Integrated Multi-Omics Reveals Significant Roles of Non-Additively Expressed Small RNAs in Heterosis for Maize Plant Height
Abstract
1. Introduction
2. Results
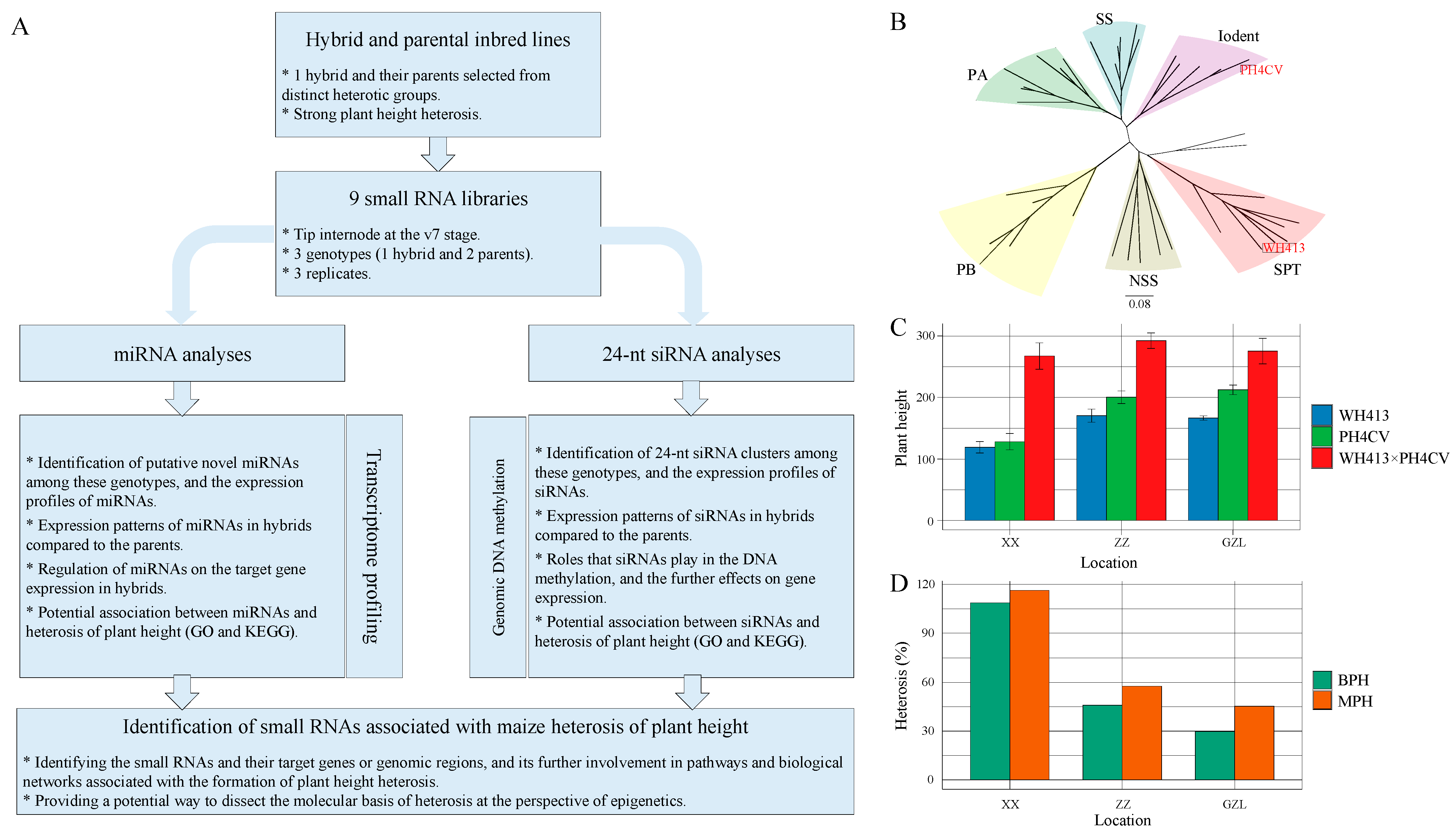
2.1. Maize Hybrids Exhibit Significant PH Heterosis
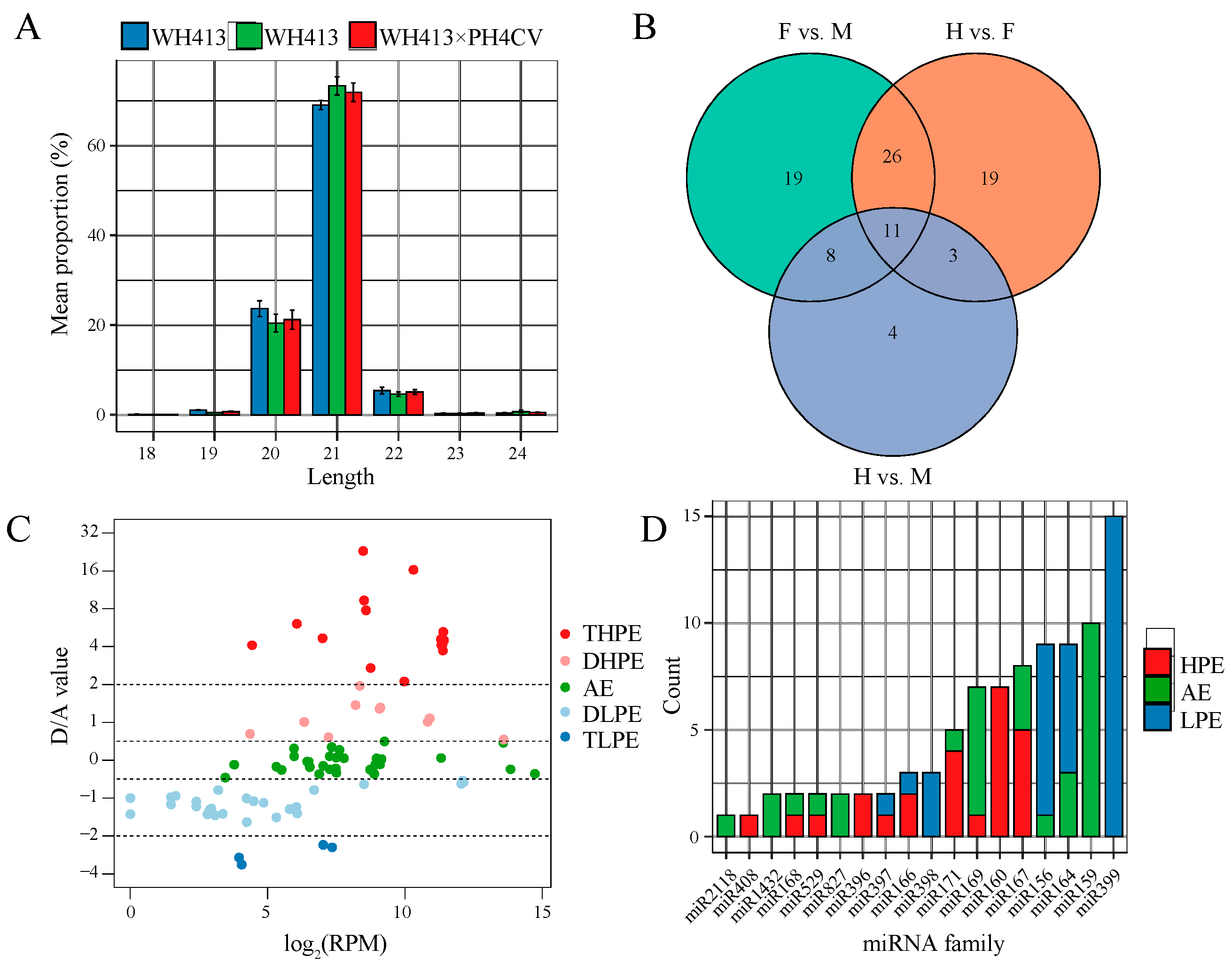
2.2. Identification and Expression Patterns of miRNAs in a Maize Hybrid
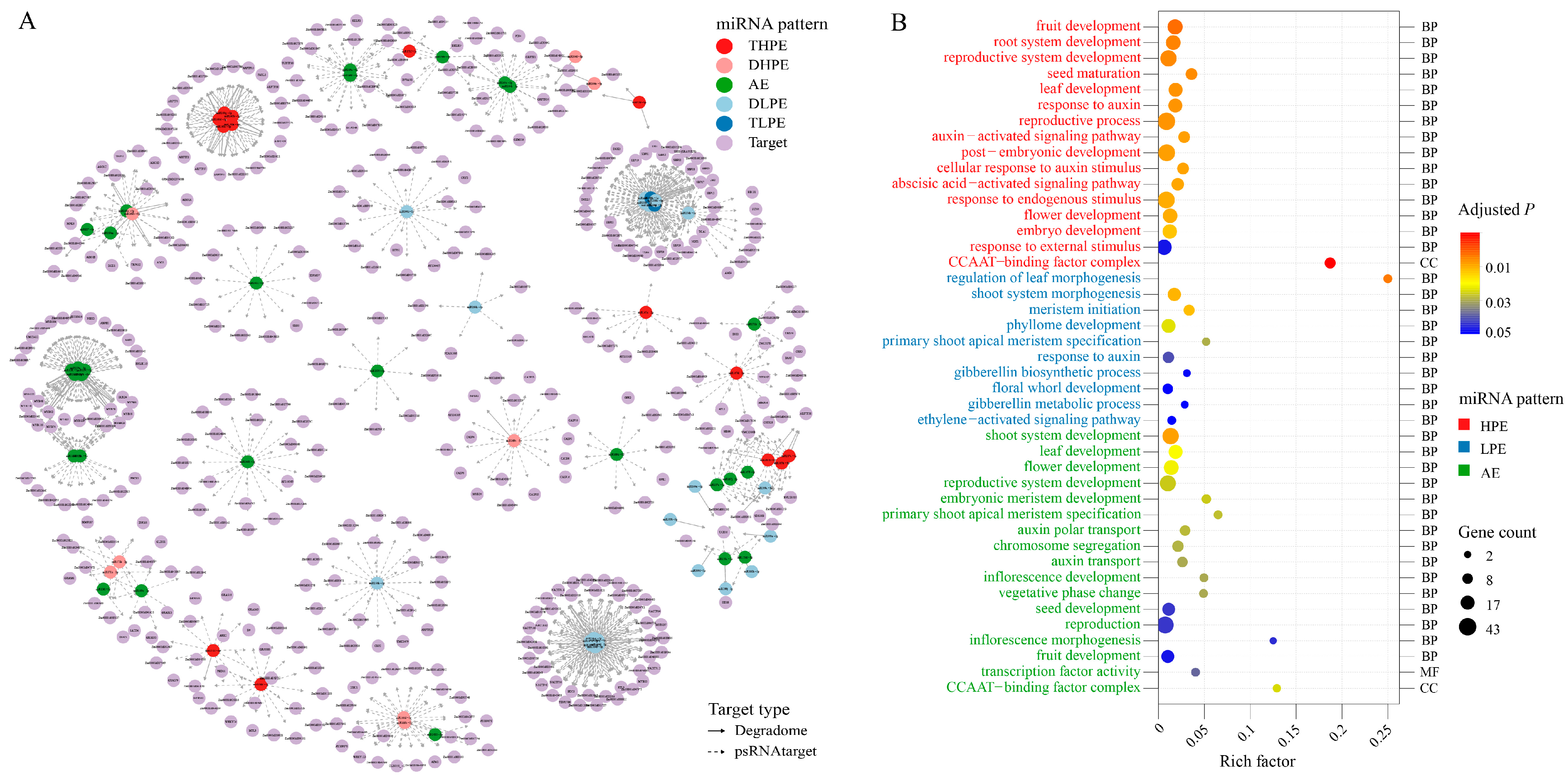
2.3. Non-Additive miRNAs Might Contribute to Heterosis by Regulating the Targets of Distinct Pathways
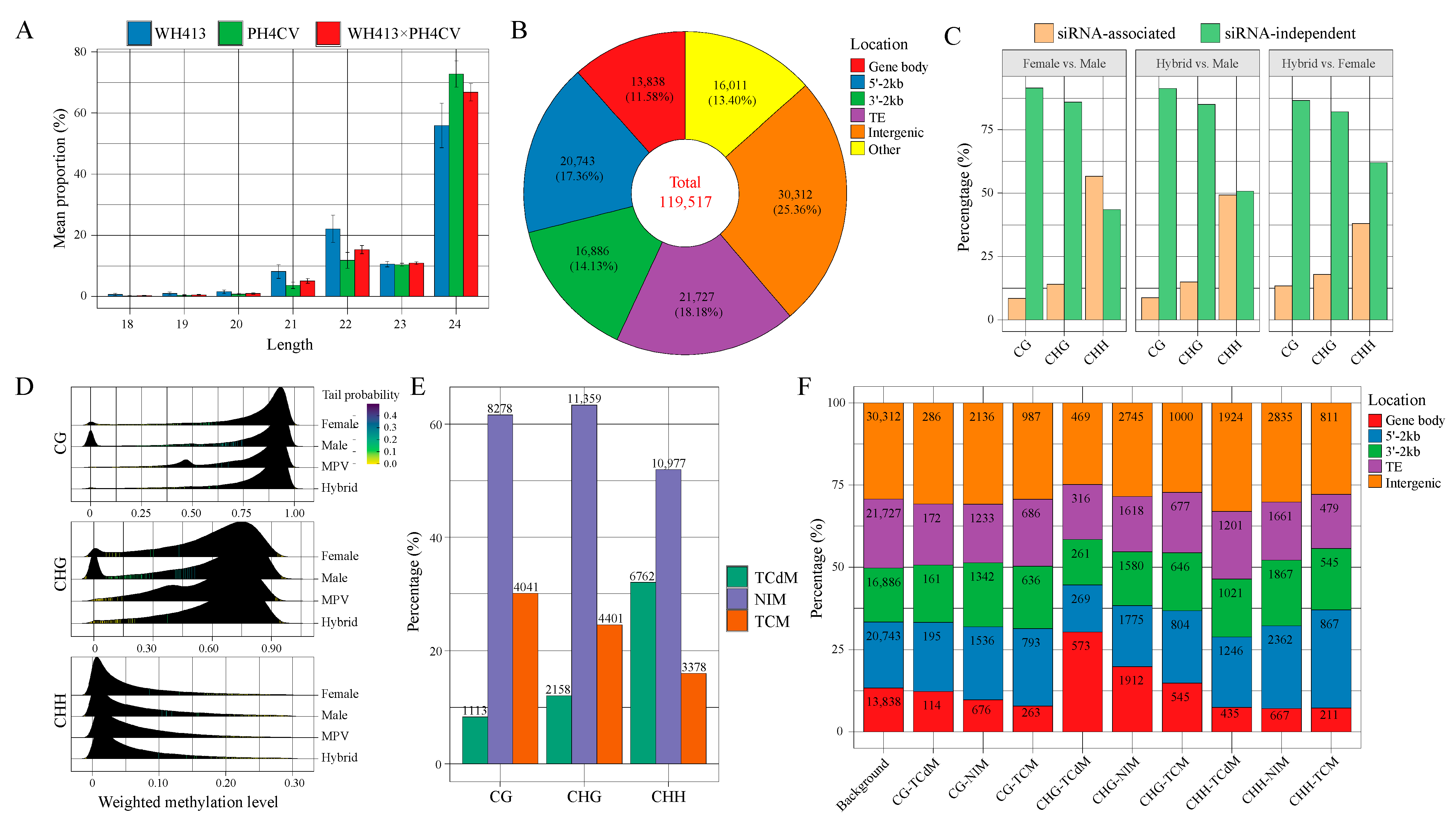
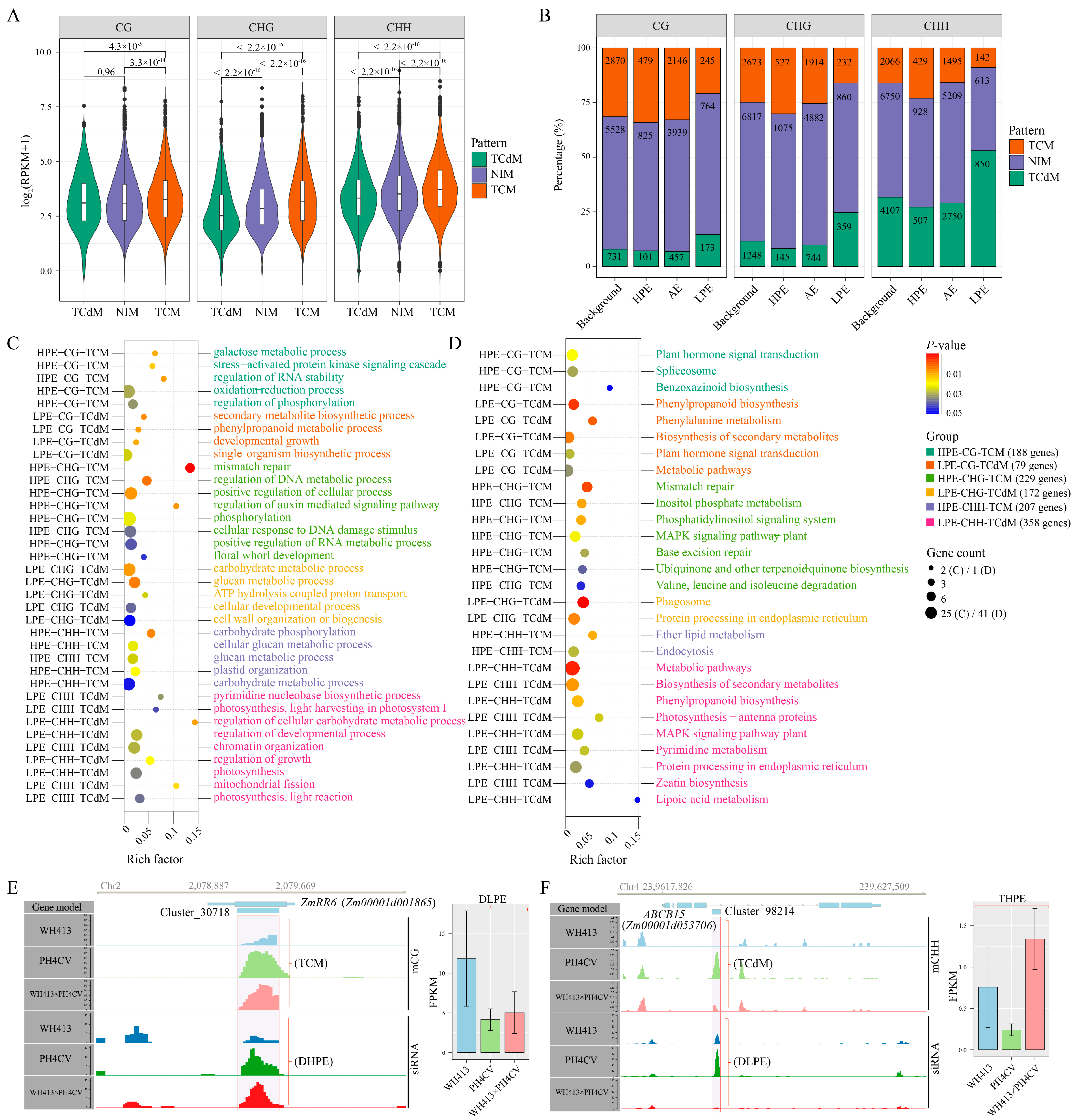
2.4. Identification of siRNA Clusters and Their Relationship with DNA Methylation in a Maize Hybrid
2.5. Non-Additively Expressed siRNA Might Contribute to PH Heterosis by Modulating the Methylation of Co-Localized Genes
3. Discussion
3.1. PH Serves as an Excellent Model Trait for Studying Maize Heterosis
3.2. miRNAs Are Critical Regulators of Maize PH Heterosis
3.3. The 24-nt siRNAs Play an Important Role in Heterosis by Altering the DNA Methylation Status of Associated Genes
4. Materials and Methods
4.1. Plant Materials and Phenotyping
4.2. Construction and Sequencing of sRNA-seq, mRNA-seq, and Degradome Libraries
4.3. Identification of miRNAs and siRNAs
4.4. Differential Expressions and Expression Patterns of miRNA and siRNA
4.5. Identification of miRNA Target Genes
4.6. Analysis of mRNA-seq Data
4.7. Construction of Bisulfite-seq Libraries and Analysis of Whole-Genome Bisulfite Sequencing (WGBS)-seq Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PH | Plant height |
| sRNA | Small RNA |
| miRNA | MicroRNA |
| siRNA | Small interfering RNA |
| HPE/LPE | High/Low-parental expressed |
| TCM/TCdM | Trans-chromosomal methylation/demethylation |
| NIM | Non-interactive methylation |
| RdDM | RNA-directed DNA methylation |
| MPH/BPH | Mid/Better-parent heterosis |
| DEM/DES/DEG | Differentially expressed miRNA/siRNA/gene |
| DMR | Differentially methylation region |
References
- Schnable, P.S.; Springer, N.M. Progress toward Understanding Heterosis in Crop Plants. Annu. Rev. Plant Biol. 2013, 64, 71–88. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Zhang, Q.; Wei, X.; Huang, X. Exploring the molecular basis of heterosis for plant breeding. J. Integr. Plant Biol. 2020, 62, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.J. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 2013, 14, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Carroll, B.J. Evolution and Diversification of Small RNA Pathways in Flowering Plants. Plant Cell Physiol. 2018, 59, 2169–2187. [Google Scholar] [CrossRef]
