Drought Resistance and Ginsenosides Biosynthesis in Response to Abscisic Acid in Panax ginseng C. A. Meyer
Abstract
1. Introduction
2. Results
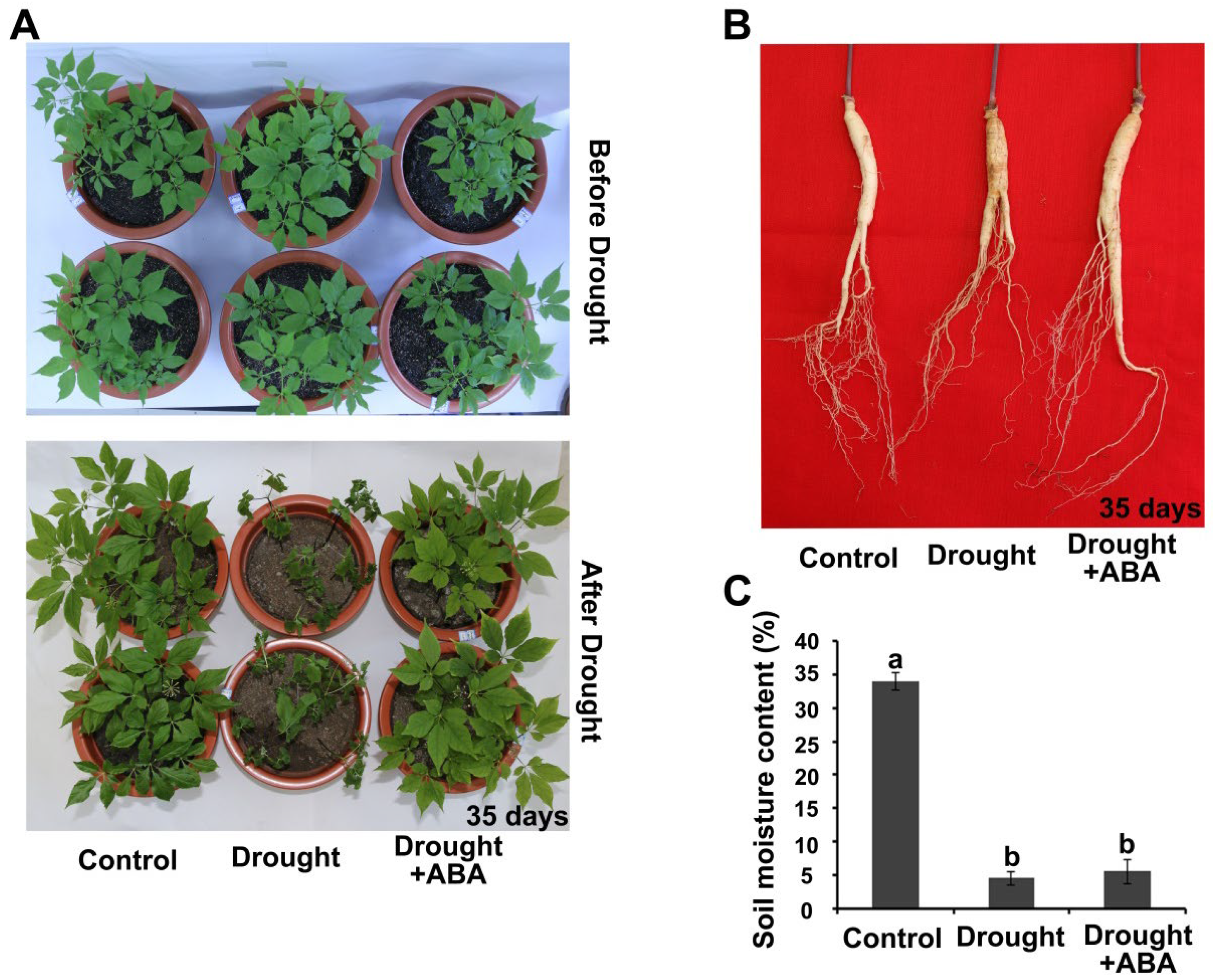
2.1. Drought Resistance Is Enhanced by ABA in Panax ginseng
2.2. Stomatal Closure under Drought Stress Is Promoted by ABA in Panax ginseng
2.3. Physiological Characteristics of Panax ginseng under Drought Stress Is Affected by ABA Treatment
2.4. The Accumulation of Ginsenoside under Drought Stress Was Enhanced by ABA Treatment in Panax ginseng Roots
2.5. ABA Treatment Induced PgHMGR1/2 Expression in Panax ginseng
3. Discussion
4. Materials and Methods
4.1. Ginseng Materials and Growth Conditions
4.2. Drought Treatment, Drought + ABA Treatment, and Stomatal Aperture Measurement
4.3. Soil Moisture Content Analysis
4.4. Determination of Chlorophyll Content, Root Activity, MDA Content and Soluble Sugar Content
4.5. Antioxidant Enzymatic Activity Analysis of SOD and CAT
4.6. Determination of Ginsenosides Content by HPLC
4.7. RNA Isolation and Quantitative RT-PCR Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Han, S.W.; Lee, S.C. The Pepper RING finger E3 ligase, CaDIR1, regulates the drought stress response via ABA-mediated signaling. Front. Plant Sci. 2017, 8, 690. [Google Scholar] [CrossRef]
- Xiong, L.; Schumaker, K.S.; Zhu, J.K. Cell signaling during cold, drought, and salt stress. Plant Cell 2002, 14 (Suppl. 1), S165–S183. [Google Scholar] [CrossRef]
- Maritim, T.K.; Kamunya, S.M.; Mireji, P.; Mwendia, C.; Muoki, R.C.; Cheruiyot, E.K.; Wachira, F.N. Physiological and biochemical response of tea (Camellia sinensis (L.) O. Kuntze) to water-deficit stress. J. Hortic. Sci. Biotechnol. 2015, 90, 395–400. [Google Scholar] [CrossRef]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and mechanism of jasmonic acid in plant responses to abiotic and biotic stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Claeys, H.; Inzé, D. The agony of choice: How plants balance growth and survival under water-limiting conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Hou, K.; Zhang, H.; Wang, X.; Wu, W. Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L. under drought stress. Ind. Crops Prod. 2020, 151, 112465. [Google Scholar] [CrossRef]
- Finkelstein, R.R.; Gampala, S.S.; Rock, C.D. Abscisic acid signaling in seeds and seedlings. Plant Cell 2002, 14 (Suppl. 1), S15–S45. [Google Scholar] [CrossRef]
- Christmann, A.; Moes, D.; Himmelbach, A.; Yang, Y.; Tang, Y.; Grill, E. Integration of abscisic acid signalling into plant responses. Plant Biol. 2006, 8, 314–325. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Dubeaux, G.; Takahashi, Y.; Schroeder, J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021, 105, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, H.; Zhang, Z.; Sun, H.; Li, M.; Shao, C.; Liang, H.; Wu, H.; Zhang, Y. Physiological and transcriptomic analyses of roots from Panax ginseng C. A. Meyer under drought stress. Ind. Crops Prod. 2023, 191, 115858. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Zhao, L.; Zhang, Z.H.; Tang, Z.H. Profiling of ginsenosides in the two medicinal Panax herbs based on ultra-performance liquid chromatography-electrospray ionization-mass spectrometry. SpringerPlus 2016, 5, 1770. [Google Scholar] [CrossRef]
