The Synergistic Mechanism of Photosynthesis and Antioxidant Metabolism between the Green and White Tissues of Ananas comosus var. bracteatus Chimeric Leaves
Abstract
:1. Introduction
2. Results
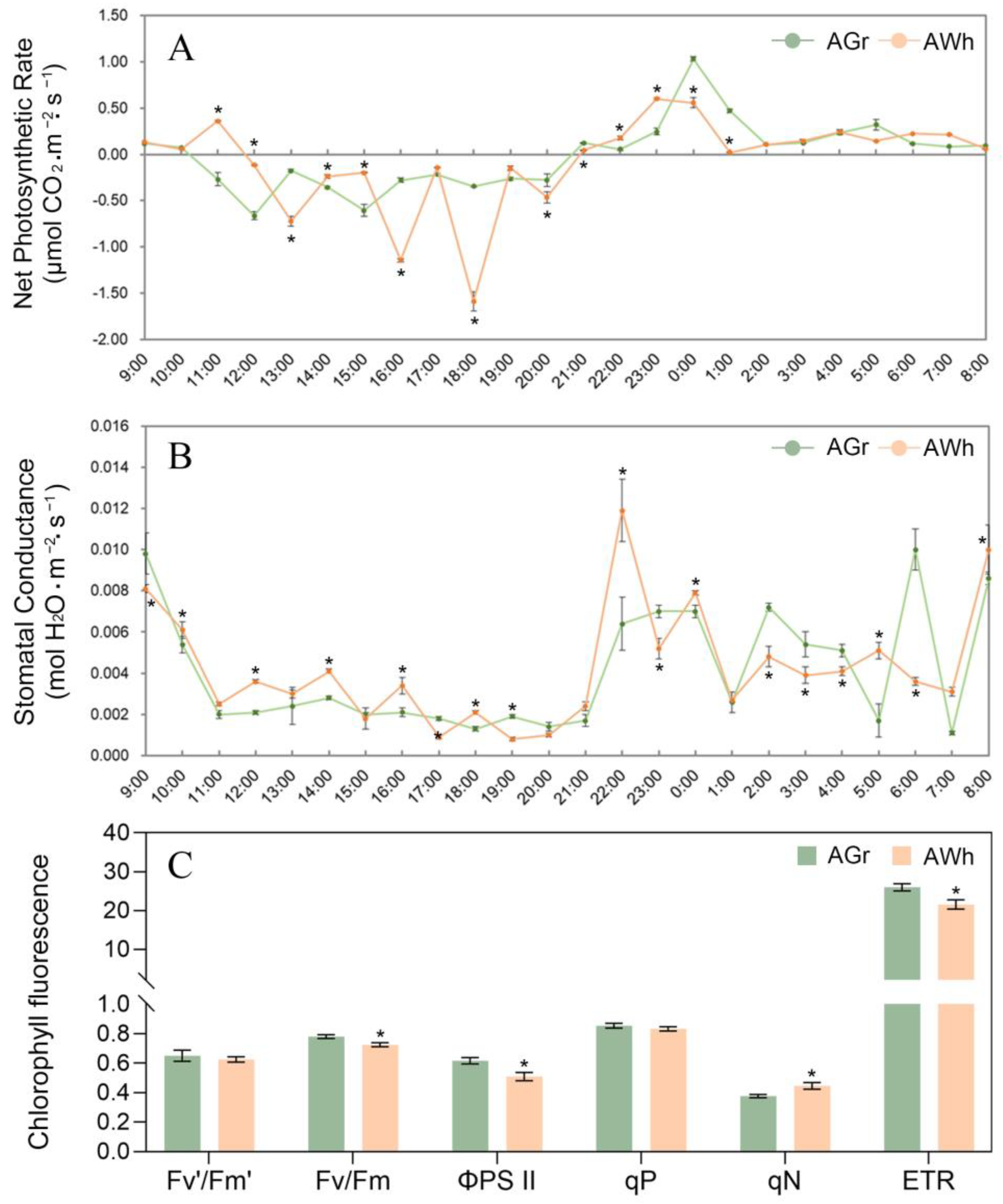
2.1. Photosynthetic Characteristics of Chimeric Leaves
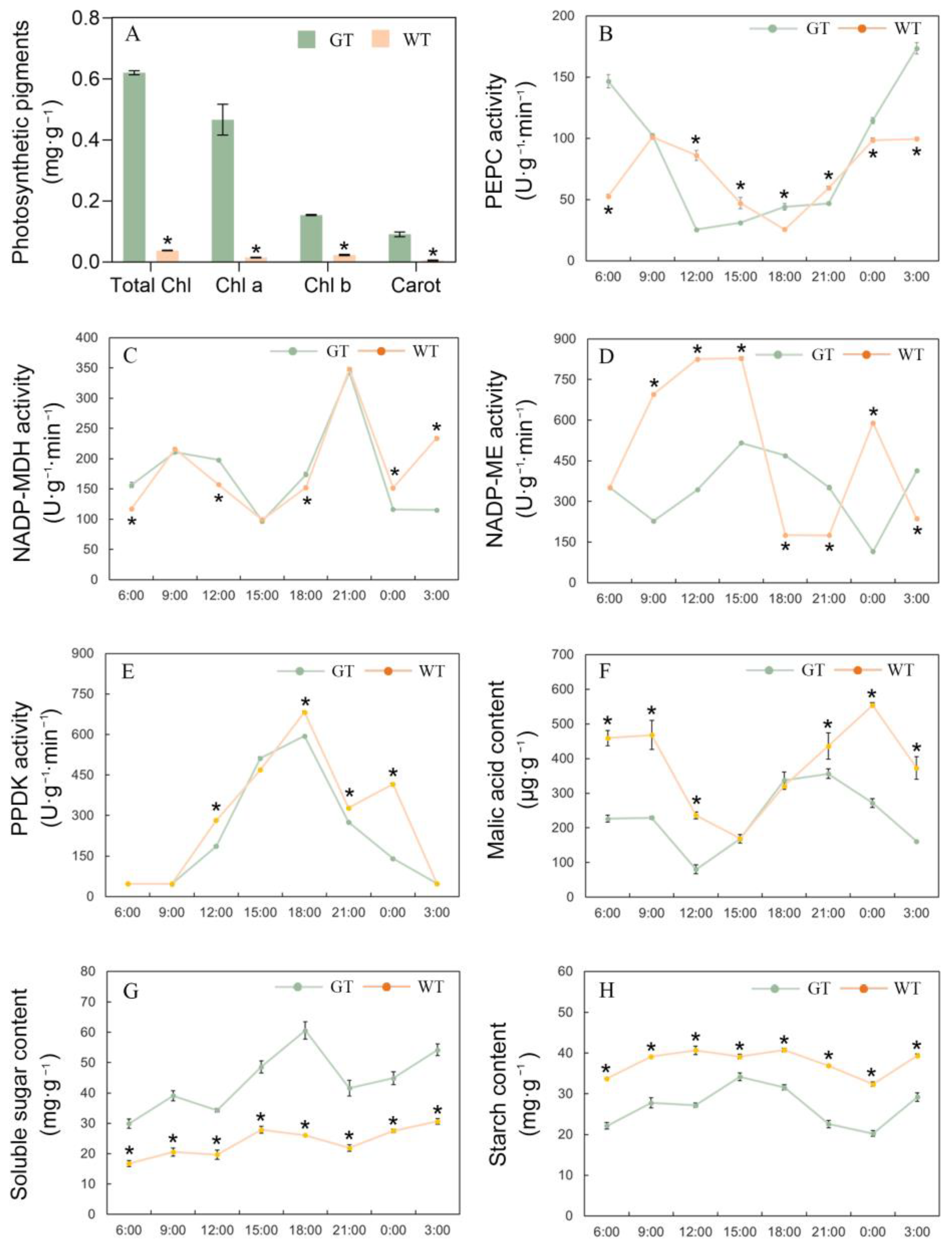
2.2. Character of Photosynthetic Pigments Accumulation in Chimeric Leaves
2.3. CO2 Utilization Mechanism of the Chimeric Leaves
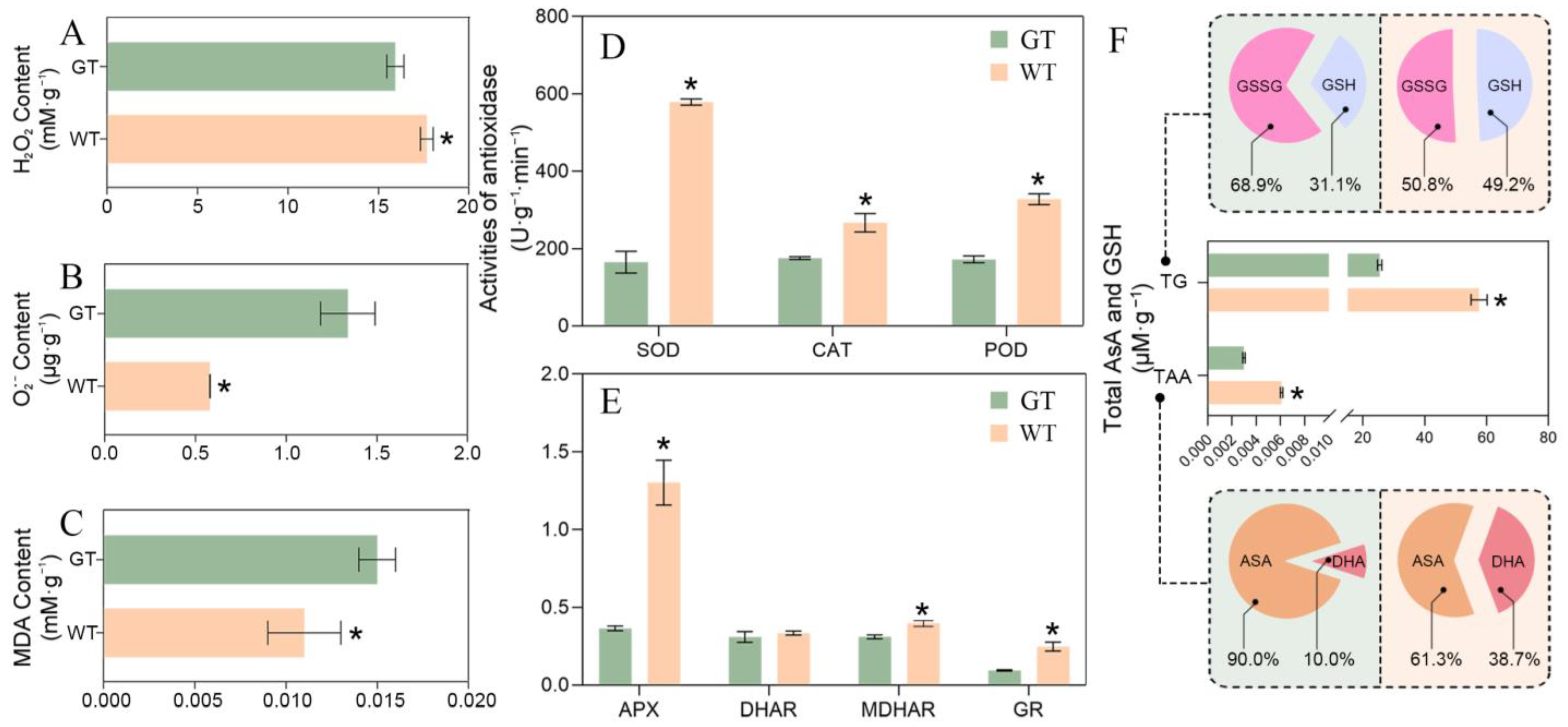
2.4. ROS Equilibrium Mechanism of Chimeric Leaves
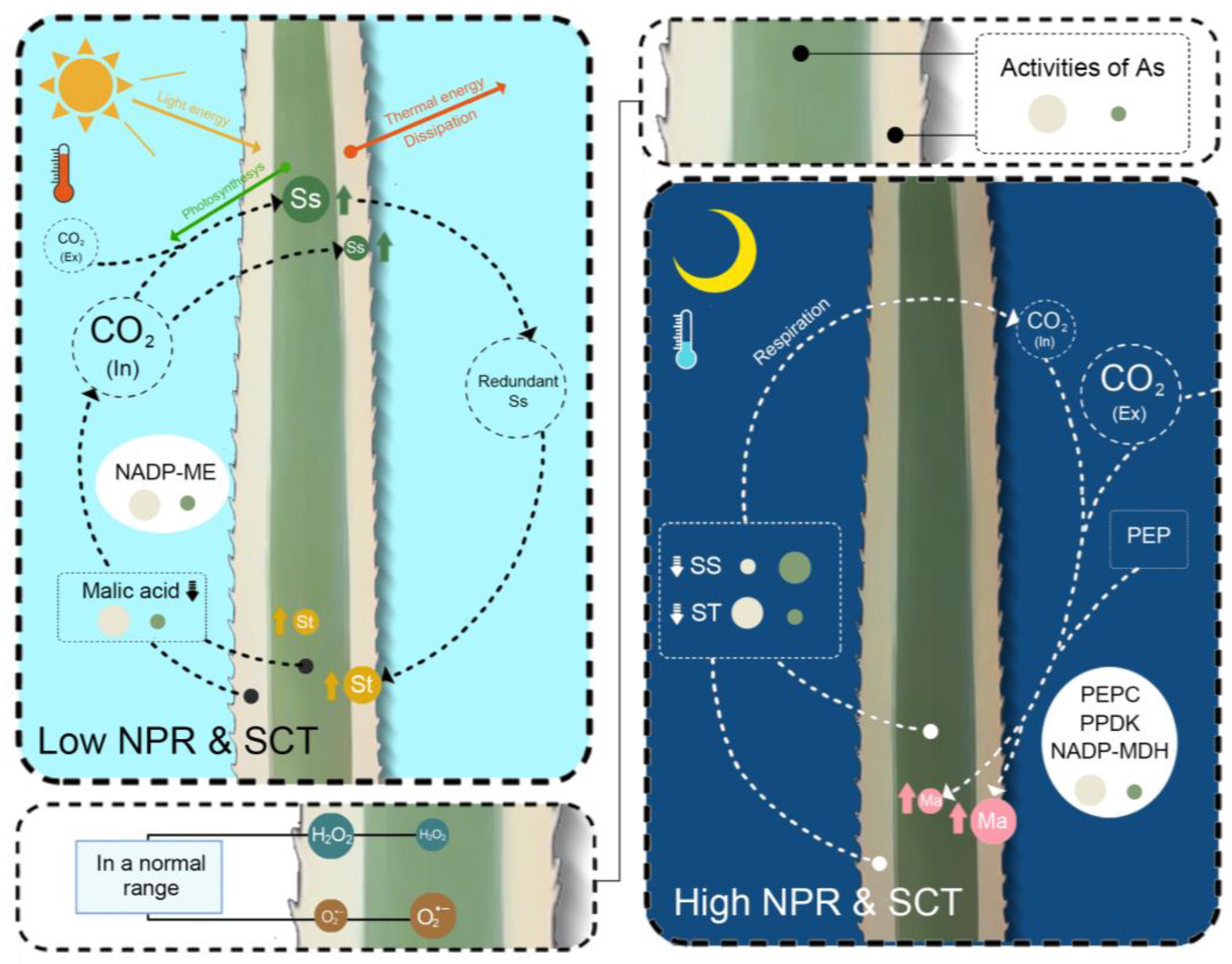
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Preparation
4.2. Measurement of Photosynthesis and Chlorophyll Fluorescence Parameters
4.3. Determination of Photosynthetic Enzyme Activities
4.4. Determination of Photosynthetic Pigments
4.5. Assays of Antioxidant Enzyme Activities
4.6. Assays of Malic Acid, Starch and Soluble Sugar Contents
4.7. Determination of H2O2, O2•−, MDA, Antioxidants and Enzyme Activities in AsA-GSH Cycle
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, C.E. Physiological ecology of the Bromeliaceae. Bot. Rev. 1994, 60, 1–82. [Google Scholar] [CrossRef]
- Xue, Y.; Ma, J.; He, Y.; Yu, S.; Lin, Z.; Xiong, Y.; Rafique, F.; Jiang, F.; Sun, L.; Ma, M.; et al. Comparative transcriptomic and proteomic analyses of the green and white parts of chimeric leaves in Ananas comosus var. bracteatus. PeerJ 2019, 7, e7261. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Xue, Y.; He, Y.; Zhou, X.; Rafique, F.; Hu, H.; Liu, J.; Feng, L.; Yang, W.; Li, X.; et al. Systematic identi-fication and comparative analysis of lysine succinylation between the green and white parts of chimeric leaves of Ananas comosus var. bracteatus. BMC Genom. 2020, 21, 383. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, D.; Sun, J.; Mu, Y.; Pu, W.; Ma, B.; Ren, F.; Yan, W.; Zhang, Z.; Li, G.; et al. Phenotypic and proteomic characteristics of sorghum (Sorghum bicolor) albino lethal mutant sbe6-a1. Plant Physiol. Biochem. 