- Muslu, T.; Akpinar, B.A.; Biyiklioglu-Kaya, S.; Yuce, M.; Budak, H. Comparative Analysis of Coding and Non-Coding Features within Insect Tolerance Loci in Wheat with Their Homologs in Cereal Genomes. Int. J. Mol. Sci. 2021, 22, 12349. [Google Scholar] [CrossRef] [PubMed]
- Budak, H.; Khan, Z.; Kantar, M. History and current status of wheat miRNAs using next-generation sequencing and their roles in development and stress. Brief. Funct. Genom. 2014, 14, 189–198. [Google Scholar] [CrossRef]
- Hou, G.; Dong, Y.; Zhu, F.; Zhao, Q.; Li, T.; Dou, D.; Ma, X.; Wu, L.; Ku, L.; Chen, Y. MicroRNA transcriptomic analysis of the sixth leaf of maize (Zea mays L.) revealed a regulatory mechanism of jointing stage heterosis. BMC Plant Biol. 2020, 20, 541. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Dai, Z.; Ma, F.; Miao, X.; Shi, Z. OsmiR396/growth regulating factor modulate rice grain size through direct regulation of embryo-specific miR408. Plant Physiol. 2021, 186, 519–533. [Google Scholar] [CrossRef]
- Dixon, L.E.; Pasquariello, M.; Badgami, R.; Levin, K.A.; Poschet, G.; Ng, P.Q.; Orford, S.; Chayut, N.; Adamski, N.M.; Brinton, J.; et al. MicroRNA-resistant alleles of HOMEOBOX DOMAIN-2 modify inflorescence branching and increase grain protein content of wheat. Sci. Adv. 2022, 8, eabn5907. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, Y.; Xie, Y.; Wang, H. Exploiting SPL genes to improve maize plant architecture tailored for high-density planting. J. Exp. Bot. 2018, 69, 4675–4688. [Google Scholar] [CrossRef]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA Methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef] [PubMed]
- Brant, E.J.; Budak, H. Plant Small Non-coding RNAs and Their Roles in Biotic Stresses. Front. Plant Sci. 2018, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Shivaprasad, P.V.; Dunn, R.M.; Santos, B.A.; Bassett, A.; Baulcombe, D.C. Extraordinary transgressive phenotypes of hybrid tomato are influenced by epigenetics and small silencing RNAs. EMBO J. 2012, 31, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Sun, S.; Hua, S.; Shen, E.; Ye, C.-Y.; Cai, D.; Timko, M.P.; Zhu, Q.-H.; Fan, L. Analysis of transcriptional and epigenetic changes in hybrid vigor of allopolyploid Brassica napus uncovers key roles for small RNAs. Plant J. Cell Mol. Biol. 2017, 91, 874–893. [Google Scholar] [CrossRef]
