. Can. Med. Assoc. Open Access J. 2020, 8, E83–E89. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, S.; Wewers, M.E.; Koletar, S.; Reynolds, N.; Ferketich, A.; Diaz, P. Cigarette smoking in the HIV-infected population. Proc. Am. Thorac. Soc. 2011, 8, 313–319. [Google Scholar] [CrossRef]
- Unwalla, H.M.A. Trachebronchial Mucociliary Dysfunction in HIV. J. Neuroimmune Pharm. 2015, 10 (Suppl. S2), S106. [Google Scholar]
- Lifson, A.R.; Neuhaus, J.; Arribas, J.R.; van den Berg-Wolf, M.; Labriola, A.M.; Read, T.R.; Group, I.S.S. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am. J. Public Health 2010, 100, 1896–1903. [Google Scholar] [CrossRef]
- Gingo, M.R.; George, M.P.; Kessinger, C.J.; Lucht, L.; Rissler, B.; Weinman, R.; Slivka, W.A.; McMahon, D.K.; Wenzel, S.E.; Sciurba, F.C. Pulmonary function abnormalities in HIV-infected patients during the current antiretroviral therapy era. Am. J. Respir. Crit. Care Med. 2010, 182, 790–796. [Google Scholar] [CrossRef]
- van Riel, S.E.; Klipstein-Grobusch, K.; Barth, R.E.; Grobbee, D.E.; Feldman, C.; Shaddock, E.; Stacey, S.L.; Venter, W.D.; Vos, A.G. Predictors of impaired pulmonary function in people living with HIV in an urban African setting. South. Afr. J. HIV Med. 2021, 22, 1–8. [Google Scholar] [CrossRef]
- Zifodya, J.S.; Triplette, M.; Shahrir, S.; Attia, E.F.; Akgun, K.M.; Hoo, G.W.S.; Rodriguez-Barradas, M.C.; Wongtrakool, C.; Huang, L.; Crothers, K. A cross-sectional analysis of diagnosis and management of chronic obstructive pulmonary disease in people living with HIV: Opportunities for improvement. Medicine 2021, 100, e27124. [Google Scholar] [CrossRef]
- Kunisaki, K.M. Recent advances in HIV-associated chronic lung disease clinical research. Curr. Opin. HIV AIDS 2021, 16, 156–162. [Google Scholar] [CrossRef]
- Modi, P.; Cascella, M. Diffusing Capacity of the Lungs for Carbon Monoxide; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Besutti, G.; Santoro, A.; Scaglioni, R.; Neri, S.; Zona, S.; Malagoli, A.; Orlando, G.; Beghè, B.; Ligabue, G.; Torricelli, P. Significant chronic airway abnormalities in never-smoking HIV-infected patients. HIV Med. 2019, 20, 657–667. [Google Scholar] [CrossRef]
- Mirani, G.; Williams, P.L.; Chernoff, M.; Abzug, M.J.; Levin, M.J.; Seage III, G.R.; Oleske, J.M.; Purswani, M.U.; Hazra, R.; Traite, S. Changing trends in complications and mortality rates among US youth and young adults with HIV infection in the era of combination antiretroviral therapy. Clin. Infect. Dis. 2015, 61, 1850–1861. [Google Scholar] [CrossRef]
- Kirenga, B.J.; Mugenyi, L.; de Jong, C.; Lucian Davis, J.; Katagira, W.; van der Molen, T.; Kamya, M.R.; Boezen, M. The impact of HIV on the prevalence of asthma in Uganda: A general population survey. Respir. Res. 2018, 19, 184. [Google Scholar] [CrossRef]
- Barton, J.H.; Ireland, A.; Fitzpatrick, M.; Kessinger, C.; Camp, D.; Weinman, R.; McMahon, D.; Leader, J.K.; Holguin, F.; Wenzel, S.E. Adiposity influences airway wall thickness and the asthma phenotype of HIV-associated obstructive lung disease: A cross-sectional study. BMC Pulm. Med. 2016, 16, 111. [Google Scholar] [CrossRef]