2019, 139, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, H.; Guo, Z.; Meng, Y.; Yang, M.; Li, X.; Yang, Q. Adaptation responses in C4 photosynthesis of sweet maize (Zea mays L.) exposed to nicosulfuron. Ecotoxicol. Environ. Saf. 2021, 214, 112096. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Heckathorn, S.A.; Barua, D.; Joshi, P.; Hamilton, E.W.; LaCroix, J.J. Effects of elevated CO2 on the tolerance of photosynthesis to acute heat stress in C3, C4, and CAM species. Am. J. Bot. 2008, 95, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Borland, A.M.; Griffiths, H.; Hartwell, J.; Smith, J.A.C. Exploiting the potential of plants with crassulacean acid metabolism for bioenergy production on marginal lands. J. Exp. Bot. 2009, 60, 2879–2896. [Google Scholar] [CrossRef]
- Borland, A.M.; Hartwell, J.; Weston, D.J.; Schlauch, K.A.; Tschaplinski, T.J.; Tuskan, G.A.; Yang, X.; Cushman, J.C. Engineering crassulacean acid metabolism to improve water-use efficiency. Trends Plant Sci. 2014, 19, 327–338. [Google Scholar] [CrossRef]
- Winter, K.; Holtum, J.A.M. How Closely Do the δ13C Values of Crassulacean Acid Metabolism Plants Reflect the Pro-portion of CO2 Fixed during Day and Night? Plant Physiol. 2002, 129, 1834–1851. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F.H. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Schreiber, U.; Klughammer, C.; Kolbowski, J. Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth. Res. 2012, 113, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shi, M.; Zhang, Y.; Xie, X.; Sun, P.; Fang, C.; Zhao, J. FvMYB24, a strawberry R2R3-MYB transcription factor, improved salt stress tolerance in transgenic Arabidopsis. Biochem. Biophys. Res. Commun. 2021, 569, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ishitani, M.; Nakamura, T.; Han, S.Y.; Takabe, T. Expression of the betaine aldehyde dehydrogenase gene in barley in response to osmotic stress and abscisic acid. Plant Mol. Biol. 1995, 27, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Nolte, K.D.; Hanson, A.D.; Gage, D.A. Proline Accumulation and Methylation to Proline Betaine in Citrus: Implications for Genetic Engineering of Stress Resistance. J. Am. Soc. Hortic. Sci. 1997, 122, 8–13. [Google Scholar] [CrossRef]