- Li, P.; Su, T.; Zhang, D.; Wang, W.; Xin, X.; Yu, Y.; Zhao, X.; Yu, S.; Zhang, F. Genome-wide analysis of changes in miRNA and target gene expression reveals key roles in heterosis for Chinese cabbage biomass. Hortic. Res. 2021, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Greaves, I.K.; Eichten, S.R.; Groszmann, M.; Wang, A.; Ying, H.; Peacock, W.J.; Dennis, E.S. Twenty-four-nucleotide siRNAs produce heritable trans-chromosomal methylation in F1 Arabidopsis hybrids. Proc. Natl. Acad. Sci. USA 2016, 113, E6895–E6902. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Wang, L.; Han, T.; Ye, W.; Liu, Y.; Sun, Y.; Moose, S.P.; Song, Q.; Chen, Z.J. Small RNAs mediate transgenerational inheritance of genome-wide trans-acting epialleles in maize. Genome Biol. 2022, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Singh, V.K.; Saxena, R.K.; Kale, S.M.; Li, Y.; Garg, V.; Meifang, T.; Khan, A.W.; Kim, K.D.; Chitikineni, A.; et al. Genome-wide analysis of epigenetic and transcriptional changes associated with heterosis in pigeonpea. Plant Biotechnol. J. 2020, 18, 1697–1710. [Google Scholar] [CrossRef]
- Swanson-Wagner, R.A.; Eichten, S.R.; Kumari, S.; Tiffin, P.; Stein, J.C.; Ware, D.; Springer, N.M. Pervasive gene content variation and copy number variation in maize and its undomesticated progenitor. Genome Res. 2010, 20, 1689–1699. [Google Scholar] [CrossRef]
- Crow, J.F. 90 years ago: The beginning of hybrid maize. Genetics 1998, 148, 923–928. [Google Scholar] [CrossRef]
- Li, C.; Guan, H.; Jing, X.; Li, Y.; Wang, B.; Li, Y.; Liu, X.; Zhang, D.; Liu, C.; Xie, X.; et al. Genomic insights into historical improvement of heterotic groups during modern hybrid maize breeding. Nat. Plants 2022, 8, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, J.A.; Romay, M.C.; Gore, M.A.; Flint-Garcia, S.A.; Zhang, Z.; Millard, M.J.; Gardner, C.A.C.; McMullen, M.D.; Holland, J.B.; Bradbury, P.J.; et al. The genetic architecture of maize height. Genetics 2014, 196, 1337–1356. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Conrad, C.; Chen, Y.; Wilde, S.C.; Murray, S.C.; Anderson Ii, S.L.; Subramanian, N.K. Validation of functional polymorphisms affecting maize plant height by unoccupied aerial systems discovers novel temporal phenotypes. G3 2021, 11, jkab075. [Google Scholar] [CrossRef] [PubMed]
- Xing, A.; Gao, Y.; Ye, L.; Zhang, W.; Cai, L.; Ching, A.; Llaca, V.; Johnson, B.; Liu, L.; Yang, X.; et al. A rare SNP mutation in Brachytic2 moderately reduces plant height and increases yield potential in maize. J. Exp. Bot. 2015, 66, 3791–3802. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, L.; Liu, M.; Dong, Z.; Li, Q.; Fei, S.; Xiang, H.; Liu, B.; Jin, W. Maize Plant Architecture Is Regulated by the Ethylene Biosynthetic Gene ZmACS7. Plant Physiol. 2020, 183, 1184–1199. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, D.; Xue, M.; Qian, J.; He, Y.; Wang, S. Overexpression of the maize GRF10, an endogenous truncated growth-regulating factor protein, leads to reduction in leaf size and plant height. J. Integr. Plant Biol. 2014, 56, 1053–1063. [Google Scholar] [CrossRef]