- Adrish, M.; Gomez, G.R.; Rodriguez, E.C.; Mantri, N. Influence of HIV status on the management of acute asthma exacerbations. BMJ Open Respir. Res. 2019, 6, e000472. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.E.; Wong, J.; Taljaard, M.; Glazier, R.H.; Hogg, W.; Younger, J.; Manuel, D.G. A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 2014, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Gingo, M.R.; Wenzel, S.E.; Steele, C.; Kessinger, C.J.; Lucht, L.; Lawther, T.; Busch, M.; Hillenbrand, M.E.; Weinman, R.; Slivka, W.A. Asthma diagnosis and airway bronchodilator response in HIV-infected patients. J. Allergy Clin. Immunol. 2012, 129, 708–714.e8. [Google Scholar] [CrossRef]
- Fernandez-Botran, R.; Vega, A.R.; García, Y.; Tirumala, C.C.; Srisailam, P.; Raghuram, A.; Peyrani, P.; Furmanek, S.; Tella, M.A.; Ritzhentaler, J.D. The elevated systemic cytokine levels in HIV patients are not associated with an elevated pulmonary cytokine environment. Cytokine 2020, 126, 154874. [Google Scholar] [CrossRef]
- Poirier, C.D.; Inhaber, N.; Lalonde, R.G.; Ernst, P. Prevalence of bronchial hyperresponsiveness among HIV-infected men. Am. J. Respir. Crit. Care Med. 2001, 164, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Kanmogne, G.D.; Primeaux, C.; Grammas, P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: Implications for the pathogenesis of HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2005, 64, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Presta, M. HIV-1 Tat protein and endothelium: From protein/cell interaction to AIDS-associated pathologies. Angiogenesis 2002, 5, 141–151. [Google Scholar] [CrossRef]
- Sehgal, P.B.; Mukhopadhyay, S.; Patel, K.; Xu, F.; Almodóvar, S.; Tuder, R.M.; Flores, S.C. Golgi dysfunction is a common feature in idiopathic human pulmonary hypertension and vascular lesions in SHIV-nef-infected macaques. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 297, L729–L737. [Google Scholar] [CrossRef]
- Shiels, M.S.; Pfeiffer, R.M.; Gail, M.H.; Hall, H.I.; Li, J.; Chaturvedi, A.K.; Bhatia, K.; Uldrick, T.S.; Yarchoan, R.; Goedert, J.J. Cancer burden in the HIV-infected population in the United States. J. Natl. Cancer Inst. 2011, 103, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Lau, B.; Achenbach, C.J.; Jing, Y.; Althoff, K.N.; D’Souza, G.; Engels, E.A.; Hessol, N.A.; Brooks, J.T.; Burchell, A.N. Cumulative incidence of cancer among persons with HIV in North America: A cohort study. Ann. Intern. Med. 2015, 163, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Hleyhel, M. Writing Committee of the Cancer Risk Group of the French Hospital Database on HIV. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: Results from a French cohort. Aids 2014, 28, 2109–2118. [Google Scholar] [CrossRef]
- Barnes, P.J. Circadian variation in airway function. Am. J. Med. 1985, 79, 5–9. [Google Scholar] [CrossRef]
- Angelis, N.; Porpodis, K.; Zarogoulidis, P.; Spyratos, D.; Kioumis, I.; Papaiwannou, A.; Pitsiou, G.; Tsakiridis, K.; Mpakas, A.; Arikas, S. Airway inflammation in chronic obstructive pulmonary disease. J. Thorac. Dis. 2014, 6 (Suppl. S1), S167. [Google Scholar]
- Sundar, I.K.; Ahmad, T.; Yao, H.; Hwang, J.-W.; Gerloff, J.; Lawrence, B.P.; Sellix, M.T.; Rahman, I. Influenza A virus-dependent remodeling of pulmonary clock function in a mouse model of COPD. Sci. Rep. 2015, 5, 9927. [Google Scholar] [CrossRef]
