Catechins and Proanthocyanidins Involvement in Metabolic Syndrome
Abstract
:1. Introduction
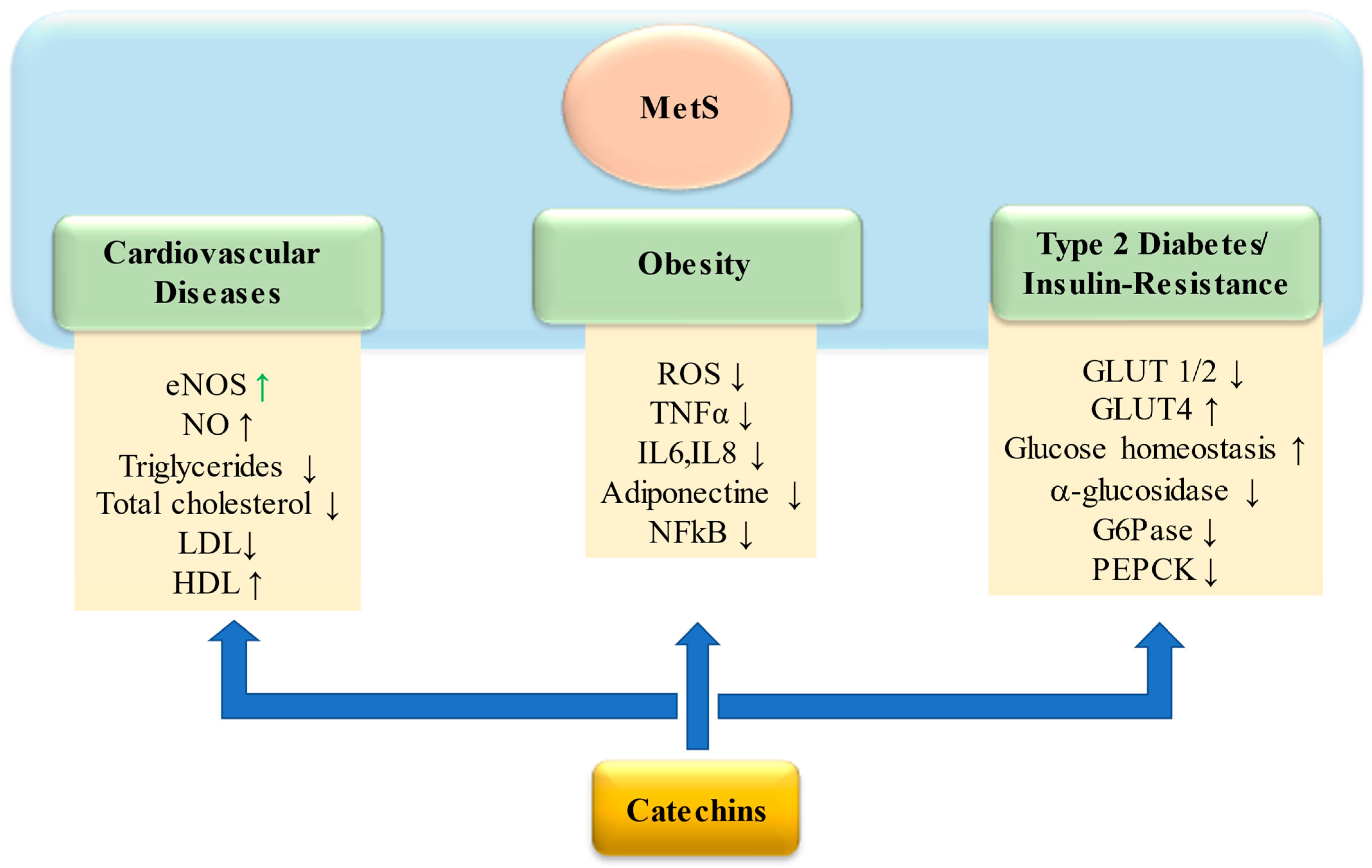
2. Metabolic Syndrome (MetS)
3. Chemical Structure and Classification of Flavonoids
3.1. Bioavailability of Catechin and Their Polymeric Structures
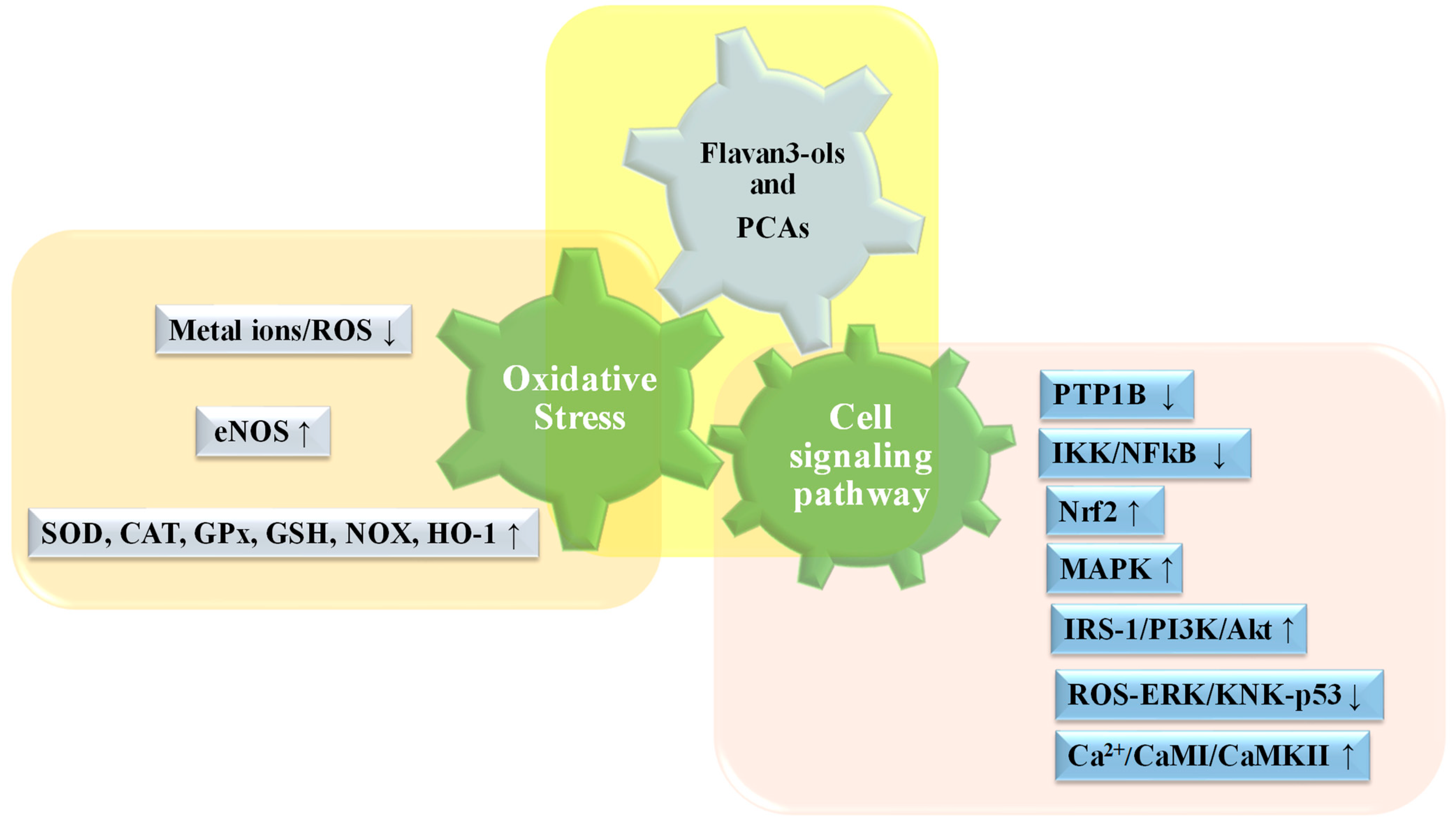
3.2. Biochemical and Functional Aspects of Catechin and Their Polymers
4. Catechins in Metabolic Syndrome
5. Proanthocyanidins in Mets
6. Human Studies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Middleton, E., Jr. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar]
- Santos, E.; Maia, B.; Ferriani, A.; Teixeira, S. ‘Flavonoids: Classification, Biosynthesis and Chemical Ecology’; Flavonoids, C., Justino, G.C., Eds.; InTechOpen: Rijeka, Croatia, 2017; Chapter 1. [Google Scholar] [CrossRef]
- Brodowska, K. Natural Flavonoids: Classification, Potential Role, and Application of Flavonoid Analogues. Europ. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14835. [Google Scholar] [CrossRef]
- Gouveia, H.J.C.B.; Urquiza-Martínez, M.V.; Manhães-de-Castro, R.; Costa-de-Santana, B.J.R.; Villarreal, J.P.; Mercado-Camargo, R.; Torner, L.; de Souza Aquino, J.; Toscano, A.E.; Guzmán-Quevedo, O. Effects of the Treatment with Flavonoids on Metabolic Syndrome Components in Humans: A Systematic Review Focusing on Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 8344. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1 diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than bmi: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trend. Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Zhang, K.; Pan, H.; Wang, L.; Yang, H.; Zhu, H.; Gong, F. Adipose Tissue Insulin Resistance is Closely Associated with Metabolic Syndrome in Northern Chinese Populations. Diabetes Metab. Syndr. Obes. 2021, 14, 1117–1128. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Tylutka, A.; Morawin, B.; Walas, Ł.; Michałek, M.; Gwara, A.; Zembron-Lacny, A. Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci. Rep. 2023, 13, 89. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Stelmaszczyk-Emmel, A.; Plywaczewski, R.; Stoklosa, A.; Gorecka, D.; Pyrzak, B.; Demkow, U. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 120–122. [Google Scholar] [CrossRef]
- Lackey, D.E.; Olefsky, J.M. Regulation of metabolism by the innate immune system. Nat. Rev. Endocrinol. 2016, 12, 15–28. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Abdelazeem, A.H.; Abuelsaad, A.S.A.; Abdel-Moniem, A.; Abdel-Gabbar, M. Association of metabolic syndrome components with alterations in oxidative stress and cytokines expression. J. Taibah Univ. Sci. 2021, 15, 928–940. [Google Scholar] [CrossRef]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability and Epidemiology. Nutrition Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- Welch, A.A.; Hardcastle, A.C. The effects of flavonoids on bone. Curr. Osteoporos. Rep. 2014, 12, 205–210. [Google Scholar] [CrossRef]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The Second Life of Citrus Fruit Waste: A Valuable Source of Bioactive Compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef]
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.I.; Notas, G. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action breast. Cancer Res. 2004, 6, R63–R74. [Google Scholar]
- Xie, D.Y.; Dixon, R.A. Proanthocyanidin biosynthesis–still more questions than answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef]
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528. [Google Scholar] [CrossRef]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; Wiley: Oxford, UK, 1999; Volume 2. [Google Scholar]
- Harborne, J.B. The Flavonoids: Advances in Research since 1986; Chapman and Hall: London, UK, 1993. [Google Scholar]
- Thompson, R.S.; Jacques, D.; Haslam, E.; Tanner, R.J.N. Plant proanthocyanidins. Part. I. Introduction: The isolation, structure and distribution in nature of plant procyanidins. J. Chem. Soc. (PI) 1972, 1, 1387–1399. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free Rad. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef]