- Zhao, P.; Ding, D.; Zhang, F.; Zhao, X.; Xue, Y.; Li, W.; Fu, Z.; Li, H.; Tang, J. Investigating the molecular genetic basis of heterosis for internode expansion in maize by microRNA transcriptomic deep sequencing. Funct. Integr. Genom. 2015, 15, 261–270. [Google Scholar] [CrossRef]
- Wang, F.; Yu, Z.; Zhang, M.; Wang, M.; Lu, X.; Liu, X.; Li, Y.; Zhang, X.; Tan, B.-C.; Li, C.; et al. ZmTE1 promotes plant height by regulating intercalary meristem formation and internode cell elongation in maize. Plant Biotechnol. J. 2022, 20, 526–537. [Google Scholar] [CrossRef]
- Kir, G.; Ye, H.; Nelissen, H.; Neelakandan, A.K.; Kusnandar, A.S.; Luo, A.; Inzé, D.; Sylvester, A.W.; Yin, Y.; Becraft, P.W. RNA Interference Knockdown of BRASSINOSTEROID INSENSITIVE1 in Maize Reveals Novel Functions for Brassinosteroid Signaling in Controlling Plant Architecture. Plant Physiol. 2015, 169, 826–839. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Singh, R.; Zheng, Y.; Cao, Z.; Li, M.; Lunde, C.; Hake, S.; Zhang, Z. GRF-interacting factor1 Regulates Shoot Architecture and Meristem Determinacy in Maize. Plant Cell 2018, 30, 360–374. [Google Scholar] [CrossRef]
- Ng, D.W.-K.; Lu, J.; Chen, Z.J. Big roles for small RNAs in polyploidy, hybrid vigor, and hybrid incompatibility. Curr. Opin. Plant Biol. 2012, 15, 154–161. [Google Scholar] [CrossRef]
- Li, A.; Liu, D.; Wu, J.; Zhao, X.; Hao, M.; Geng, S.; Yan, J.; Jiang, X.; Zhang, L.; Wu, J.; et al. mRNA and Small RNA Transcriptomes Reveal Insights into Dynamic Homoeolog Regulation of Allopolyploid Heterosis in Nascent Hexaploid Wheat. Plant Cell 2014, 26, 1878–1900. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. Cell Mol. Biol. 2008, 53, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Gonzalez-Mula, A.; Taconnat, L.; Clement, G.; Faure, D. The plant GABA signaling downregulates horizontal transfer of the Agrobacterium tumefaciens virulence plasmid. New Phytol. 2016, 210, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wang, K.; Zou, C.; Xu, C.; Li, W.-X. The PILNCR1-miR399 Regulatory Module Is Important for Low Phosphate Tolerance in Maize. Plant Physiol. 2018, 177, 1743–1753. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, J.; Dong, Z.; Richardson, A.; Patterson, E.; Mangrum, S.; Bybee, S.; Bertolini, E.; Bartlett, M.; Chuck, G.; et al. Boundary domain genes were recruited to suppress bract growth and promote branching in maize. Sci. Adv. 2022, 8, eabm6835. [Google Scholar] [CrossRef]