- Asada, K.; Takahashi, M. Production and scavenging of active oxygen in photosynthesis. Photoinhib. Top. Photo-Synth. 1987, 227–287. [Google Scholar]
- Niewiadomska, E.; Miszalski, Z. Does CO2 modify the effect of SO2 on variegated leaves of Chlorophytum comosum (Thunb) Bak. New Phytol. 1995, 130, 461–466. [Google Scholar] [CrossRef]
- Peltzer, D.; Schwanz, P.; Polle, A. Preliminary studies of ascorbatemetabolism in gree n and albino regions of variegated leaves of Coleus blumei Benth. Free. Radic. Res. 1999, 31, 181–185. [Google Scholar] [CrossRef]
- Tartoura, K.A.; Youssef, S.A. Stimulation of ROS-scavenging systems in squash (Cucurbita pepo L.) plants by compost supplementation under normal and low temperature conditions. Sci. Hortic. 2011, 130, 862–868. [Google Scholar] [CrossRef]
- Jia, L.; Qin, X.; Lyu, D.; Qin, S.; Zhang, P. ROS production and scavenging in three cherry rootstocks under short-term waterlogging conditions. Sci. Hortic. 2019, 257, 108647. [Google Scholar] [CrossRef]
- Farooq, M.; Ahmad, R.; Shahzad, M.; Sajjad, Y.; Hassan, A.; Shah, M.M.; Naz, S.; Khan, S.A. Differential variations in total flavonoid content and antioxidant enzymes activities in pea under different salt and drought stresses. Sci. Hortic. 2021, 287, 110258. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; Malik, A.U.; Khan, A.S.; Hasan, M.U.; Anwar, R.; Ali, S.; Haider, M.W. Combined application of hot water treatment and methyl salicylate mitigates chilling injury in sweet pepper (Capsicum annuum L.) fruits. Sci. Hortic. 2021, 283, 110113. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Jung, H.-I.; Lee, B.-R.; Chae, M.-J.; Lee, E.-J.; Lee, T.-G.; Jung, G.-B.; Kim, M.-S.; Lee, J. Ascorbate-Mediated Modulation of Cadmium Stress Responses: Reactive Oxygen Species and Redox Status in Brassica napus. Front. Plant Sci. 2020, 11, 586547. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Ma, Y.; Qing, Y.; Wang, H.; Huang, X. The highly drought-tolerant pitaya (Hylocereus undatus) is a non-facultative CAM plant under both well-watered and drought conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 643–652. [Google Scholar] [CrossRef]
