COVID-19: The Course, Vaccination and Immune Response in People with Multiple Sclerosis: Systematic Review
Abstract
:1. Introduction
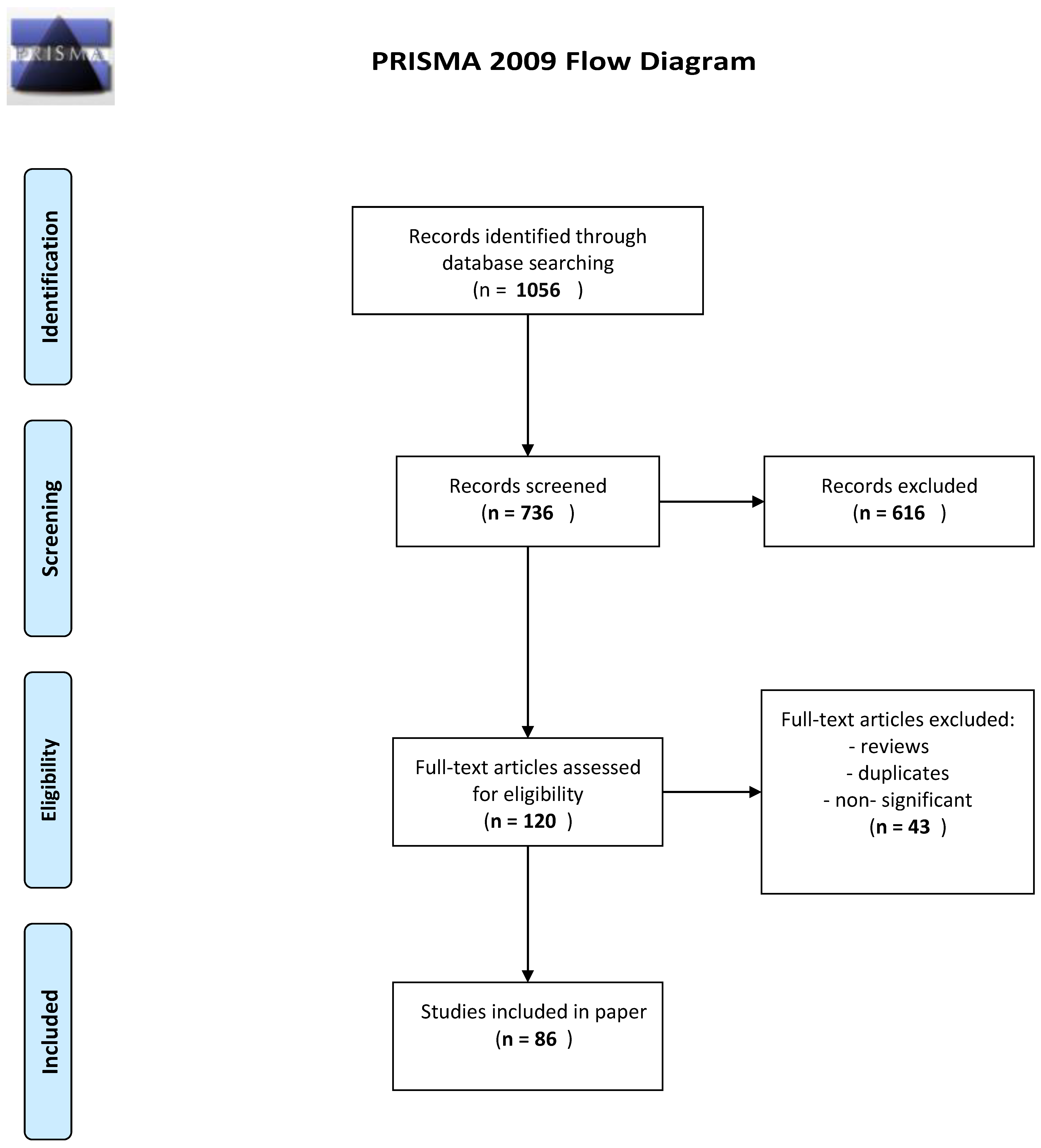
2. Material and Methods
3. Results and Discussion
3.1. Risk, Course, and Mortality of COVID-19 in PwMS
3.2. Multiple Sclerosis Treatment and COVID-19
3.3. Vaccination and Immune Response
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Lancet Neurology. Multiple sclerosis under the spotlight. Lancet Neurol. 2021, 20, 497. [Google Scholar] [CrossRef] [PubMed]
- Juryńczyk, M.; Jakuszyk, P.; Kurkowska-Jastrzębska, I.; Palace, J. Increasing role of imaging in differentiating MS from non-MS and defining indeterminate borderline cases. Neurol. Neurochir. Pol. 2022, 56, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Hart, F.M.; Bainbridge, J. Current and emerging treatment of multiple sclerosis. Am. J. Manag. Care 2016, 22 (Suppl. S6), S159–S170. [Google Scholar]
- Gudowska-Sawczuk, M.; Mroczko, B. What Is Currently Known about the Role of CXCL10 in SARS-CoV-2 Infection? Int. J. Mol. Sci. 2022, 23, 3673. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://portal.who.int/report/eios-covid19-counts/ (accessed on 16 April 2023).