- Yang, J.; Tian, L.; Sun, M.-X.; Huang, X.-Y.; Zhu, J.; Guan, Y.-F.; Jia, Q.-S.; Yang, Z.-N. AUXIN RESPONSE FACTOR17 is essential for pollen wall pattern formation in Arabidopsis. Plant Physiol. 2013, 162, 720–731. [Google Scholar] [CrossRef]
- Berka, M.; Kopecká, R.; Berková, V.; Brzobohatý, B.; Černý, M. Regulation of heat shock proteins 70 and their role in plant immunity. J. Exp. Bot. 2022, 73, 1894–1909. [Google Scholar] [CrossRef]
- Castiglioni, P.; Bell, E.; Lund, A.; Rosenberg, A.F.; Galligan, M.; Hinchey, B.S.; Bauer, S.; Nelson, D.E.; Bensen, R.J. Identification of GB1, a gene whose constitutive overexpression increases glycinebetaine content in maize and soybean. Plant Direct 2018, 2, e00040. [Google Scholar] [CrossRef]
- Groszmann, M.; Greaves, I.K.; Albertyn, Z.I.; Scofield, G.N.; Peacock, W.J.; Dennis, E.S. Changes in 24-nt siRNA levels in Arabidopsis hybrids suggest an epigenetic contribution to hybrid vigor. Proc. Natl. Acad. Sci. USA 2011, 108, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Greaves, I.K.; Groszmann, M.; Wang, A.; Peacock, W.J.; Dennis, E.S. Inheritance of Trans Chromosomal Methylation patterns from Arabidopsis F1 hybrids. Proc. Natl. Acad. Sci. USA 2014, 111, 2017–2022. [Google Scholar] [CrossRef]
- Sham, A.; Moustafa, K.; Al-Ameri, S.; Al-Azzawi, A.; Iratni, R.; AbuQamar, S. Identification of Arabidopsis candidate genes in response to biotic and abiotic stresses using comparative microarrays. PLoS ONE 2015, 10, e0125666. [Google Scholar] [CrossRef]
- Zhu, D.; Li, R.; Liu, X.; Sun, M.; Wu, J.; Zhang, N.; Zhu, Y. The positive regulatory roles of the TIFY10 proteins in plant responses to alkaline stress. PLoS ONE 2014, 9, e111984. [Google Scholar] [CrossRef]
- Giri, M.K.; Singh, N.; Banday, Z.Z.; Singh, V.; Ram, H.; Singh, D.; Chattopadhyay, S.; Nandi, A.K. GBF1 differentially regulates CAT2 and PAD4 transcription to promote pathogen defense in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2017, 91, 802–815. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Makita, N.; Kojima, M.; Kamada-Nobusada, T.; Sakakibara, H. Overexpression of a type-A response regulator alters rice morphology and cytokinin metabolism. Plant Cell Physiol. 2007, 48, 523–539. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Du, Y.; Shen, X.; Ning, Q.; Li, Y.; Liu, D.; Xiong, Q.; Zhang, Z. The Coordinated KNR6-AGAP-ARF1 Complex Modulates Vegetative and Reproductive Traits by Participating in Vesicle Trafficking in Maize. Cells 2021, 10, 2601. [Google Scholar] [CrossRef]