- Alañón, M.E.; Castle, S.M.; Serra, G.; Lévèques, A.; Poquet, L.; Actis-Goretta, L.; Spencer, J.P.E. Acute study of dose-dependent effects of (-)-epicatechin on vascular function in healthy male volunteers: A randomized controlled trial. Clin. Nutr. 2020, 39, 746–754. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Remesy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radical Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Li, D.; Ho, C.T.; Li, J.; Wan, X. The absorption, distribution, metabolism and excretion of procyanidins. Food Funct. 2016, 7, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed]
- Bittner, K.; Kemme, T.; Peters, K.; Kersten, S.; Dänicke, S.; Humpf, H.U. Systemic absorption and metabolism of dietary procyanidin B4 inpigs. Mol. Nut. Food Res. 2014, 58, 2261–2273. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, K.M.; Smithson, A.T.; Ickes, A.K.; Neilson, A.P. Pan-colonic pharmacokinetics of catechins and procyanidins in male Sprague–Dawley rats. J. Nutr. Biochem. 2015, 26, 1007–1014. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Kwik-Uribe, C.; Keen, C.L.; Schroeter, H. Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans. Am. J. Clin. Nutr. 2012, 95, 851–858. [Google Scholar] [CrossRef]
- Wiese, S.; Esatbeyoglu, T.; Winterhalter, P.; Kruse, H.P.; Winkler, S.; Bub, A.; Kulling, S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015, 59, 610–621. [Google Scholar] [CrossRef]
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Naumovski, N.; Blades, B.L.; Roach, P. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Andreu-Fernández, V.; Almeida Toledano, L.; Pizarro, N.; Navarro-Tapia, E.; Gómez-Roig, M.D.; de la Torre, R.; García-Algar, Ó. Bioavailability of Epigallocatechin Gallate Administered with Different Nutritional Strategies in Healthy Volunteers. Antioxidants 2020, 9, 440. [Google Scholar] [CrossRef]
- Hodges, J.K.; Zhu, J.; Yu, Z.; Vodovotz, Y.; Brock, G.; Sasaki, G.Y.; Dey, P.; Bruno, R.S. Intestinal-level anti-inflammatory bioactivities of catechin-rich green tea: Rationale, design, and methods of a double-blind, randomized, placebo-controlled crossover trial in metabolic syndrome and healthy adults. Contemp. Clin. Trials Commun. 2019, 17, 100495. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Li, D.; Cao, F. The Role of Catechins in Regulating Diabetes: An Update Review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Waheed, J.A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-rich Foods (frf): A Promising Nutraceutical Approach Against Lifespan-shortening Diseases. Iran. J. Basic. Med. Sci. Feb. 2020, 23, 140–153. [Google Scholar]
- During, A.; Larondelle, Y. The O-methylation of chrysin markedly improves its intestinal anti-inflammatory properties: Structure-activity relationships of flavones. Biochem. Pharmacol. 2013, 86, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A. Structure-Activity Relationships Analysis of Monomeric and Polymeric Polyphenols (Quercetin, Rutin and Catechin) Obtained by Various Polymerization Methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef]
- Atrahimovich, D.; Vaya, J.; Khatib, S. The effects and mechanism of flavonoid–rePON1 interactions. Structure– activity relationship study. Bioorg. Med. Chem. 2013, 21, 3348–3355. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Ishii, T.; Ichikawa, T.; Minoda, K.; Kusaka, K.; Ito, S.; Suzuki, Y.; Akagawa, M.; Mochizuki, K.; Goda, T.; Nakayama, T. Human Serum Albumin as an Antioxidant in the Oxidation of (−)-Epigallocatechin Gallate: Participation of Reversible Covalent Binding for Interaction and Stabilization. Biosci. Biotechnol. Biochem. 2011, 75, 100–106. [Google Scholar] [CrossRef]
- Maiti, T.K.; Ghosh, K.S.; and Dasgupta, S. Inter- action of (-)-epigallocatechin- 3-gallate with human serum albumin: Fluorescence, fourier transform infrared, circular dichroism, and docking studies. Proteins 2006, 64, 355–362. [Google Scholar] [CrossRef]