- Ehlers, A.; Xie, W.; Agapov, E.; Brown, S.; Steinberg, D.; Tidwell, R.; Sajol, G.; Schutz, R.; Weaver, R.; Yu, H. BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunol. 2018, 11, 97–111. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. The mammalian circadian timing system and the suprachiasmatic nucleus as its pacemaker. Biology 2019, 8, 13. [Google Scholar] [CrossRef]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, R.; Tsuchiya, Y.; Tokuda, I.; Matsuo, T.; Sato, M.; Node, K.; Nishida, E.; Akashi, M. The mammalian circadian clock protein period counteracts cryptochrome in phosphorylation dynamics of circadian locomotor output cycles kaput (CLOCK). J. Biol. Chem. 2014, 289, 32064–32072. [Google Scholar] [CrossRef] [PubMed]
- Parico, G.C.G.; Perez, I.; Fribourgh, J.L.; Hernandez, B.N.; Lee, H.-W.; Partch, C.L. The human CRY1 tail controls circadian timing by regulating its association with CLOCK: BMAL1. Proc. Natl. Acad. Sci. USA 2020, 117, 27971–27979. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, P.D.; DuBois, D.C.; Almon, R.R.; Jusko, W.J. Modeling circadian variability of core-clock and clock-controlled genes in four tissues of the rat. PLoS ONE 2018, 13, e0197534. [Google Scholar] [CrossRef] [PubMed]
- Rajendrasozhan, S.; Yang, S.-R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef]
- Hwang, J.-W.; Sundar, I.K.; Yao, H.; Sellix, M.T.; Rahman, I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014, 28, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, M.; Zhao, C.; Cheng, Y.; Liu, C.; Shi, M. Circadian clock gene Clock-Bmal1 regulates cellular senescence in Chronic obstructive pulmonary disease. BMC Pulm. Med. 2022, 22, 435. [Google Scholar] [CrossRef]
- Lechasseur, A.; Jubinville, É.; Routhier, J.; Bérubé, J.C.; Hamel-Auger, M.; Talbot, M.; Lamothe, J.; Aubin, S.; Paré, M.È.; Beaulieu, M.J. Exposure to electronic cigarette vapors affects pulmonary and systemic expression of circadian molecular clock genes. Physiol. Rep. 2017, 5, e13440. [Google Scholar] [CrossRef] [PubMed]
- Vitaterna, M.H.; King, D.P.; Chang, A.-M.; Kornhauser, J.M.; Lowrey, P.L.; McDonald, J.D.; Dove, W.F.; Pinto, L.H.; Turek, F.W.; Takahashi, J.S. Mutagenesis and mapping of a mouse gene, Clock, essential for circadian behavior. Science 1994, 264, 719–725. [Google Scholar] [CrossRef]
- Deng, W.; Zhu, S.; Zeng, L.; Liu, J.; Kang, R.; Yang, M.; Cao, L.; Wang, H.; Billiar, T.R.; Jiang, J. The circadian clock controls immune checkpoint pathway in sepsis. Cell Rep. 2018, 24, 366–378. [Google Scholar] [CrossRef]
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 2012, 36, 251–261. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wright, C.J.; Hinson, M.D.; Fernando, A.P.; Sengupta, S.; Biswas, C.; La, P.; Dennery, P.A. Oxidative stress and inflammation modulate Rev-erbα signaling in the neonatal lung and affect circadian rhythmicity. Antioxid. Redox Signal. 2014, 21, 17–32. [Google Scholar] [CrossRef]
- Griffin, P.; Dimitry, J.M.; Sheehan, P.W.; Lananna, B.V.; Guo, C.; Robinette, M.L.; Hayes, M.E.; Cedeño, M.R.; Nadarajah, C.J.; Ezerskiy, L.A. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 5102–5107. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chen, Y.-C.; Wang, T.-N.; Fang, W.-F.; Chang, Y.-C.; Chen, Y.-M.; Chen, I.-Y.; Lin, M.-C.; Yang, M.-Y. Disrupted expression of circadian clock genes in patients with bronchial asthma. J. Asthma Allergy 2021, 14, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Cory, T.J.; Schacker, T.W.; Stevenson, M.; Fletcher, C.V. Overcoming pharmacologic sanctuaries. Curr. Opin. HIV AIDS 2013, 8, 190–195. [Google Scholar] [CrossRef]