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Mroczko, B. The Role of Neuropilin-1 (NRP-1) in SARS-CoV-2 Infection: Review. J. Clin. Med. 2021, 10, 2772. [Google Scholar] [CrossRef]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis -A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Gupta, N.; Augustine, S.; Narayan, T.; O’Riordan, A.; Das, A.; Kumar, D.; Luong, J.H.T.; Malhotra, B.D. Point-of-Care PCR Assays for COVID-19 Detection. Biosensors 2021, 11, 141. [Google Scholar] [CrossRef]
- Green, K.; Winter, A.; Dickinson, R.; Graziadio, S.; Wolff, R.; Mallett, S.; Allen, A.J.; Park, E.B. What Tests Could Potentially Be Used for the Screening, Diagnosis and Monitoring of COVID-19 and What Are Their Advantages and Disadvantages. 2020. Available online: https://www.cebm.net/covid-19/what-tests-could-potentially-be-used-for-the-screening-diagnosis-and-monitoring-of-covid-19-and-what-are-their-advantages-and-disadvantages/ (accessed on 16 April 2023).
- Sterlin, D.; Mathian, A.; Miyara, M.; Mohr, A.; Anna, F.; Claër, L.; Quentric, P.; Fadlallah, J.; Ghillani, P.; Gunn, C.; et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021, 13, eabd2223. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals; CellPress: Palo Alto, CA, USA, 2020. [Google Scholar]
- Hellerstein, M. What are the roles of antibodies versus a durable, high quality T-cell response in protective immunity against SARS-CoV-2? Vaccine X 2020, 6, 100076. [Google Scholar] [CrossRef]
- REDONE.br—Neuroimmunology Brazilian Study Group Focused on COVID-19 and MS; Ferreira, L.C.; Sousa, N.A.D.C.; Ferreira, M.L.B.; Dias-Carneiro, R.P.C.; Mendes, M.F.; Piccolo, A.C.; Thomaz, R.B.; Vasconcelos, C.C.F.; Alves-Leon, S.V.; et al. Incidence and clinical outcome of Coronavirus disease 2019 in a cohort of 11,560 Brazilian patients with multiple sclerosis. Mult. Scler. J. 2021, 27, 1615–1619. [Google Scholar] [CrossRef]
- Solomon, J.M.; Jones, A.; Hohol, M.; Krysko, K.M.; Muccilli, A.; Roll, A.; Rotstein, D.; Schneider, R.; Selchen, D.; Vosoughi, R.; et al. Clinical characteristics and outcomes of multiple sclerosis patients with COVID-19 in Toronto, Canada. Mult. Scler. Relat. Disord. 2022, 58, 103509. [Google Scholar] [CrossRef]
- Naghavi, S.; Kavosh, A.; Adibi, I.; Shaygannejad, V.; Arabi, S.; Rahimi, M.; Mazaheri, S.; Ashtari, F. COVID-19 infection and hospitalization rate in Iranian multiple sclerosis patients: What we know by May 2021. Mult. Scler. Relat. Disord. 2022, 57, 103335. [Google Scholar] [CrossRef]
- Evangelou, N.; Garjani, A.; dasNair, R.; Hunter, R.; Tuite-Dalton, K.A.; Craig, E.M.; Rodgers, W.J.; Coles, A.; Dobson, R.; Duddy, M.; et al. Self-diagnosed COVID-19 in people with multiple sclerosis: A community-based cohort of the UK MS Register. J. Neurol. Neurosurg. Psychiatry 2020, 92, 107–109. [Google Scholar] [CrossRef]
- Moreno-Torres, I.; Meca Lallana, V.; Costa-Frossard, L.; Oreja-Guevara, C.; Aguirre, C.; Alba Suárez, E.M.; Gómez Moreno, M.; Borrega Canelo, L.; Sabín Muñoz, J.; Aladro, Y.; et al. Risk and outcomes of COVID-19 in patients with multiple sclerosis. Eur. J. Neurol. 2021, 28, 3712–3721. [Google Scholar] [CrossRef]
- Ghadiri, F.; Sahraian, M.A.; Shaygannejad, V.; Ashtari, F.; Ghalyanchi Langroodi, H.; Baghbanian, S.M.; Mozhdehipanah, H.; Majdi-Nasab, N.; Hosseini, S.; Poursadeghfard, M.; et al. Characteristics of COVID-19 in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 57, 103437. [Google Scholar] [CrossRef]