- Kluge, M.; Ting, I.P. Crassulacean acid metabolism: Analysis of an ecological adaptation. Q. Rev. Biol. 1978. [Google Scholar]
- Castelfranco, P.A.; Beale, S.I. Chlorophyll Biosynthesis. Photosynthesis 1981, 47, 375–421. [Google Scholar]
- Hansson, M.; Lundqvist, J.; Sirijovski, N.; Al-Karadaghi, S. Magnesium Chelatase: The Molecular Motor of Chlo-rophyll Biosynthesis. Handb. Porphyrin. Sci. 2013, 28, 41–84. [Google Scholar]
- Semer, J.; Navrátil, M.; Špunda, V.; Štroch, M. Chlorophyll fluorescence parameters to assess utilization of excitation energy in photosystem II independently of changes in leaf absorption. J. Photochem. Photobiol. B Biol. 2019, 197, 111535. [Google Scholar] [CrossRef]
- Henriques, F.S. Photosynthetic characteristics of light-sensitive, chlorophyll-deficient leaves from sectorially chimeric stinging-nettle. Studies 2008, 49, 235–241. [Google Scholar]
- Khalekuzzaman, M.D.; Kim, K.J.; Kim, H.J.; Jung, H.H.; Jang, H.S. Comparison of green and variegated foliage plant species based on chlorophyll fluorescence parameters under different light intensities. Pak. J. Bot 2015, 47, 1709–1715. [Google Scholar]
- Murchie, E.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Yun, B.-W.; Choi, J.S.; Kim, T.-J.; Kwak, S.-S.; Cho, K.-Y. Death mechanisms caused by carotenoid biosynthesis inhibitors in green and in undeveloped plant tissues. Pestic. Biochem. Physiol. 2004, 78, 127–139. [Google Scholar] [CrossRef]
- Adams, W.W.; Stewart, J.J.; Demmig-Adams, B. Photosynthetic Modulation in Response to Plant Activity and Environment. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; pp. 493–563. [Google Scholar] [CrossRef]
- Ayre, B.G.; Turgeon, R. Export of Photosynthates from the Leaf. Photosynthesis 2018, 55–79. [Google Scholar] [CrossRef]
- Yaronskaya, E.B.; Shalygo, N.V. Activity of an antioxidant system in albino tissue of streptomycin-treated barley (hordeum vulgare) seedlings. In Proceedings of the National Academy of Sciences of Belarus; Bio-Logical Series; National Academy of Sciences: Washington, DC, USA, 2010. [Google Scholar]