- Barreca, D.; Laganà, G.; Toscano, G.; Calandra, P.; Kiselev, M.A.; Lombardo, D.; Bellocco, E. The interaction and binding of flavonoids to human serum albumin modify its conformation, stability and resistance against aggregation and oxidative injuries. Biochim Biophys Acta Gen Subj. 2017, 1861 Pt B, 3531–3539. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Xue, C.; Hu, Z.; Chen, X.; Sheng, F. Effect of Chinese medicine alpinetin on the structure of human serum albumin. Bioorg. Med. Chem. 2005, 13, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.L.; Iv, Z.; Liu, Z.G.; Hu, Z.; Zou, G.L. Study on interaction between apigenin and human serum albumin by spectroscopy and molecular modeling. J. Photochem. Photobiol. A Chem. 2007, 191, 104–113. [Google Scholar] [CrossRef]
- Tian, J.; Liu, J.; Hu, Z.; Chen, X. Interaction of wogonin with bovine serum albumin. Bioorg. Med. Chem. 2005, 13, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Suzuki, M.; Jiang, X.; Chen, X.; Yamamoto, K.; Ren, F.; Xu, M.J. Influence of B-ring hydroxylation on interactions of flavonols with bovine serum albumin. Agric. Food Chem. 2005, 56, 2350. [Google Scholar] [CrossRef] [PubMed]
- Trnková, L.; Boušová, I.; Staňková, V.; Dršata, J. Study on the interaction of catechins with human serum albumin using spectroscopic and electrophoretic techniques. J. Mol. Struct. 2011, 985, 243–250. [Google Scholar] [CrossRef]
- Guo, X.J.; Zhang, L.; Sun, X.D.; Han, X.W.; Guo, C.; Kang, P.L. Spectroscopic studies on the interaction between sodium ozagrel and bovine serum albumin. J. Mol. Struct. 2009, 928, 114–120. [Google Scholar] [CrossRef]
- Bi, S.; Ding, L.; Tian, Y.; Song, D.; Zhou, X.; Liu, X.; Zhang, H. Investigation of the interaction between flavonoids and human serum albumin. J. Mol. Struct. 2004, 703, 37–45. [Google Scholar] [CrossRef]
- Bae, M.J.; Ishii, T.; Minoda, K.; Kawada, Y.; Ichikawa, T.; Mori, T.; Kamihira, M.; Nakayama, T. Albumin stabilizes (–)-epigallocatechin gallate in human serum: Binding capacity and antioxidant property. Mol. Nutr. Food Res. 2009, 53, 709–715. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Wang, G.; Lu, Y. Study of interaction between human serum albumin and three antioxidants: Ascorbic acid, α-tocopherol, and proanthocyanidins. Eu. J. Med. Chem. 2013, 70, 22–36. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, H.; Katsuki, A.; Sumida, Y.; Gabazza, E.C.; Murashima, S.; Morioka, K.; Maruyama, N.; Kitagawa, N.; Tanaka, T.; Hori, Y.; et al. Oxidative stress is associated with adiposity and insulin resistance in men. J. Clinic. End. Met. 2003, 88, 4673–4676. [Google Scholar] [CrossRef] [PubMed]
- Gealekman, O.; Guseva, N.; Hartigan, C.; Apotheker, S.; Gorgoglione, M.; Gurav, K.; Tran, K.V.; Straubhaar, J.; Nicoloro, S.; Czech, M.P.; et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation 2011, 123, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bastard, J.P.; Maachi, M.; Lagathu, C.; Kim, M.J.; Caron, M.; Vidal, H.; Capeau, J.; Feve, B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur. Cytokine Netw. 2006, 17, 4–12. [Google Scholar]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Oliveira-Marques, V.; Marinho, H.S.; Cyrne, L.; Antunes, F. Role of hydrogen peroxide in NF-kappaB activation: From inducer to modulator. Antioxid. Redox Signal. 2009, 11, 2223–2243. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal. Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell. Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef]
- Pang, Y.; Wu, D.; Ma, Y.; Cao, Y.; Liu, Q.; Tang, M.; Pu, Y.; Zhang, T. Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation involvement in low-dose CdTe QDs exposure-induced hepatotoxicity. Redox Biol. 2021, 47, 102157. [Google Scholar] [CrossRef]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Pereira, R.B.; Sousa, C.; Costa, A.; Andrade, P.B.; Valentão, P. Glutathione and the Antioxidant Potential of Binary Mixtures with Flavonoids: Synergisms and Antagonisms. Molecules 2013, 18, 8858–8872. [Google Scholar] [CrossRef] [PubMed]