- Zhiponova, M.K.; Morohashi, K.; Vanhoutte, I.; Machemer-Noonan, K.; Revalska, M.; Van Montagu, M.; Grotewold, E.; Russinova, E. Helix-loop-helix/basic helix-loop-helix transcription factor network represses cell elongation in Arabidopsis through an apparent incoherent feed-forward loop. Proc. Natl. Acad. Sci. USA 2014, 111, 2824–2829. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Benning, C. Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. Cell Mol. Biol. 2005, 41, 243–256. [Google Scholar] [CrossRef]
- Jan, A.; Nakamura, H.; Handa, H.; Ichikawa, H.; Matsumoto, H.; Komatsu, S. Gibberellin regulates mitochondrial pyruvate dehydrogenase activity in rice. Plant Cell Physiol. 2006, 47, 244–253. [Google Scholar] [CrossRef]
- Ham, B.-K.; Park, J.M.; Lee, S.-B.; Kim, M.J.; Lee, I.-J.; Kim, K.-J.; Kwon, C.S.; Paek, K.-H. Tobacco Tsip1, a DnaJ-type Zn finger protein, is recruited to and potentiates Tsi1-mediated transcriptional activation. Plant Cell 2006, 18, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress1[W][OA]. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef]
- Martin, G.T.; Seymour, D.K.; Gaut, B.S. CHH Methylation Islands: A Nonconserved Feature of Grass Genomes That Is Positively Associated with Transposable Elements but Negatively Associated with Gene-Body Methylation. Genome Biol. Evol. 2021, 13, evab144. [Google Scholar] [CrossRef]
- German, M.A.; Luo, S.; Schroth, G.; Meyers, B.C.; Green, P.J. Construction of Parallel Analysis of RNA Ends (PARE) libraries for the study of cleaved miRNA targets and the RNA degradome. Nat. Protoc. 2009, 4, 356–362. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef]
- Kuang, Z.; Wang, Y.; Li, L.; Yang, X. miRDeep-P2: Accurate and fast analysis of the microRNA transcriptome in plants. Bioinformatics 2019, 35, 2521–2522. [Google Scholar] [CrossRef]
- Meyers, B.C.; Axtell, M.J.; Bartel, B.; Bartel, D.P.; Baulcombe, D.; Bowman, J.L.; Cao, X.; Carrington, J.C.; Chen, X.; Green, P.J.; et al. Criteria for annotation of plant MicroRNAs. Plant Cell 2008, 20, 3186–3190. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.R.; Yeoh, J.M.; Coruh, C.; Axtell, M.J. Improved Placement of Multi-mapping Small RNAs. G3 2016, 6, 2103–2111. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Addo-Quaye, C.; Miller, W.; Axtell, M.J. CleaveLand: A pipeline for using degradome data to find cleaved small RNA targets. Bioinformatics 2009, 25, 130–131. [Google Scholar] [CrossRef]