- Sousa, R.H.; Carvalho, F.E.; Ribeiro, C.W.; Passaia, G.; Cunha, J.R.; Lima-Melo, Y.U.G.O.; Pinheiro, M.-M.; Silveira, J.A.G. Peroxisomal apx knockdown triggers antioxidant mechanisms fa-vourable for coping with high photorespiratory H2O2 induced by cat deficiency in rice. Plant Cell Environ. 2015, 38, 499–513. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Wu, J.; Jie, L.; Chen, J.; Cai, L. Effects of exogenous no on asa-gsh circulation metabolism in young loquat fruit mitochondria under low temperature stress. Pak. J. Bot. 2012, 44, 847–851. [Google Scholar]
- Deng, G.; Huang, X.; Xie, L.; Tan, S.; Gbokie, J.T.; Bao, Y.; Xie, Z.; Yi, K. Identification and Expression of SAUR Genes in the CAM Plant Agave. Genes 2019, 10, 555. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Ai, P.; Cui, X.; Zhang, Z. ALM1, encoding a Fe-superoxide dismutase, is critical for rice chloroplast biogenesis and drought stress response. Crop. J. 2020, 9, 1018–1029. [Google Scholar] [CrossRef]
- Ma, H.; Wu, J.; Zhang, H.; Tang, H.; Wan, Y. Identification and expression profiling of genes involved in circadian clock regulation in red dragon fruit (Hylocereus polyrhizus) by full-length transcriptome sequencing. Plant Signal. Behav. 2021, 16, 1907054. [Google Scholar] [CrossRef]
- A Raven, J.; Cockell, C.S.; De La Rocha, C.L. The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 2641–2650. [Google Scholar] [CrossRef]
- Klavsen, S.K.; Madsen, T.V.; Maberly, S.C. Crassulacean acid metabolism in the context of other carbon-concentrating mechanisms in freshwater plants: A review. Photosynth. Res. 2011, 109, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Buckland, C.E.; Thomas, D.S. Analysing the potential for CAM-fed bio-economic uses in sub-Saharan Africa. Appl. Geogr. 2021, 132, 102463. [Google Scholar] [CrossRef]
- Crayn, D.M.; Winter, K.; Schulte, K.; Smith, J.A. Photosynthetic pathways in Bromeliaceae: Phylogenetic and ecological signifificance of CAM and C3 based on carbon isotope ratios for 1893 species. Bot. J. Linnean. Soc. 2015, 178, 169–221. [Google Scholar] [CrossRef]