- Sahraian, M.A.; Azimi, A.; Navardi, S.; Ala, S.; Naser Moghadasi, A. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 46, 102472. [Google Scholar] [CrossRef]
- Prosperini, L.; Tortorella, C.; Haggiag, S.; Ruggieri, S.; Galgani, S.; Gasperini, C. Determinants of COVID-19-related lethality in multiple sclerosis: A meta-regression of observational studies. J. Neurol. 2022, 269, 2275–2285. [Google Scholar] [CrossRef]
- Sormani, M.P.; De Rossi, N.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Moiola, L.; Radaelli, M.; Immovilli, P.; Capobianco, M.; Trojano, M.; et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021, 89, 780–789. [Google Scholar] [CrossRef]
- Alroughani, R.; Inshasi, J.; Al-Hashel, J.; Alkhaboury, J.; Alsalti, A.; Al Suwaidi, R.; Hassino, L.H.; Farouk Ahmed, S. Prevalence, severity, outcomes, and risk factors of COVID-19 in multiple sclerosis: An observational study in the Middle East. J. Clin. Neurosci. 2022, 99, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, P.; Lucisano, G.; Manni, A.; Paolicelli, D.; Patti, F.; Capobianco, M.; Brescia Morra, V.; Sola, P.; Pesci, I.; Lus, G.; et al. Italian MS Register. Risk of Getting COVID-19 in People with Multiple Sclerosis: A Case-Control Study. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1141. [Google Scholar] [CrossRef] [PubMed]
- Dalla Costa, G.; Leocani, L.; Montalban, X.; Guerrero, A.I.; Sørensen, P.S.; Magyari, M.; Dobson, R.J.B.; Cummins, N.; Narayan, V.A.; Hotopf, M.; et al. RADAR-CNS consortium. Real-time assessment of COVID-19 prevalence among multiple sclerosis patients: A multicenter European study. Neurol. Sci. 2020, 41, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE); Royal College of General Practitioners. Healthcare Improvement Scotland SIGN. In COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; National Institute for Health and Care Excellence: London, UK, 2022. [Google Scholar]
- Garjani, A.; Middleton, R.M.; Nicholas, R.; Evangelou, N. Recovery From COVID-19 in Multiple Sclerosis: A Prospective and Longitudinal Cohort Study of the United Kingdom Multiple Sclerosis Register. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1118. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, E.; Kister, I.; Charvet, L.; Sammarco, C.; Saha, V.; Charlson, R.E.; Howard, J.; Gutman, J.M.; Gottesman, M.; Abou-Fayssal, N.; et al. COVID-19 outcomes in MS: Observational study of early experience from NYU Multiple Sclerosis Comprehensive Care Center. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e835. [Google Scholar] [CrossRef]
- Czarnowska, A.; Kapica-Topczewska, K.; Zajkowska, O.; Adamczyk-Sowa, M.; Kubicka-Bączyk, K.; Niedziela, N.; Warmus, P.; Kalinowska-Łyszczarz, A.; Kania, K.; Słowik, A.; et al. Symptoms after COVID-19 Infection in Individuals with Multiple Sclerosis in Poland. J. Clin. Med. 2021, 10, 5225. [Google Scholar] [CrossRef]
- Safavi, F.; Nourbakhsh, B.; Azimi, A.R. B-cell depleting therapies may affect susceptibility to acute respiratory illness among patients with multiple sclerosis during the early COVID-19 epidemic in Iran. Mult. Scler. Relat. Disord. 2020, 43, 102195. [Google Scholar] [CrossRef]
- Sormani, M.P.; Italian Study Group on COVID-19 infection in multiple sclerosis. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020, 19, 481–482, Erratum in Lancet Neurol. 2020. [Google Scholar] [CrossRef]