- Palmer, S.; Josefsson, L.; Coffin, J.M. HIV reservoirs and the possibility of a cure for HIV infection. J. Intern. Med. 2011, 270, 550–560. [Google Scholar] [CrossRef]
- Buzon, M.J.; Massanella, M.; Llibre, J.M.; Esteve, A.; Dahl, V.; Puertas, M.C.; Gatell, J.M.; Domingo, P.; Paredes, R.; Sharkey, M.; et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat. Med. 2010, 16, 460–465. [Google Scholar] [CrossRef]
- Hatano, H.; Strain, M.C.; Scherzer, R.; Bacchetti, P.; Wentworth, D.; Hoh, R.; Martin, J.N.; McCune, J.M.; Neaton, J.D.; Tracy, R.P.; et al. Increase in 2-long terminal repeat circles and decrease in D-dimer after raltegravir intensification in patients with treated HIV infection: A randomized, placebo-controlled trial. J. Infect. Dis. 2013, 208, 1436–1442. [Google Scholar] [CrossRef]
- Twigg, H.L.; Soliman, D.M.; Day, R.B.; Knox, K.S.; Anderson, R.J.; Wilkes, D.S.; Schnizlein-Bick, C.T. Lymphocytic alveolitis, bronchoalveolar lavage viral load, and outcome in human immunodeficiency virus infection. Am. J. Respir. Crit. Care Med. 1999, 159 Pt 1, 1439–1444. [Google Scholar] [CrossRef]
- Nakata, K.; Weiden, M.; Harkin, T.; Ho, D.; Rom, W.N. Low copy number and limited variability of proviral DNA in alveolar macrophages from HIV-1-infected patients: Evidence for genetic differences in HIV-1 between lung and blood macrophage populations. Mol. Med. 1995, 1, 744–757. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.M.; Krivine, A.; Pinkston, P.; Gillis, J.M.; Huang, A.; Hammer, S.M. Frequent identification of HIV-1 DNA in bronchoalveolar lavage cells obtained from individuals with the acquired immunodeficiency syndrome. Am. Rev. Respir. Dis. 1991, 143 Pt 1, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Devadoss, D.; Singh, S.P.; Acharya, A.; Do, K.C.; Periyasamy, P.; Manevski, M.; Mishra, N.; Tellez, C.; Ramakrishnan, S.; Belinsky, S.; et al. Lung Bronchial Epithelial Cells are HIV Targets for Proviral Genomic Integration. bioRxiv 2020. bioRxiv:2020.06.01.126821. [Google Scholar]
- Chand, H.S.; Vazquez-Guillamet, R.; Royer, C.; Rudolph, K.; Mishra, N.; Singh, S.P.; Hussain, S.S.; Barrett, E.; Callen, S.; Byrareddy, S.N.; et al. Cigarette smoke and HIV synergistically affect lung pathology in cynomolgus macaques. J. Clin. Investig. 2018, 128, 5428–5433. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Dutta, R.K.; Nair, M.; Chand, H.S.; Rahman, I.; Unwalla, H.J. TGF-beta1 increases viral burden and promotes HIV-1 latency in primary differentiated human bronchial epithelial cells. Sci. Rep. 2019, 9, 12552. [Google Scholar] [CrossRef] [PubMed]
- Chinnapaiyan, S.; Dutta, R.; Bala, J.; Parira, T.; Agudelo, M.; Nair, M.; Unwalla, H.J. Cigarette smoke promotes HIV infection of primary bronchial epithelium and additively suppresses CFTR function. Sci. Rep. 2018, 8, 7984. [Google Scholar] [CrossRef]
- Collora, J.A.; Ho, Y.C. The loud minority: Transcriptionally active HIV-1-infected cells survive, proliferate, and persist. Cell 2022, 185, 227–229. [Google Scholar] [CrossRef]
- Einkauf, K.B.; Osborn, M.R.; Gao, C.; Sun, W.; Sun, X.; Lian, X.; Parsons, E.M.; Gladkov, G.T.; Seiger, K.W.; Blackmer, J.E.; et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 2022, 185, 266–282.e15. [Google Scholar] [CrossRef]