- Niechayev, N.A.; Pereira, P.N.; Cushman, J.C. Understanding trait diversity associated with crassulacean acid metabolism (CAM). Curr. Opin. Plant Biol. 2019, 49, 74–85. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Soldatova, O.; Apchelimov, A.; Radukina, N.; Ezhova, T.; Shestakov, S.; Ziemann, V.; Hedtke, B.; Grimm, B. An Arabidopsis mutant that is resistant to the protoporphyrinogen oxidase inhibitor acifluorfen shows regulatory changes in tetrapyrrole biosynthesis. Mol. Genet. Genom. 2005, 273, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Hu, G.; Wang, X.; Chen, H.; Shi, Q.; Xiang, J.; Zhang, Y.; Zhu, D.; Zhang, Y. Reduced bioactive gibberellin content in rice seeds under low temperature leads to decreased sugar consumption and low seed germination rates. Plant Physiol. Biochem. 2018, 133, 1–10. [Google Scholar] [CrossRef]
- Salin, M.L. Chloroplast and Mitochondrial Mechanisms for Protection Against Oxygen Toxicity. Free. Radic. Res. Commun. 1991, 13, 851–858. [Google Scholar] [CrossRef]
- Ay, A.A.Y.; Dawood, H.D. Chilling injury, fruit color maturity stages, and antioxidant enzyme activities of lemon ‘baladi CV’ fruits under cold storage stress. Sci. Hortic. 2019, 257, 108676. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.-M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Chojak-Koźniewska, J.; Kuźniak, E.; Linkiewicz, A.; Sowa, S. Primary carbon metabolism-related changes in cucumber exposed to single and sequential treatments with salt stress and bacterial infection. Plant Physiol. Biochem. 2018, 123, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, D.H.; Iglesias, A.A.; Andreo, C.S. On the Regulation of Phosphoenolpyruvate Carboxylase Activity from Maize Leaves by L-malate. Effect of pH. J. Plant Physiol. 1984, 116, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Penninckx, I.A.; Eggermont, K.; Terras, F.R.; Thomma, B.P.; De Samblanx, G.W.; Buchala, A.; Métraux, J.P.; Manners, J.M.; Broekaert, W.F. Pathogen-induced systemic activation of a plant defensin gene in Arabidopsis follows a salicylic acid-independent pathway. Plant Cell 1996, 8, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.T.; Kennedy, R.A. Photosynthetic Enzyme Activities and Localization in Mollugo verticillata Populations Differing in the Levels of C(3) and C(4) Cycle Operation. Plant Physiol. 