- Alonso, R.; Silva, B.; Garcea, O.; Diaz, P.E.C.; Dos Passos, G.R.; Navarro, D.A.R.; Valle, L.A.G.; Salinas, L.C.R.; Negrotto, L.; Luetic, G.; et al. COVID-19 in multiple sclerosis neuromyelitis optica spectrum disorder patients in Latin America. Mult. Scler. Relat. Disord. 2021, 51, 102886. [Google Scholar] [CrossRef]
- Louapre, C.; Collongues, N.; Stankoff, B.; Giannesini, C.; Papeix, C.; Bensa, C.; Deschamps, R.; Créange, A.; Wahab, A.; Pelletier, J.; et al. Clinical Characteristics and Outcomes in Patients with Coronavirus Disease 2019 and Multiple Sclerosis. JAMA Neurol. 2020, 77, 1079–1088. [Google Scholar] [CrossRef]
- Freedman, M.S.; Jack, D.; Murgašová, Z.; Todorović, M.; Seitzinger, A. Outcomes of COVID-19 among patients treated with subcutaneous interferon beta-1a for multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103283. [Google Scholar] [CrossRef]
- Landi, D.; Cola, G.; Mantero, V.; Balgera, R.; Moiola, L.; Nozzolillo, A.; Dattola, V.; Sinisi, L.; Fantozzi, R.; Di Lemme, S.; et al. Safety of Natalizumab infusion in multiple sclerosis patients during active SARS-CoV-2 infection. Mult. Scler. Relat. Disord. 2022, 57, 103345. [Google Scholar] [CrossRef]
- Sullivan, R.; Kilaru, A.; Hemmer, B.; Campbell Cree, B.A.; Greenberg, B.M.; Kundu, U.; Hach, T.; DeLasHeras, V.; Ward, B.J.; Berger, J. COVID-19 Infection in Fingolimod- or Siponimod-Treated Patients: Case Series. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1092. [Google Scholar] [CrossRef]
- Reder, A.T.; Centonze, D.; Naylor, M.L.; Nagpal, A.; Rajbhandari, R.; Altincatal, A.; Kim, M.; Berdofe, A.; Radhakrishnan, M.; Jung, E.; et al. COVID-19 in Patients with Multiple Sclerosis: Associations with Disease-Modifying Therapies. CNS Drugs. 2021, 35, 317–330. [Google Scholar] [CrossRef]
- Simpson-Yap, S.; De Brouwer, E.; Kalincik, T.; Rijke, N.; Hillert, J.A.; Walton, C.; Edan, G.; Moreau, Y.; Spelman, T.; Geys, L.; et al. Associations of Disease-Modifying Therapies with COVID-19 Severity in Multiple Sclerosis. Neurology 2021, 97, e1870–e1885. [Google Scholar] [CrossRef]
- Spelman, T.; Forsberg, L.; McKay, K.; Glaser, A.; Hillert, J. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: A study of the Swedish multiple sclerosis registry. Mult. Scler. J. 2022, 28, 1051–1059. [Google Scholar] [CrossRef]
- McKay, K.A.; Piehl, F.; Englund, S.; He, A.; Langer-Gould, A.; Hillert, J.; Frisell, T. Rituximab Infusion Timing, Cumulative Dose, and Hospitalization for COVID-19 in Persons with Multiple Sclerosis in Sweden. JAMA Netw. Open 2021, 4, e2136697. [Google Scholar] [CrossRef]
- Czarnowska, A.; Brola, W.; Zajkowska, O.; Rusek, S.; Adamczyk-Sowa, M.; Kubicka-Bączyk, K.; Kalinowska-Łyszczarz, A.; Kania, K.; Słowik, A.; Wnuk, M.; et al. Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies—The Polish experience. Neurol. Neurochir. Pol. 2021, 55, 212–222. [Google Scholar] [CrossRef]
- Alshamrani, F.; Alnajashi, H.; AlJumah, M.; Almuaigel, M.; Almalik, Y.; Makkawi, S.; Alsalman, S.; Almejally, M.; Qureshi, S.; Aljarallah, S.; et al. Registry of patients with multiple sclerosis and COVID-19 infection in Saudi Arabia. Mult. Scler. Relat. Disord. 2021, 52, 103004. [Google Scholar] [CrossRef]
- NMoghadasi, A.; Shabany, M.; Heidari, H.; Eskandarieh, S. Can pulse steroid therapy increase the risk of infection by COVID-19 in patients with multiple sclerosis? Clin. Neurol. Neurosurg. 2021, 203, 106563. [Google Scholar] [CrossRef]