- Brückner, M.; Westphal, S.; Domschke, W.; Kucharzik, T.; Lügering, A. Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis. J. Crohns Colitis 2008, 2, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Velalar, C.N.; Ruan, R. Effects of epigallocatechin-3-gallate on mitochondrial integrity and antioxidative enzyme activity in the aging process of human fibroblast. Free Radic. Biol. Med. 2008, 44, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar] [PubMed]
- Martín, M.A.; Serrano, A.B.; Ramos, S.; Pulido, M.I.; Bravo, L.; Goya, L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J. Nutr. Biochem. 2010, 21, 196–205. [Google Scholar] [CrossRef]
- Ran, Z.H.; Chen, C.; Xiao, S.D. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed. Pharmacother. 2008, 3, 189–196. [Google Scholar] [CrossRef]
- Chiou, Y.S.; Huang, Q.; Ho, C.T.; Wang, Y.J.; Pan, M.H. Directly interact with Keap1 and LPS is involved in the anti-inflammatory mechanisms of (–)-epicatechin-3-gallate in LPS-induced macrophages and endotoxemia. Free Radic. Biol. Med. 2016, 94, 1–16. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, B.; Li, M.; Shen, S.; Xin, W. Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim. Biophys. Acta 1996, 1304, 210–222. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Kumamoto, M.; Sonda, T.; Nagayama, K.; Tabata, M. Effects of pH and Metal Ions on Antioxidative Activities of Catechins. Biosci. Biotechnol. Biochem. 2001, 65, 126–132. [Google Scholar] [CrossRef]
- Maassen, J.A.T.H.L.; Van Essen, E.; Heine, R.J.; Nijpels, G.; Jahangir, T.R.; Raap, A.K.; Janssen, G.M.; Lemkes, H.H. Mitochondrial diabetes: Molecular mechanisms and clinical presentation. Diabetes 2004, 53 (Suppl. S1), S103–S109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kumar, A. Protective effects of epigallocatechin gallate following 3-nitropropionic acid-induced brain damage: Possible nitric oxide mechanisms. Psychopharmacol. 2009, 207, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Tan, J.; Cao, C.; Shytle, R.D.; Bradshaw, P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J. Alzheimer’s Dis. 2011, 26, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Vazquez Prieto, M.A.; Rodriguez Lanzi, C.; Miatello, R.M.; Haj, F.G.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin mitigates high-fructose-associated insulin resistance by modulating redox signaling and endoplasmic reticulum stress. Free Radic. Biol. Med. 2014, 72, 247–256. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Maya, L.; Ceballos, G.; Villarreal, F. (-)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension 2010, 55, 1398–1405. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Yip, S.C.; Saha, S.; Chernoff, J. PTP1B: A double agent in metabolism and oncogenesis. Trends Biochem. Sci. 2010, 35, 442–449. [Google Scholar] [CrossRef]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; Mcclain, C.J. A Pilot Study to Evaluate the Safety and Efficacy of an Oral Dose of (–)-Epigallocatechin-3-Gallate-Rich Polyphenon E in Patients with Mild to Moderate Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef]
- Fleming, I.; Busse, R. Signal transduction of eNOS activation. Cardiovasc. Res. 1999, 43, 532–541. [Google Scholar] [CrossRef]
- Forstermann, U.; Munzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory effect of epigallocatechin-3-O-gallate on-glucosidase and its hypoglycemic effect via targeting PI3K/AKT signaling pathway in L6 skeletal muscle cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Boue, S.M.; Daigle, K.W.; Chen, M.H.; Cao, H.; Heiman, M.L. Antidiabetic potential of purple and red rice (Oryza sativa L.) bran extracts. J. Agric. Food Chem. 2016, 64, 5345–5353. [Google Scholar] [CrossRef]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), A Green Tea Polyphenol, Suppresses Hepatic Gluconeogenesis through 50-AMP-activated Protein Kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef]