1979, 64, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Camp, P.J.; Huber, S.C.; Burke, J.J.; Moreland, D.E. Biochemical Changes that Occur during Senescence of Wheat Leaves. Plant Physiol. 1982, 70, 1641–1646. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res. 2002, 73, 149–156. [Google Scholar] [CrossRef]
- Abassi, N.; Kushad, M.; Endress, A. Active oxygen-scavenging enzymes activities in developing apple flowers and fruits. Sci. Hortic. 1998, 74, 183–194. [Google Scholar] [CrossRef]
- Yin, J.; Bai, S.; Wu, F.; Lu, G.; Yang, H. Effect of nitric oxide on the activity of phenylalanine ammonia-lyase and antioxidative response in sweetpotato root in relation to wound-healing. Postharvest Biol. Technol. 2012, 74, 125–131. [Google Scholar] [CrossRef]
- Lee, M.Y.; Kim, S.S. Characteristics of six isoperoxidases from Korean radish root. Phytochemistry 1994, 35, 287–290. [Google Scholar] [CrossRef]
- Kou, X.-H.; Wang, S.; Zhang, Y.; Guo, R.-Z.; Wu, M.-S.; Chen, Q.; Xue, Z.-H. Effects of chitosan and calcium chloride treatments on malic acid-metabolizing enzymes and the related gene expression in post-harvest pear cv. ‘Huang guan’. Sci. Hortic. 2014, 165, 252–259. [Google Scholar] [CrossRef]
- Hansen, J.; Møller, I. Percolation of starch and soluble carbohydrates from plant tissue for quantitative determination with anthrone. Anal. Biochem. 1975, 68, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Li, Q.; Chen, H.; Zeng, Y.; Li, B.; Zhong, X.; Wang, L.; Ren, W. Relationship between chalkiness and the structural and thermal properties of rice starch after shading during grain-filling stage. Carbohydr. Polym. 2020, 252, 117212. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Selenium Pretreatment Upregulates the Antioxidant Defense and Methylglyoxal Detoxification System and Confers Enhanced Tolerance to Drought Stress in Rapeseed Seedlings. Biol. Trace Element Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef]