- Abbasi, J. COVID-19 and mRNA Vaccines—First Large Test for a New Approach. JAMA 2020, 324, 1125–1127. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Chisari, C.G.; Patti, F. Multiple Sclerosis, COVID-19 and Vaccines: Making the Point. Neurol. Ther. 2021, 10, 627–649. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Kim, C.J.; Mateus, J.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Weiskopf, D.; Sette, A.; Fries, L.; Glenn, G.; et al. NVX-CoV2373 vaccination induces functional SARS-CoV-2-specific CD4+ and CD8+ T cell responses. J. Clin. Investig. 2022, 132, e160898. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Livingston, E.H.; Malani, P.N.; Creech, C.B. The Johnson & Johnson Vaccine for COVID-19. JAMA 2021, 325, 1575. [Google Scholar] [CrossRef]
- Witman Tsur, S.; Adrian Zaher, E.; Tsur, M.; Kania, K.; Kalinowska-Łyszczarz, A. Current Immunological and Clinical Perspective on Vaccinations in Multiple Sclerosis Patients: Are They Safe after All? Int. J. Mol. Sci. 2021, 22, 3859. [Google Scholar] [CrossRef]
- Abbasi, N.; Ghadiri, F.; Moghadasi, A.N.; Azimi, A.; Navardi, S.; Heidari, H.; Karaminia, M.; Sahraian, M.A. COVID-19 vaccine hesitancy in Iranian patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 60, 103723. [Google Scholar] [CrossRef]
- Cohen, J.A.; Bermel, R.A.; Grossman, C.I.; Hersh, C.M.; Hyland, M.; Mowry, E.M.; Naismith, R.; Naylor, M.L.; Nicholas, J.; Rajbhandar, R.; et al. Immunoglobulin G immune response to SARS-CoV-2 vaccination in people living with multiple sclerosis within Multiple Sclerosis Partners Advancing Technology and Health Solutions. Mult. Scler. 2022, 28, 1131–1137. [Google Scholar] [CrossRef]
- Marrie, R.A.; Dolovich, C.; Cutter, G.R.; Fox, R.J.; Salter, A. Attitudes toward coronavirus disease 2019 vaccination in people with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173221102067. [Google Scholar] [CrossRef]
- Ciotti, J.R.; Perantie, D.C.; Moss, B.P.; Fitzgerald, K.C.; Cohen, J.A.; Mowry, E.M.; Naismith, R.T.; Chahin, S. Perspectives and experiences with COVID-19 vaccines in people with MS. Mult. Scler. J. Exp. Transl. Clin. 2022, 8, 20552173221085242. [Google Scholar] [CrossRef]
- Allen-Philbey, K.; Stennett, A.; Begum, T.; Johnson, A.C.; MacDougall, A.; Green, S.; Dobson, R.; Giovannoni, G.; Gnanapavan, S.; Marta, M.; et al. Did it hurt? COVID-19 vaccination experience in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 65, 104022. [Google Scholar] [CrossRef]
- Lotan, I.; Wilf-Yarkoni, A.; Friedman, Y.; Stiebel-Kalish, H.; Steiner, I.; Hellmann, M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): Early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021, 28, 3742–3748. [Google Scholar] [CrossRef]
- Briggs, F.B.S.; Mateen, F.J.; Schmidt, H.; Currie, K.M.; Siefers, H.M.; Crouthamel, S.; Bebo, B.F.; Fiol, J.; Racke, M.K.; O’Connor, K.C.; et al. COVID-19 Vaccination Reactogenicity in Persons with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2021, 9, e1104. [Google Scholar] [CrossRef]
- Dreyer-Alster, S.; Menascu, S.; Mandel, M.; Shirbint, E.; Magalashvili, D.; Dolev, M.; Flechter, S.; Givon, U.; Guber, D.; Stern, Y.; et al. COVID-19 vaccination in patients with multiple sclerosis: Safety and humoral efficacy of the third booster dose. J. Neurol. Sci. 2022, 434, 120155. [Google Scholar] [CrossRef]