- Long, M.; Yang, S.H.; Han, J.X.; Li, P.; Zhang, Y.; Dong, S.; Chen, X.; Guo, J.; Wang, J.; He, J.B. The protective effect of grapeseed proanthocyanidin extract on oxidative damage induced by zearalenone in kunming mice liver. Int. J. Mol. Sci. 2016, 17, 808. [Google Scholar] [CrossRef]
- El-Alfy, A.T.; Ahmed, A.A.E.; Fatani, A.J. Protective effect of red grape seeds proanthocyanidins against induction of diabetes by alloxan in rats. Pharmacol. Res. 2005, 52, 264–270. [Google Scholar] [CrossRef]
- Sapwarobol, S.; Adisakwattana, S.; Changpeng, S.; Ratanawachirin, W.; Tanruttanawong, K.; Boonyarit, W. Postprandial blood glucose response to grape seed extract in healthy participants: A pilot study. Pharmacogn. Mag. 2012, 8, 192–196. [Google Scholar] [CrossRef]
- De Los Santos, S.; García-Pérez, V.; Hernández-Reséndiz, S.; Palma-Flores, C.; González-Gutiérrez, C.J.; Zazueta, C.; Canto, P.; Coral-Vázquez, R.M. (-)-Epicatechin induces physiological cardiac growth by activation of the PI3K/Akt pathway in mice. Mol. Nutr. Food Res. 2017, 61, 2. [Google Scholar] [CrossRef]
- Waltner-Law, M.E.; Wang, X.L.; Law, B.K.; Hall, R.K.; Nawano, M.; Granner, D.K. Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J. Biol. Chem. 2002, 277, 34933–34940. [Google Scholar] [CrossRef]
- Zhong, H.; Xue, Y.; Lu, X.; Shao, Q.; Cao, Y.; Wu, Z.; Chen, G. The effects of different degrees of procyanidin polymerization on the nutrient absorption and digestive enzyme activity in mice. Molecules 2018, 23, 2916. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.N. Type 2 diabetes, mitochondrial biology and the heart. J. Mol. Cell. Cardiol. 2009, 46, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ulloa, A.; Miranda-Cervantes, A.; Licea-Navarro, A.; Mansour, C.; Beltrán-Partida, E.; Donis-Maturano, L.; Delgado De la Herrán, H.C.; Villarreal, F.; Álvarez-Delgado, C. (−)-Epicatechin stimulates mitochondrial biogenesis and cell growth in C2C12 myotubes via the G-protein coupled estrogen receptor. Eur. J. Pharmacol. 2018, 822, 95–107. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Savickas, A.; Vetchý, D.; Masteikova, R.; Kasauskas, A.; Bernatoniene, J. Direct effects of (-)-hepicatechin and procyanidin B2 on the respiration of rat heart mitochondria. Biomed. Res. Int. 2015, 2015, 232836. [Google Scholar] [CrossRef]
- Shi, W.; Li, L.; Ding, Y.; Yang, K.; Chen, Z.; Fan, X.; Jiang, S.; Guan, Y.; Liu, Z.; Xu, D.; et al. The critical role of epigallocatechin gallate in regulating mitochondrial metabolism. Future Med. Chem. 2018, 10, 795–809. [Google Scholar] [CrossRef]
- Mohan, T.; Narasimhan, K.K.S.; Ravi, D.B.; Velusamy, P.; Chandrasekar, N.; Chakrapani, L.N.; Srinivasan, A.; Karthikeyan, P.; Kannan, P.; Tamilarasan, B.; et al. Role of Nrf2 dysfunction in the pathogenesis of diabetic nephropathy: Therapeutic prospect of epigallocatechin-3-gallate. Free Rad. Biol. Med. 2020, 160, 227–238. [Google Scholar] [CrossRef]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef]
- Cremonini, E.; Oteiza, P.I. (-)-Epicatechin and its metabolites prevent palmitate-induced NADPH oxidase upregulation, oxidative stress and insulin resistance in HepG2 cells. Arch. Biochem. Biophys. 2018, 646, 55–63. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell. Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Klaman, L.D.; Boss, O.; Peroni, O.D.; Kim, J.K.; Martino, J.L.; Zabolotny, J.M.; Moghal, N.; Lubkin, M.; Kim, Y.B.; Sharpe, A.H.; et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase1B-deficient mice. Mol. Cell. Biol. 2000, 20, 5479–5489. [Google Scholar] [CrossRef] [PubMed]
- Haruta, T.; Uno, T.; Kawahara, J.; Takano, A.; Egawa, K.; Sharma, P.M.; Olefsky, J.M.; Kobayashi, M. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol. Endocrinol. 2000, 14, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of apple polyphenols on inflammatory gene expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Lu, C.H.; Kuo, Y.Y.; Chen, W.T.; Chao, C.Y. Studies on the non-invasive anticancer remedy of the triple combination of epigallocatechin gallate, pulsed electric field, and ultrasound. PLoS ONE 2018, 13, e0201920. [Google Scholar] [CrossRef] [PubMed]