- Murthy, H.N.; Georgiev, M.I.; Kim, Y.S.; Jeong, C.S.; Kim, S.J.; Park, S.Y.; Paek, K.Y. Ginsenosides: Prospective for sustainable biotechnological production. Appl. Microbiol. Biotechnol. 2014, 98, 6243–6254. [Google Scholar] [CrossRef]
- Lee, J.D.; Le, K.C.; Park, Y.K.; Murthy, H.N.; Paek, K.Y.; Park, S.Y. Cell culture system versus adventitious root culture system in Asian and American ginseng: A collation. Plant Cell Tiss. Org. 2018, 132, 295–302. [Google Scholar] [CrossRef]
- Attele, A.S.; Wu, J.A.; Yuan, C.S. Ginseng pharmacology: Multiple constituents and multiple actions. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef]
- Ru, W.; Wang, D.; Xu, Y.; He, X.; Sun, Y.E.; Qian, L.; Zhou, X.; Qin, Y. Chemical constituents and bioactivities of Panax ginseng (C. A. Mey.). Drug Discov. Ther. 2015, 9, 23–32. [Google Scholar] [CrossRef]
- Hou, M.; Wang, R.; Zhao, S.; Wang, Z. Ginsenosides in Panax genus and their biosynthesis. Acta. Pharm. Sin. B 2021, 11, 1813–1834. [Google Scholar] [CrossRef]
- Yin, X.; Hu, H.; Shen, X.; Li, X.; Pei, J.; Xu, J. Ginseng omics for ginsenoside biosynthesis. Curr. Pharm. Biotechnol. 2021, 22, 570–578. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, O.R.; Oh, J.Y.; Jang, M.G.; Yang, D.C. Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme a reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol. 2014, 165, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Hyun, D.Y.; Park, C.G.; Kim, T.S.; Yeon, B.Y.; Kim, C.G.; Cha, S.W. Effect of soil moisture content on photosynthesis and root yield of Panax ginseng C. A. Meyer Seedling. Korean J. Med. Crop Sci. 2007, 15, 367–370. [Google Scholar]
- Zhang, T.; Han, M.; Yang, L.; Han, Z.; Cheng, L.; Sun, Z.; Yang, L. The effects of environmental factors on ginsenoside biosynthetic enzyme gene expression and saponin abundance. Molecules 2018, 24, 14. [Google Scholar] [CrossRef]
- Ding, L.; Li, Y.; Wang, Y.; Gao, L.; Wang, M.; Chaumont, F.; Shen, Q.; Guo, S. Root ABA accumulation enhances rice seedling drought resistance under ammonium supply: Interaction with aquaporins. Front. Plant Sci. 2016, 7, 1206. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Yang, L.; Tai, F.; Hu, X.; Wang, W. “Omics” of maize stress response for sustainable food production: Opportunities and challenges. OMICS 2014, 18, 714–732. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Kochan, E.; Balcerczak, E.; Szymczyk, P.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Szymańska, G. Abscisic Acid Regulates the 3-Hydroxy-3-methylglutaryl CoA Reductase Gene Promoter and Ginsenoside Production in Panax quinquefolium Hairy Root Cultures. Int. J. Mol. Sci. 2019, 20, 1310. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Yang, L.; Ge, N.; Jia, J.S.; Huang, R.M.; Chen, C.; Meng, Z.G.; Li, L.G.; Chen, J.W. Exogenous abscisic acid prolongs the dormancy of recalcitrant seed of Panax notoginseng. Front. Plant Sci. 2023, 14, 1054736. [Google Scholar] [CrossRef]
- Ievinsh, G. Water content of plant tissues: So simple that almost forgotten? Plants 2023, 12, 1238. [Google Scholar] [CrossRef]
- An, Z.; Jing, W.; Liu, Y.; Zhang, W. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 2008, 59, 815–825. [Google Scholar] [CrossRef]