- Zabalza, A.; Cárdenas-Robledo, S.; Tagliani, P.; Arrambide, G.; Otero-Romero, S.; Carbonell-Mirabent, P.; Rodriguez-Barranco, M.; Rodríguez-Acevedo, B.; Restrepo Vera, J.L.; Resina-Salles, M.; et al. COVID-19 in multiple sclerosis patients: Susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2021, 28, 3384–3395. [Google Scholar] [CrossRef]
- van Kempen, Z.L.E.; Strijbis, E.M.M.; Al, M.M.C.T.; Steenhuis, M.; Uitdehaag, B.M.J.; Rispens, T.; Killestein, J. SARS-CoV-2 Antibodies in Adult Patients with Multiple Sclerosis in the Amsterdam MS Cohort. JAMA Neurol. 2021, 78, 880–882. [Google Scholar] [CrossRef]
- Bsteh, G.; Dürauer, S.; Assar, H.; Hegen, H.; Heschl, B.; Leutmezer, F.; Pauli, F.D.; Gradl, C.; Traxler, G.; Zulehner, G.; et al. Humoral immune response after COVID-19 in multiple sclerosis: A nation-wide Austrian study. Mult. Scler. 2021, 27, 2209–2218. [Google Scholar] [CrossRef]
- Iannetta, M.; Landi, D.; Cola, G.; Malagnino, V.; Teti, E.; Fraboni, D.; Buccisano, F.; Grelli, S.; Coppola, L.; Campogiani, L.; et al. T-cell responses to SARS-CoV-2 in multiple sclerosis patients treated with ocrelizumab healed from COVID-19 with absent or low anti-spike antibody titers. Mult. Scler. Relat. Disord. 2021, 55, 103157. [Google Scholar] [CrossRef]
- Schwarz, T.; Otto, C.; Jones, T.C.; Pache, F.; Schindler, P.; Niederschweiberer, M.; Schmidt, F.A.; Drosten, C.; Corman, V.M.; Ruprecht, K. Preserved T cell responses to SARS-CoV-2 in anti-CD20 treated multiple sclerosis. Mult. Scler. 2022, 28, 1041–1050. [Google Scholar] [CrossRef]
- Achiron, A.; Dolev, M.; Menascu, S.; Zohar, D.N.; Dreyer-Alster, S.; Miron, S.; Shirbint, E.; Magalashvili, D.; Flechter, S.; Givon, U.; et al. COVID-19 vaccination in patients with multiple sclerosis: What we have learnt by February 2021. Mult. Scler. 2021, 27, 864–870. [Google Scholar] [CrossRef]
- Coyle, P.K.; Gocke, A.; Vignos, M.; Newsome, S.D. Vaccine Considerations for Multiple Sclerosis in the COVID-19 Era. Adv. Ther. 2021, 38, 3550–3588, Erratum in Adv. Ther. 2022, 39, 822–830. [Google Scholar] [CrossRef]
- Ciampi, E.; Uribe-San-Martin, R.; Soler, B.; García, L.; Guzman, J.; Pelayo, C.; Jürgensen, L.; Guzman, I.; Vera, F.; Galleguillos, L.; et al. Safety and humoral response rate of inactivated and mRNA vaccines against SARS-CoV-2 in patients with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 59, 103690. [Google Scholar] [CrossRef]
- Capone, F.; Lucchini, M.; Ferraro, E.; Bianco, A.; Rossi, M.; Cicia, A.; Cortese, A.; Cruciani, A.; De Arcangelis, V.; De Giglio, L.; et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics 2022, 19, 325–333. [Google Scholar] [CrossRef]
- Altieri, M.; Capuano, R.; Conte, M.; Donnarumma, G.; Grimaldi, E.; Coppola, N.; Galdiero, M.; d’Ambrosio, A.; Tedeschi, G.; Gallo, A. Six-month humoral response to BNT162b2 mRNA COVID-19 vaccine in people with multiple sclerosis treated with natalizumab. Neurol Sci. 2022, 43, 2947–2949. [Google Scholar] [CrossRef]
- Pitzalis, M.; Idda, M.L.; Lodde, V.; Loizedda, A.; Lobina, M.; Zoledziewska, M.; Virdis, F.; Delogu, G.; Pirinu, F.; Marini, M.G.; et al. Effect of Different Disease-Modifying Therapies on Humoral Response to BNT162b2 Vaccine in Sardinian Multiple Sclerosis Patients. Front. Immunol. 2021, 12, 781843. [Google Scholar] [CrossRef]
- Tortorella, C.; Aiello, A.; Gasperini, C.; Agrati, C.; Castilletti, C.; Ruggieri, S.; Meschi, S.; Matusali, G.; Colavita, F.; Farroni, C.; et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with MS Using Different Disease-Modifying Therapies. Neurology 2022, 98, e541–e554. [Google Scholar] [CrossRef]