- Kurita, I.; Kim, J.H.; Auger, C.; Kinoshita, Y.; Miyase, T.; Ito, T.; Schini-Kerth, V.B. Hydroxylation of (-)-epigallocatechin-3-O-gallate at 311, but not 411, is essential for the PI3-kinase/Akt-dependent phosphorylation of endothelial NO synthase in endothelial cells and relaxation of coronary artery rings. Food Funct. 2013, 4, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Snoussi, C.; Ducroc, R.; Hamdaoui, M.H.; Dhaouadi, K.; Abaidi, H.; Cluzeaud, F.; Nazaret, C.; Le Gall, M.; Bado, A. Green tea decoction improves glucose tolerance and reduces weight gain of rats fed normal and high-fat diet. J. Nutr. Biochem. 2014, 25, 557–564. [Google Scholar] [CrossRef]
- Yan, J.; Feng, Z.; Liu, J.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Long, J. Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: Ameliorating effects of ()-epigallocatechin-3-gallate. J. Nutr. Biochem. 2012, 23, 716–724. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, B. (-)-Epicatechin and β-glucan from highland barley grain modulated glucose metabolism and showed synergistic effect via Akt pathway. J. Func. Foods 2021, 87, 104793. [Google Scholar] [CrossRef]
- Wang, X.H.; Huang, L.L.; Yu, T.T.; Zhu, J.H.; Shen, B.; Zhang, Y.; Wang, H.Z.; Gao, S. Effects of oligomeric grape seed proanthocyanidins on heart, aorta, kidney in DOCA-salt mice: Role of oxidative stress. Phytother. Res. 2013, 27, 869–876. [Google Scholar] [CrossRef]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Oxidative stress induced by cadmium in the plasma, erythrocytes, and lymphocytes of rats: Attenuation by grape seed proanthocyanidins. Hum. Exp. Tox. 2016, 35, 428–447. [Google Scholar] [CrossRef]
- Sun, Y.; Xiu, C.; Liu, W.; Tao, Y.; Wang, J.; Qu, Y. Grape seed proanthocyanidin extract protects the retina against early diabetic injury by activating the Nrf2 pathway. Exp. Therap. Med. 2016, 11, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Denis, M.C.; Desjardins, Y.; Furtos, A.; Marcil, V.; Dudonne, S.; Montoudis, A.; Garofalo, C.; Delvin, E.; Marette, A.; Levy, E. Prevention of oxidative stress, inflammation, and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin. Sci. 2015, 128, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.J.; Truong, V.L.; Ko, S.Y.; Nguyen, X.N.; Ingkasupart, P.; Jun, M.; Shin, J.Y.; Jeong, W.S. Antioxidant and hepatoprotective effects of procyanidins from wild grape (Vitis amurensis) seeds in ethanol-induced cells and rats. Int. J. Mol. Sci. 2016, 17, 758. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, E.G.; Alfeky, N.H.; Ibrahim, A.O. Upregulation of GLUT4 and PI3K, and downregulation of GSK3 mediate the anti-hyperglycemic effects of proanthocyanidins. Med. Int. 2022, 2, 14. [Google Scholar] [CrossRef]
- Zhang, H.J.; Ji, B.P.; Chen, G.; Zhou, F.; Luo, Y.C.; Yu, H.Q.; Gao, F.Y.; Zhang, Z.P.; Li, H.Y. A combination of grape seed-derived procyanidins and gypenosides alleviates insulin resistance in mice and HepG2 cells. J. Food Sci. 2007, 74, H1–H7. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.; Ma, W.; Li, D.; Shi, R.; Wang, M. Proanthocyanidin B2 attenuates postprandial blood glucose and its inhibitory effect on alpha-glucosidase: Analysis by kinetics, fluorescence spectroscopy, atomic force microscopy and molecular docking. Food Funct. 2018, 9, 4673–4682. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as -Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Yogalakshmi, B.; Bhuvaneswari, S.; Sreeja, S.; Anuradha, C.V. Grape seed proanthocyanidins and metformin act by different mechanisms to promote insulin signaling in rats fed high calorie diet. J. Cell. Commun. Signal. 2013, 6, 13–22. [Google Scholar]