- Malone, J.L.; Oldfield III, E.C.; Wagner, K.F.; Simms, T.E.; Daly, R.; O’Brian, J.; Burke, D.S. Abnormalities of morning serum cortisol levels and circadian rhythms of CD4+ lymphocyte counts in human immunodeficiency virus type 1-infected adult patients. J. Infect. Dis. 1992, 165, 185–186. [Google Scholar] [CrossRef]
- Lee, K.A.; Gay, C.; Byun, E.; Lerdal, A.; Pullinger, C.R.; Aouizerat, B.E. Circadian regulation gene polymorphisms are associated with sleep disruption and duration, and circadian phase and rhythm in adults with HIV. Chronobiol. Int. 2015, 32, 1278–1293. [Google Scholar] [CrossRef]
- Kwon, H.-S.; Brent, M.M.; Getachew, R.; Jayakumar, P.; Chen, L.-F.; Schnolzer, M.; McBurney, M.W.; Marmorstein, R.; Greene, W.C.; Ott, M. Human immunodeficiency virus type 1 Tat protein inhibits the SIRT1 deacetylase and induces T cell hyperactivation. Cell Host Microbe 2008, 3, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, V.; Tartaglia, E.; Refolo, G.; Sacchi, A.; Grassi, G.; Antinori, A.; Fimia, G.M.; Agrati, C. Per2 upregulation in circulating hematopoietic progenitor cells during chronic hiv infection. Front. Cell. Infect. Microbiol. 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.K.; Chinnapaiyan, S.; Unwalla, H. Aberrant microRNAomics in pulmonary complications: Implications in lung health and diseases. Mol. Ther.-Nucleic Acids 2019, 18, 413–431. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Lewis, B.P.; Burge, C.B.; Bartel, D.P. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005, 120, 15–20. [Google Scholar] [CrossRef]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef]
- Griffiths-Jones, S.; Grocock, R.J.; Van Dongen, S.; Bateman, A.; Enright, A.J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006, 34 (Suppl. S1), D140–D144. [Google Scholar] [CrossRef]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Adcock, I.M.; Garssen, J.; Mortaz, E.; Varahram, M.; Mirsaeidi, M.; Velayati, A. The roles of miRNAs as potential biomarkers in lung diseases. Eur. J. Pharmacol. 2016, 791, 395–404. [Google Scholar] [CrossRef]
- Su, B.; Fu, Y.; Liu, Y.; Wu, H.; Ma, P.; Zeng, W.; Zhang, T.; Lian, S.; Wu, H. Potential application of microRNA profiling to the diagnosis and prognosis of HIV-1 infection. Front. Microbiol. 2018, 9, 3185. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Takahashi, J.S. Circadian clock genes and the transcriptional architecture of the clock mechanism. J. Mol. Endocrinol. 2019, 63, R93. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, T.; Zhu, J.; Wang, X.; Tong, J.; Li, Z.; Dong, J. The link between circadian clock genes and autophagy in chronic obstructive pulmonary disease. Mediat. Inflamm. 2021, 2021, 2689600. [Google Scholar] [CrossRef]
- Suárez, Y.; Sessa, W.C. MicroRNAs as novel regulators of angiogenesis. Circ. Res. 2009, 104, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.-Y.M.; Papp, J.W.; Varlamova, O.; Dziema, H.; Russell, B.; Curfman, J.P.; Nakazawa, T.; Shimizu, K.; Okamura, H.; Impey, S. microRNA modulation of circadian-clock period and entrainment. Neuron 2007, 54, 813–829. [Google Scholar] [CrossRef]
- Shende, V.R.; Neuendorff, N.; Earnest, D.J. Role of miR-142-3p in the post-transcriptional regulation of the clock gene Bmal1 in the mouse SCN. PLoS ONE 2013, 8, e65300. [Google Scholar] [CrossRef] [PubMed]