- Dai, X.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server. Nucleic Acids Res. 2011, 39, W155–W159. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Krueger, F.; Andrews, S.R. Bismark: A flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 2011, 27, 1571–1572. [Google Scholar] [CrossRef]
- Jühling, F.; Kretzmer, H.; Bernhart, S.H.; Otto, C.; Stadler, P.F.; Hoffmann, S. Metilene: Fast and sensitive calling of differentially methylated regions from bisulfite sequencing data. Genome Res. 2016, 26, 256–262. [Google Scholar] [CrossRef]
- Schultz, M.D.; Schmitz, R.J.; Ecker, J.R. ‘Leveling’ the playing field for analyses of single-base resolution DNA methylomes. Trends Genet. TIG 2012, 28, 583–585. [Google Scholar] [CrossRef]
| Family | Representative miRNAs | Express Pattern of miRNA a | D/A Ratio of miRNA b | Target Gene ID | Gene Name | Express Pattern of Target c | D/A Ratio of Target d |
|---|---|---|---|---|---|---|---|
| miR164 | zma-miR164f-5p | DLPE | −0.79 | Zm00001d002101 | GA20ox2 | DHPE | 0.92 |
| Zm00001d042438 | PROT1 | DHPE | 1.33 | ||||
| DLPE | −0.79 | Zm00001d047973 | CYP18-1 | DHPE | 0.77 | ||
| miR399 | zma-miR399c-3p | DLPE | −1.37 | Zm00001d038972 | PHO2 | THPE | 19.8 |
| zma-miR399i-5p | DLPE | −1 | Zm00001d003477 | CRY2 | THPE | 10.59 | |
| miR156 | zma-miR156k-5p | DLPE | −0.64 | Zm00001d022275 | NPSN13 | DHPE | 1.19 |
| zma-miR156h-5p | TLPE | −3.39 | Zm00001d031451 | UB2 | THPE | 8.51 | |
| miR160 | zma-miR160g-5p | THPE | 5.23 | Zm00001d002929 | ARF17 | TLPE | −15.86 |
| Zm00001d025225 | - | DLPE | −1.25 | ||||
| miR167 | zma-miR167d-3p | THPE | 4.66 | Zm00001d028630 | HSP70-6 | DLPE | −0.95 |
| zma-miR167c-3p | THPE | 16.29 | Zm00001d029408 | GB1 | DLPE | −1.81 | |
| Zm00001d040731 | GDSL-like lipase | TLPE | −2.1 |
| Group a | siRNA ID | Express Pattern of siRNA Cluster | D/A Ratio of siRNA Cluster | Cytosine Context | Methylation Pattern | D/A Ratio of Methylation Level | Feature b | Gene ID | Gene Name | Expression Pattern of Gene | D/A Ratio of Gene |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPE-CG-TCM | Cluster_30718 | HPE | 0.85 | CG | TCM | 0.55 | Gene body | Zm00001d001865 | ZmRR6 | DLPE | −0.77 |
| HPE-CG-TCM | Cluster_137039 | HPE | 0.62 | CG | TCM | 0.65 | 5’-2kb | Zm00001d039065 | GBF3 | DLPE | −0.71 |
| HPE-CG-TCM | Cluster_28403 | HPE | 0.72 | CG | TCM | 0.8 | 3’-2kb | Zm00001d034356 | GSTU25 | DLPE | −1.94 |
| HPE-CG-TCM | Cluster_80610 | HPE | 1.07 | CG | TCM | 1.02 | 3’-2kb | Zm00001d049630 | Galactose oxidase | TLPE | −4.55 |
| HPE-CG-TCM | Cluster_127623 | HPE | 1.11 | CG | TCM | 0.92 | 5’-2kb | Zm00001d036602 | KNR6 | DLPE | −0.87 |