- Xu, M.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, Y.; Zheng, P.; Yu, J.; He, J. Grape seed proanthocyanidin extract promotes skeletal muscle fiber type transformation via AMPK signaling pathway. J. Nutr. Biochem. 2020, 84, 108462. [Google Scholar] [CrossRef]
- Huang, P.L.; Chi, C.W.; Liu, T.Y. Areca nut procyanidins ameliorate streptozocin-induced hyperglycemia by regulating gluconeogenesis. Food Chem. Toxicol. 2013, 55, 137–143. [Google Scholar] [CrossRef]
- DalBó, S.; Jürgensen, S.; Horst, H.; Soethe, D.N.; Santos, A.R.; Pizzolatti, M.G.; Ribeiro-do-Valle, R.M. Analysis of the antinociceptive effect of the proanthocyanidin-rich fraction obtained from Croton celtidifolius barks: Evidence for a role of the dopaminergic system. Pharmacol. Biochem. Behav. 2006, 85, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sankar, J.; Rathinavel, A.; Mohammed, S.S.S.; Devaraj, S.N. Oligomeric proanthocyanidins mitigate cholesterol and cholic acid diet-induced hepatic dysfunction in male Sprague Dawley rats. J. Biochem. Mol. Toxicol. 2018, 1, e22234. [Google Scholar] [CrossRef] [PubMed]
- Rigotti, M.; Cerbaro, A.F.; da Silva, I.D.R.; Agostini, F.; Branco, C.S.; Moura, S.; Salvador, M. Grape seed proanthocyanidins prevent H2O2-induced mitochondrial dysfunction and apoptosis via SIRT 1 activation in embryonic kidney cells. J. Food Biochem. 2020, 44, e13147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, M.; Zhang, W.; Liu, C.; Chen, S. Natural Polyphenols in Metabolic Syndrome: Protective Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6110. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No. 1924/20061. EFSA J. 2012, 10, 2809. [Google Scholar]
- Brown, A.L.; Lane, J.; Coverly, J.; Stocks, J.; Jackson, S.; Stephen, A.; Bluck, L.; Coward, A.; Hendrickx, H. Effects of Dietary Supplementation with the Green Tea Polyphenol Epigallocatechin-3-Gallate on Insulin Resistance and Associated Metabolic Risk Factors: Randomized Controlled Trial. Br. J. Nutr. 2009, 101, 886–894. [Google Scholar] [CrossRef]
- Chatree, S.; Sitticharoon, C.; Maikaew, P.; Pongwattanapakin, K.; Keadkraichaiwat, I.; Churintaraphan, M.; Sripong, C.; Sririwichitchai, R.; Tapechum, S. Epigallocatechin Gallate Decreases Plasma Triglyceride, Blood Pressure, and Serum Kisspeptin in Obese Human Subjects. Exp. Biol. Med. 2021, 246, 163–176. [Google Scholar] [CrossRef]
- Hodges, J.; Zeng, M.; Cao, S.; Pokala, A.; Rezaei, S.; Sasaki, G.; Vodovotz, Y.; Bruno, R. Catechin-Rich Green Tea Extract Reduced Intestinal Inflammation and Fasting Glucose in Metabolic Syndrome and Healthy Adults: A Randomized, Controlled, Crossover Trial. Curr. Develop. Nutr. 2022, 6, 981. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C.H. Effects of the Pure Flavonoids Epicatechin and Quercetin on Vascular Function and Cardiometabolic Health: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Esser, D.; Geleijnse, J.M.; Matualatupauw, J.C.; Dower, J.I.; Kromhout, D.; Hollman, P.C.H.; Afman, L.A. Pure Flavonoid Epicatechin and Whole Genome Gene Expression Profiles in Circulating Immune Cells in Adults with Elevated Blood Pressure: A Randomised Double-Blind, Placebo-Controlled, Crossover Trial. PLoS ONE 2018, 13, e0194229. [Google Scholar] [CrossRef]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.J.; Liu, C.Y.; Chiu, J.P.; Hsu, C.H. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2016, 35, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; Aston, C.E.; Lyons, T.J. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Hibi, M.; Takase, H.; Iwasaki, M.; Osaki, N.; Katsuragi, Y. Efficacy of tea catechin-rich beverages to reduce abdominal adiposity and metabolic syndrome risks in obese and overweight subjects: A pooled analysis of 6 human trials. Nutr. Res. 2018, 55, 1–10. [Google Scholar] [CrossRef]
- Bajerska, J.; Mildner-Szkudlarz, S.; Walkowiak, J. Effects of rye bread enriched with green tea extract on weight maintenance and the characteristics of metabolic syndrome following weight loss: A pilot study. J. Med. Food 2015, 18, 698–705. [Google Scholar] [CrossRef]