- Shende, V.R.; Goldrick, M.M.; Ramani, S.; Earnest, D.J. Expression and rhythmic modulation of circulating microRNAs targeting the clock gene Bmal1 in mice. PLoS ONE 2011, 6, e22586. [Google Scholar] [CrossRef]
- Tan, X.; Zhang, P.; Zhou, L.; Yin, B.; Pan, H.; Peng, X. Clock-controlled mir-142-3p can target its activator, Bmal1. BMC Mol. Biol. 2012, 13, 27. [Google Scholar] [CrossRef]
- Chaudhuri, A.D.; Yelamanchili, S.V.; Marcondes, M.C.G.; Fox, H.S. Up-regulation of microRNA-142 in simian immunodeficiency virus encephalitis leads to repression of sirtuin1. FASEB J. 2013, 27, 3720–3729. [Google Scholar] [CrossRef]
- Zhang, H.-S.; Chen, X.-Y.; Wu, T.-C.; Sang, W.-W.; Ruan, Z. MiR-34a is involved in Tat-induced HIV-1 long terminal repeat (LTR) transactivation through the SIRT1/NFκB pathway. FEBS Lett. 2012, 586, 4203–4207. [Google Scholar] [CrossRef]
- Cai, X.; Hagedorn, C.H.; Cullen, B.R. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. Rna 2004, 10, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Colangelo, T.; Panza, A.; Rubino, R.; Tiberio, C.; Palumbo, O.; Carella, M.; Trombetta, D.; Gentile, A.; Tavano, F. Analysis of clock gene-miRNA correlation networks reveals candidate drivers in colorectal cancer. Oncotarget 2016, 7, 45444–45461. [Google Scholar] [CrossRef]
- Hasakova, K.; Reis, R.; Vician, M.; Zeman, M.; Herichova, I. Expression of miR-34a-5p is up-regulated in human colorectal cancer and correlates with survival and clock gene PER2 expression. PLoS ONE 2019, 14, e0224396. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Khurana, N.; Williams, J.; Dowd Greene, C.; Uhlig, R.; Middleton, F.A. Diurnal oscillations in human salivary microRNA and microbial transcription: Implications for human health and disease. PLoS ONE 2018, 13, e0198288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, X.; Cheng, S.; Xie, Y.; Wang, Z.; Liu, Y.; Jiang, Z.; Xiao, J.; Guo, H.; Wang, Y. MiR-29a/b/c regulate human circadian gene hPER1 expression by targeting its 3′UTR. Acta Biochim. Biophys. Sin. 2014, 46, 313–317. [Google Scholar] [CrossRef]
- Landskroner-Eiger, S.; Qiu, C.; Perrotta, P.; Siragusa, M.; Lee, M.Y.; Ulrich, V.; Luciano, A.K.; Zhuang, Z.W.; Corti, F.; Simons, M. Endothelial miR-17∼92 cluster negatively regulates arteriogenesis via miRNA-19 repression of WNT signaling. Proc. Natl. Acad. Sci. USA 2015, 112, 12812–12817. [Google Scholar] [CrossRef]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA. org resource: Targets and expression. Nucleic Acids Res. 2008, 36 (Suppl. S1), D149–D153. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, L.; Yang, S.-Y.; Cao, J.-M. A novel role of microRNA 17-5p in the modulation of circadian rhythm. Sci. Rep. 2016, 6, 30070. [Google Scholar] [CrossRef]
- Triboulet, R.; Mari, B.; Lin, Y.-L.; Chable-Bessia, C.; Bennasser, Y.; Lebrigand, K.; Cardinaud, B.; Maurin, T.; Barbry, P.; Baillat, V. Suppression of microRNA-silencing pathway by HIV-1 during virus replication. Science 2007, 315, 1579–1582. [Google Scholar] [CrossRef]
- Nagel, R.; Clijsters, L.; Agami, R. The miRNA-192/194 cluster regulates the Period gene family and the circadian clock. FEBS J. 2009, 276, 5447–5455. [Google Scholar] [CrossRef]