- Suhita, D.; Raghavendra, A.S.; Kwak, J.M.; Vavasseur, A. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol. 2004, 134, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Dragun, Z.; Filipović Marijić, V.; Krasnići, N.; Ramani, S.; Valić, D.; Rebok, K.; Kostov, V.; Jordanova, M.; Erk, M. Malondialdehyde concentrations in the intestine and gills of Vardar chub (Squalius vardarensis Karaman) as indicator of lipid peroxidation. Environ. Sci. Pollut. Res. Int. 2017, 24, 16917–16926. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Ajigboye, O.O.; Lu, C.; Murchie, E.H.; Schlatter, C.; Swart, G.; Ray, R.V. Altered gene expression by sedaxane increases PSII efficiency, photosynthesis and growth and improves tolerance to drought in wheat seedlings. Pestic. Biochem. Physiol. 2017, 137, 49–61. [Google Scholar] [CrossRef]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signalling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Sugimoto, M.; Oono, Y.; Gusev, O.; Matsumoto, T.; Yazawa, T.; Levinskikh, M.A.; Sychev, V.N.; Bingham, G.E.; Wheeler, R.; Hummerick, M. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol. 2014, 14, 4. [Google Scholar] [CrossRef]
- Anderson, M.D.; Prasad, T.K.; Martin, B.A.; Stewart, C.R. Differential gene expression in chilling-acclimated maize seedlings and evidence for the involvement of abscisic acid in chilling tolerance. Plant Physiol. 1994, 105, 331–339. [Google Scholar] [CrossRef]
- Guan, L.; Scandalios, J.G. Two structurally similar maize cytosolic superoxide dismutase genes, Sod4 and Sod4A, respond differentially to abscisic acid and high osmoticum. Plant Physiol. 1998, 117, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Szkopińska, A.; Swiezewska, E.; Karst, F. The regulation of activity of main mevalonic acid pathway enzymes: Farnesyl diphosphate synthase, 3-hydroxy-3-methylglutaryl-CoA reductase, and squalene synthase in yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2000, 267, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in Citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, X.; Zhang, H.; Wang, Y.; Li, X.; Zhu, J.K.; Gong, Z. Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. Plant J. 2005, 43, 273–283. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Chen, P.; Chang, C. Drought Resistance and Ginsenosides Biosynthesis in Response to Abscisic Acid in Panax ginseng C. A. Meyer. Int. J. Mol. Sci. 2023, 24, 9194. https://doi.org/10.3390/ijms24119194
Kong L, Chen P, Chang C. Drought Resistance and Ginsenosides Biosynthesis in Response to Abscisic Acid in Panax ginseng C. A. Meyer. International Journal of Molecular Sciences. 2023; 24(11):9194. https://doi.org/10.3390/ijms24119194
Chicago/Turabian StyleKong, Lingyao, Peng Chen, and Cheng Chang. 2023. "Drought Resistance and Ginsenosides Biosynthesis in Response to Abscisic Acid in Panax ginseng C. A. Meyer" International Journal of Molecular Sciences 24, no. 11: 9194. https://doi.org/10.3390/ijms24119194
APA StyleKong, L., Chen, P., & Chang, C. (2023). Drought Resistance and Ginsenosides Biosynthesis in Response to Abscisic Acid in Panax ginseng C. A. Meyer. International Journal of Molecular Sciences, 24(11), 9194. https://doi.org/10.3390/ijms24119194