- Vieira Senger, A.E.; Schwanke, C.H.; Gomes, I.; Valle Gottlieb, M.G. Effect of green tea (Camellia sinensis) consumption on the components of metabolic syndrome in elderly. J. Nutr. Health Aging 2012, 16, 738–742. [Google Scholar] [CrossRef]

| Flavanols | Molecular Targets | Cell Culture | Dose | References |
|---|---|---|---|---|
| EC | Stimulated mitochondrial biogenesis | Mouse skeletal muscle | 3–10 μM | [80] |
| Reduced release of the cytochrome c | Isolated rat heart mitochondria | 0.23 µg/mL and 0.46 µg/mL | [81] | |
| Decreased NOX3/NOX4 liver expression and mitigated oxidative stress | C57BL/6J mice | 0 mg EC/kg | [84] | |
| Modulated NOX subunit expression and directly inhibit NOX. Mitigated HFr-induced insulin resistance | Rat model | 20 mg EC/kg | [85,86] | |
| Stimulated the cell membrane dissociation and activation of eNOS | Human coronary artery endothelial cells | 1 μmol/L | [87] | |
| Regulated glucose metabolism through IRβ receptor and IRS-1/PI3K/Akt pathway | IR-HepG2 cells | 0–250 μg/mL | [88] | |
| EGCG | Attenuated oxidative damage | Male Wistar rats | 20 mg/kg | [83] |
| Enhanced the expression of SOD | Mice | 6.9 mg/kg | [67] | |
| Increased the gene expression of SOD1,2, CAT, and GPx | IMR90 (HDF) cells | 25–50 μM | [68] | |
| Increased SOD activity | Male rats | 50 mg/kg/d | [71] | |
| Activated the Nrf2/ARE signaling pathway | Normal Rat Kidney Epithelial Cells (NRK-52E) cell | 5 μM | [89] | |
| Reduced Akt phosphorylation/activation | Human pancreatic cancer cell line PANC-1 | 0 to 20 μM | [90] | |
| Promoted GLUT4 translocation via activation of PI3K/Akt signaling pathway | Rat skeletal muscle L6 cell | 50 μM | [91] | |
| Downregulated the ROS-ERK/JNK-p53 pathway and improved glucose homeostasis | Goto–Kakizaki (GK) rat | 100 mg/kg/d | [92] | |
| Suppressed hepatic gluconeogenesis through 5′-AMP-activated protein kinase | Isolated hepatocytes | ≤1 μm | [93] | |
| Inhibited glucose production | H4IIE rat hepatoma cells | 25 μM | [94] | |
| EGCG + EGC | Reduced intestinal SGLT-1/GLUT2 ratio and enhanced adipose GLUT4 | Male Wistar rats | 4 mg EGCG+2 mg EGC | [95] |
| ECG | Attenuated the ROS level | RAW 264.7 cells | 10 μM | [72] |
| PCAs | Upregulated SOD, CAT, GPx, and hemeoxygenase-1 (HO-1) | Kunming Mice Liver | 100 mg/kg | [96] |
| Activated the NO/cGMP pathway | Rat mesenteric arterial | 0.1–100 μg/mL | [97] | |
| Increased GSH levels and reduced lipid peroxidation and total nitrite and nitrate levels | Wistar rats | 50 and 100 mg kg−1 | [98] | |
| Decreased blood glucose level, increased insulin level through regulation of the PI3K signaling pathway | Male albino rats | 300 mg/kg/day | [99] | |
| Activated the PI3K/AKT pathway | Albino Wistar rats | 100 mg/kg | [100] | |
| Activated AMPK, Sirt1, and PGC-1α | Male BALB/c mice | 100 and 200 mg/kg | [101] | |
| Activated the Nrf2 pathway | Wistar rats | 250 mg/kg | [102] | |
| Procyanidins | Reduced ROS formation, increased SOD, CAT, GPx activity; regulated MAPK kinase pathway | Sprague-Dawley (SD) rat | 10–50 mg/kg | [103] |
| Reduced the blood glucose level, PEPCK, and G6Pase | C57BL/6 male mice | 10 mg/kg | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanè, G.T.; Putaggio, S.; Tellone, E.; Barreca, D.; Ficarra, S.; Maffei, C.; Calderaro, A.; Laganà, G. Catechins and Proanthocyanidins Involvement in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 9228. https://doi.org/10.3390/ijms24119228
Patanè GT, Putaggio S, Tellone E, Barreca D, Ficarra S, Maffei C, Calderaro A, Laganà G. Catechins and Proanthocyanidins Involvement in Metabolic Syndrome. International Journal of Molecular Sciences. 2023; 24(11):9228. https://doi.org/10.3390/ijms24119228
Chicago/Turabian StylePatanè, Giuseppe Tancredi, Stefano Putaggio, Ester Tellone, Davide Barreca, Silvana Ficarra, Carlo Maffei, Antonella Calderaro, and Giuseppina Laganà. 2023. "Catechins and Proanthocyanidins Involvement in Metabolic Syndrome" International Journal of Molecular Sciences 24, no. 11: 9228. https://doi.org/10.3390/ijms24119228