- Tallantyre, E.C.; Vickaryous, N.; Anderson, V.; Asardag, A.N.; Baker, D.; Bestwick, J.; Bramhall, K.; Chance, R.; Evangelou, N.; George, K.; et al. COVID-19 Vaccine Response in People with Multiple Sclerosis. Ann. Neurol. 2022, 91, 89–100. [Google Scholar] [CrossRef]
- Krbot Skorić, M.; Rogić, D.; Lapić, I.; Šegulja, D.; Habek, M. Humoral immune response to COVID-19 vaccines in people with secondary progressive multiple sclerosis treated with siponimod. Mult. Scler. Relat. Disord. 2022, 57, 103435. [Google Scholar] [CrossRef]
- Kister, I.; Patskovsky, Y.; Curtin, R.; Pei, J.; Perdomo, K.; Rimler, Z.; Voloshyna, I.; Samanovic, M.I.; Cornelius, A.R.; Velmurugu, Y.; et al. Cellular and Humoral Immunity to SARS-CoV-2 Infection in Multiple Sclerosis Patients on Ocrelizumab and Other Disease-Modifying Therapies: A Multi-Ethnic Observational Study. Ann. Neurol. 2022, 91, 782–795. [Google Scholar] [CrossRef]
- Milo, R.; Staun-Ram, E.; Karussis, D.; Karni, A.; Hellmann, M.A.; Bar-Haim, E.; Miller, A. Israeli Neuroimmunology Study Group on COVID-19 Vaccination in Multiple Sclerosis. Humoral and Cellular Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients with Multiple Sclerosis: An Israeli Multi-Center Experience Following 3 Vaccine Doses. Front. Immunol. 2022, 13, 868915. [Google Scholar] [CrossRef]
- Wu, X.; Wang, L.; Shen, L.; Tang, K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: A meta-analysis. Ebiomedicine 2022, 81, 104102. [Google Scholar] [CrossRef]
- König, M.; Torgauten, H.M.; Tran, T.T.; Holmøy, T.; Vaage, J.T.; Lund-Johansen, F.; Nygaard, G.O. Immunogenicity and Safety of a Third SARS-CoV-2 Vaccine Dose in Patients with Multiple Sclerosis and Weak Immune Response After COVID-19 Vaccination. JAMA Neurol. 2022, 79, 307–309. [Google Scholar] [CrossRef]
- Guerrieri, S.; Lazzarin, S.; Zanetta, C.; Nozzolillo, A.; Filippi, M.; Moiola, L. Serological response to SARS-CoV-2 vaccination in multiple sclerosis patients treated with fingolimod or ocrelizumab: An initial real-life experience. J. Neurol. 2022, 269, 39–43. [Google Scholar] [CrossRef]
- Achiron, A.; Mandel, M.; Gurevich, M.; Dreyer-Alster, S.; Magalashvili, D.; Sonis, P.; Dolev, M.; Menascu, S.; Harari, G.; Flechter, S.; et al. Immune response to the third COVID-19 vaccine dose is related to lymphocyte count in multiple sclerosis patients treated with fingolimod. J. Neurol. 2022, 269, 2286–2292. [Google Scholar] [CrossRef]
- Tallantyre, E.C.; Scurr, M.J.; Vickaryous, N.; Richards, A.; Anderson, V.; Baker, D.; Chance, R.; Evangelou, N.; George, K.; Giovannoni, G.; et al. Response to COVID-19 booster vaccinations in seronegative people with multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 64, 103937. [Google Scholar] [CrossRef]
- Habek, M.; Željko, C.; Savić Mlakar, A.; Bendelja, K.; Rogić, D.; Adamec, I.; Barun, B.; Gabelić, T.; Krbot Skorić, M. Humoral and cellular immunity in convalescent and vaccinated COVID-19 people with multiple sclerosis: Effects of disease modifying therapies. Mult. Scler. Relat. Disord. 2022, 59, 103682. [Google Scholar] [CrossRef]
- Zabalza, A.; Arrambide, G.; Tagliani, P.; Cárdenas-Robledo, S.; Otero-Romero, S.; Esperalba, J.; Fernandez-Naval, C.; Trocoli Campuzano, J.; Martínez Gallo, M.; Castillo, M.; et al. Humoral and Cellular Responses to SARS-CoV-2 in Convalescent COVID-19 Patients with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1143. [Google Scholar] [CrossRef]