- Na, Y.-J.; Sung, J.H.; Lee, S.C.; Lee, Y.-J.; Choi, Y.J.; Park, W.-Y.; Shin, H.S.; Kim, J.H. Comprehensive analysis of microRNA-mRNA co-expression in circadian rhythm. Exp. Mol. Med. 2009, 41, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Watson, A.K.; Blankson, J.N.; Clements, J.E. Relationships of PBMC microRNA expression, plasma viral load, and CD4+ T-cell count in HIV-1-infected elite suppressors and viremic patients. Retrovirology 2012, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Miller, C.; Miraglia, L.J.; Romero, A.; Mure, L.S.; Panda, S.; Kay, S.A. A genome-wide microRNA screen identifies the microRNA-183/96/182 cluster as a modulator of circadian rhythms. Proc. Natl. Acad. Sci. USA 2021, 118, e2020454118. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-Z.; Yu, Y.; Zhou, Y.-F.; Yang, Y.-W.; Lei, M.-Q.; Lian, J.-P.; He, H.; Zhang, Y.-C.; Huang, W.; Chen, Y.-Q. A natural variant of miR397 mediates a feedback loop in circadian rhythm. Plant Physiol. 2020, 182, 204–214. [Google Scholar] [CrossRef]
- Chen, R.; D’Alessandro, M.; Lee, C. miRNAs are required for generating a time delay critical for the circadian oscillator. Curr. Biol. 2013, 23, 1959–1968. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Kojima, S.; Shimomura, K.; Koike, N.; Buhr, E.D.; Furukawa, T.; Ko, C.H.; Gloston, G.; Ayoub, C.; Nohara, K. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc. Natl. Acad. Sci. USA 2017, 114, E8855–E8864. [Google Scholar] [CrossRef]
- Chinnapaiyan, S.; Dutta, R.K.; Devadoss, D.; Chand, H.S.; Rahman, I.; Unwalla, H.J. Role of non-coding RNAs in lung circadian clock related diseases. Int. J. Mol. Sci. 2020, 21, 3013. [Google Scholar] [CrossRef]
- Datta Chaudhuri, A.; Yelamanchili, S.V.; Fox, H.S. MicroRNA-142 reduces monoamine oxidase A expression and activity in neuronal cells by downregulating SIRT1. PLoS ONE 2013, 8, e79579. [Google Scholar] [CrossRef]
- Dutta, R.K.; Chinnapaiyan, S.; Santiago, M.J.; Rahman, I.; Unwalla, H.J. Gene-specific MicroRNA antagonism protects against HIV Tat and TGF-beta-mediated suppression of CFTR mRNA and function. Biomed Pharm. 2021, 142, 112090. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, K.; Chinnapaiyan, S.; Rahman, M.S.; Santiago, M.J.; Black, S.M.; Unwalla, H.J. Circadian-Coupled Genes Expression and Regulation in HIV-Associated Chronic Obstructive Pulmonary Disease (COPD) and Lung Comorbidities. Int. J. Mol. Sci. 2023, 24, 9140. https://doi.org/10.3390/ijms24119140
Panda K, Chinnapaiyan S, Rahman MS, Santiago MJ, Black SM, Unwalla HJ. Circadian-Coupled Genes Expression and Regulation in HIV-Associated Chronic Obstructive Pulmonary Disease (COPD) and Lung Comorbidities. International Journal of Molecular Sciences. 2023; 24(11):9140. https://doi.org/10.3390/ijms24119140
Chicago/Turabian StylePanda, Kingshuk, Srinivasan Chinnapaiyan, Md. Sohanur Rahman, Maria J. Santiago, Stephen M. Black, and Hoshang J. Unwalla. 2023. "Circadian-Coupled Genes Expression and Regulation in HIV-Associated Chronic Obstructive Pulmonary Disease (COPD) and Lung Comorbidities" International Journal of Molecular Sciences 24, no. 11: 9140. https://doi.org/10.3390/ijms24119140
APA StylePanda, K., Chinnapaiyan, S., Rahman, M. S., Santiago, M. J., Black, S. M., & Unwalla, H. J. (2023). Circadian-Coupled Genes Expression and Regulation in HIV-Associated Chronic Obstructive Pulmonary Disease (COPD) and Lung Comorbidities. International Journal of Molecular Sciences, 24(11), 9140. https://doi.org/10.3390/ijms24119140