- Ozgur, R.; Turkan, I.; Uzilday, B.; Sekmen, A.H. Endoplasmic reticulum stress triggers ROS signalling, changes the redox state, and regulates the antioxidant defence of Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Pasquariello, M.S.; Di Patre, D.; Mastrobuoni, F.; Zampella, L.; Scortichini, M.; Petriccione, M. Influence of postharvest chitosan treatment on enzymatic browning and antioxidant enzyme activity in sweet cherry fruit. Postharvest Biol. Technol. 2015, 109, 45–56. [Google Scholar] [CrossRef]
- Rahman, A.; Hossain, S.; Mahmud, J.-A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced salt stress tolerance in rice seedlings: Regulation of ion homeostasis, antioxidant defense and glyoxalase systems. Physiol. Mol. Biol. Plants 2016, 22, 291–306. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, D.; Zhou, X.; Zhao, H.; Tao, X.; Yu, S.; Zhang, X.; Zang, Y.; Peng, L.; Yang, L.; Deng, S.; et al. The Synergistic Mechanism of Photosynthesis and Antioxidant Metabolism between the Green and White Tissues of Ananas comosus var. bracteatus Chimeric Leaves. Int. J. Mol. Sci. 2023, 24, 9238. https://doi.org/10.3390/ijms24119238
Lin D, Zhou X, Zhao H, Tao X, Yu S, Zhang X, Zang Y, Peng L, Yang L, Deng S, et al. The Synergistic Mechanism of Photosynthesis and Antioxidant Metabolism between the Green and White Tissues of Ananas comosus var. bracteatus Chimeric Leaves. International Journal of Molecular Sciences. 2023; 24(11):9238. https://doi.org/10.3390/ijms24119238
Chicago/Turabian StyleLin, Dongpu, Xuzixin Zhou, Huan Zhao, Xiaoguang Tao, Sanmiao Yu, Xiaopeng Zhang, Yaoqiang Zang, Lingli Peng, Li Yang, Shuyue Deng, and et al. 2023. "The Synergistic Mechanism of Photosynthesis and Antioxidant Metabolism between the Green and White Tissues of Ananas comosus var. bracteatus Chimeric Leaves" International Journal of Molecular Sciences 24, no. 11: 9238. https://doi.org/10.3390/ijms24119238
APA StyleLin, D., Zhou, X., Zhao, H., Tao, X., Yu, S., Zhang, X., Zang, Y., Peng, L., Yang, L., Deng, S., Li, X., Mao, X., Luan, A., He, J., & Ma, J. (2023). The Synergistic Mechanism of Photosynthesis and Antioxidant Metabolism between the Green and White Tissues of Ananas comosus var. bracteatus Chimeric Leaves. International Journal of Molecular Sciences, 24(11), 9238. https://doi.org/10.3390/ijms24119238