- Satyanarayan, S.; Safi, N.; Sorets, T.; Filomena, S.; Zhang, Y.; Klineova, S.; Fabian, M.; Horng, S.; Tankou, S.; Miller, A.; et al. Differential antibody response to COVID-19 vaccines across immunomodulatory therapies for multiple sclerosis. Mult. Scler. Relat. Disord. 2022, 62, 103737. [Google Scholar] [CrossRef]
- Giossi, R.; Consonni, A.; Torri Clerici, V.; Zito, A.; Rigoni, E.; Antozzi, C.; Brambilla, L.; Crisafulli, S.G.; Bellino, A.; Frangiamore, R.; et al. Anti-Spike IgG in multiple sclerosis patients after BNT162b2 vaccine: An exploratory case-control study in Italy. Mult. Scler. Relat. Disord. 2022, 58, 103415. [Google Scholar] [CrossRef]
- Sormani, M.P.; Schiavetti, I.; Inglese, M.; Carmisciano, L.; Laroni, A.; Lapucci, C.; Visconti, V.; Serrati, C.; Gandoglia, I.; Tassinari, T.; et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. Ebiomedicine 2022, 80, 104042. [Google Scholar] [CrossRef]
- Gombolay, G.Y.; Dutt, M.; Tyor, W. Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: A systematic review/meta-analysis. Ann. Clin. Transl. Neurol. 2022, 9, 1321–1331. [Google Scholar] [CrossRef]
| DMT | SARS-CoV-2 Infection Risk | COVID-19 Hospitalization Rate | References |
|---|---|---|---|
| Anti-CD20 | higher (vs. PwMS with DMT treatment) | higher (in PwMS with DMT treatment)/ lower (vs. PwMS without DMT treatment) | [35,36] |
| Anti CD52 | higher (vs. PwMS with DMT treatment) | lower (in PwMS with DMT treatment)/ lower (vs. PwMS without DMT) | [35,36] |
| Anti-VLA-4 | higher (vs. PwMS with DMT treatment) | lower (in PwMS with DMT treatment)/ lower (vs. PwMS without DMT) | [35,36] |
| Fumarate dimethyl | slightly higher (vs PwMS with DMT treatment) | higher/ lower(in PwMS with DMT treatment)/ lower (vs PwMS without DMT treatment) | [35,36] |
| IFNβ | lower (vs. PwMS with DMT treatment) | higher (in PwMS with DMT treatment)/ lower (vs. PwMS without DMT treatment) | [35,36] |
| Glatiramer acetate | lower (vs. PwMS with DMT treatment) | higher/ lower (in PwMS with DMT treatment)/lower (vs. PwMS without DMT) | [35,36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazylewicz, M.; Gudowska-Sawczuk, M.; Mroczko, B.; Kochanowicz, J.; Kułakowska, A. COVID-19: The Course, Vaccination and Immune Response in People with Multiple Sclerosis: Systematic Review. Int. J. Mol. Sci. 2023, 24, 9231. https://doi.org/10.3390/ijms24119231
Bazylewicz M, Gudowska-Sawczuk M, Mroczko B, Kochanowicz J, Kułakowska A. COVID-19: The Course, Vaccination and Immune Response in People with Multiple Sclerosis: Systematic Review. International Journal of Molecular Sciences. 2023; 24(11):9231. https://doi.org/10.3390/ijms24119231
Chicago/Turabian StyleBazylewicz, Marcin, Monika Gudowska-Sawczuk, Barbara Mroczko, Jan Kochanowicz, and Alina Kułakowska. 2023. "COVID-19: The Course, Vaccination and Immune Response in People with Multiple Sclerosis: Systematic Review" International Journal of Molecular Sciences 24, no. 11: 9231. https://doi.org/10.3390/ijms24119231
APA StyleBazylewicz, M., Gudowska-Sawczuk, M., Mroczko, B., Kochanowicz, J., & Kułakowska, A. (2023). COVID-19: The Course, Vaccination and Immune Response in People with Multiple Sclerosis: Systematic Review. International Journal of Molecular Sciences, 24(11), 9231. https://doi.org/10.3390/ijms24119231