| HPE-CHG-TCM | Cluster_28403 | HPE | 0.72 | CHG | TCM | 0.68 | 3’-2kb | Zm00001d034356 | GSTU25 | DLPE | −1.94 |
| HPE-CHG-TCM | Cluster_153805 | HPE | 0.59 | CHG | TCM | 0.68 | 3’-2kb | Zm00001d022530 | EIN3 | DLPE | −1.07 |
| HPE-CHH-TCM | Cluster_8216 | HPE | 0.63 | CHH | TCM | 1.06 | 5’-2kb | Zm00001d029448 | TIFY10B | DLPE | −0.98 |
| HPE-CHH-TCM | Cluster_186180 | HPE | 0.78 | CHH | TCM | 1.24 | 3’-2kb | Zm00001d048145 | PDK | DHPE | 1.03 |
| LPE-CG-TCdM | Cluster_191145 | LPE | −0.95 | CG | TCdM | −0.55 | 5’-2kb | Zm00001d024166 | IBL1 | THPE | 11.05 |
| LPE-CG-TCdM | Cluster_56715 | LPE | −0.74 | CG | TCdM | −0.53 | Gene body | Zm00001d040061 | Pectinacetylesterase | DHPE | 0.96 |
| LPE-CHH-TCdM | Cluster_98214 | LPE | −0.92 | CHH | TCdM | −0.9 | Gene body | Zm00001d053706 | ABCB15 | THPE | 3.24 |
| LPE-CHH-TCdM | Cluster_8459 | LPE | −0.95 | CHH | TCdM | −1 | Gene body | Zm00001d029502 | G6PD2 | THPE | 16.39 |
| LPE-CHH-TCdM | Cluster_146598 | LPE | −0.62 | CHH | TCdM | −0.51 | Gene body | Zm00001d020538 | TSIP1 | DLPE | −0.64 |
| LPE-CHH-TCdM | Cluster_87940 | LPE | −0.96 | CHH | TCdM | −1 | Gene body | Zm00001d051161 | PAL | TLPE | −2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Xie, Y.; Zhang, H.; He, C.; Wang, X.; Cui, Y.; Heng, Y.; Lin, Y.; Gu, R.; Wang, J.; et al. Integrated Multi-Omics Reveals Significant Roles of Non-Additively Expressed Small RNAs in Heterosis for Maize Plant Height. Int. J. Mol. Sci. 2023, 24, 9150. https://doi.org/10.3390/ijms24119150
Zhang J, Xie Y, Zhang H, He C, Wang X, Cui Y, Heng Y, Lin Y, Gu R, Wang J, et al. Integrated Multi-Omics Reveals Significant Roles of Non-Additively Expressed Small RNAs in Heterosis for Maize Plant Height. International Journal of Molecular Sciences. 2023; 24(11):9150. https://doi.org/10.3390/ijms24119150
Chicago/Turabian StyleZhang, Jie, Yuxin Xie, Hongwei Zhang, Cheng He, Xiaoli Wang, Yu Cui, Yanfang Heng, Yingchao Lin, Riliang Gu, Jianhua Wang, and et al. 2023. "Integrated Multi-Omics Reveals Significant Roles of Non-Additively Expressed Small RNAs in Heterosis for Maize Plant Height" International Journal of Molecular Sciences 24, no. 11: 9150. https://doi.org/10.3390/ijms24119150
APA StyleZhang, J., Xie, Y., Zhang, H., He, C., Wang, X., Cui, Y., Heng, Y., Lin, Y., Gu, R., Wang, J., & Fu, J. (2023). Integrated Multi-Omics Reveals Significant Roles of Non-Additively Expressed Small RNAs in Heterosis for Maize Plant Height. International Journal of Molecular Sciences, 24(11), 9150. https://doi.org/10.3390/ijms24119150









