Plasma-Induced Oxygen Vacancies in N-Doped Hollow NiCoPBA Nanocages Derived from Prussian Blue Analogue for Efficient OER in Alkaline Media
Abstract
1. Introduction
2. Results and Discussion
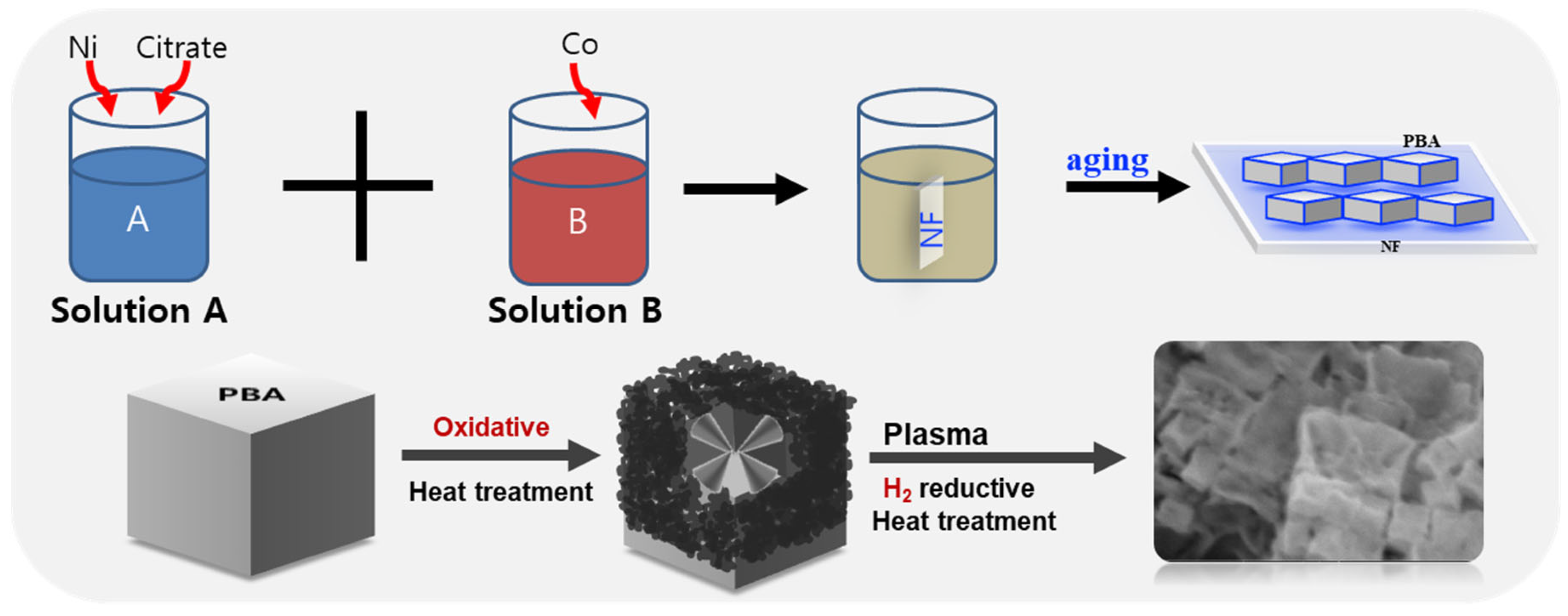
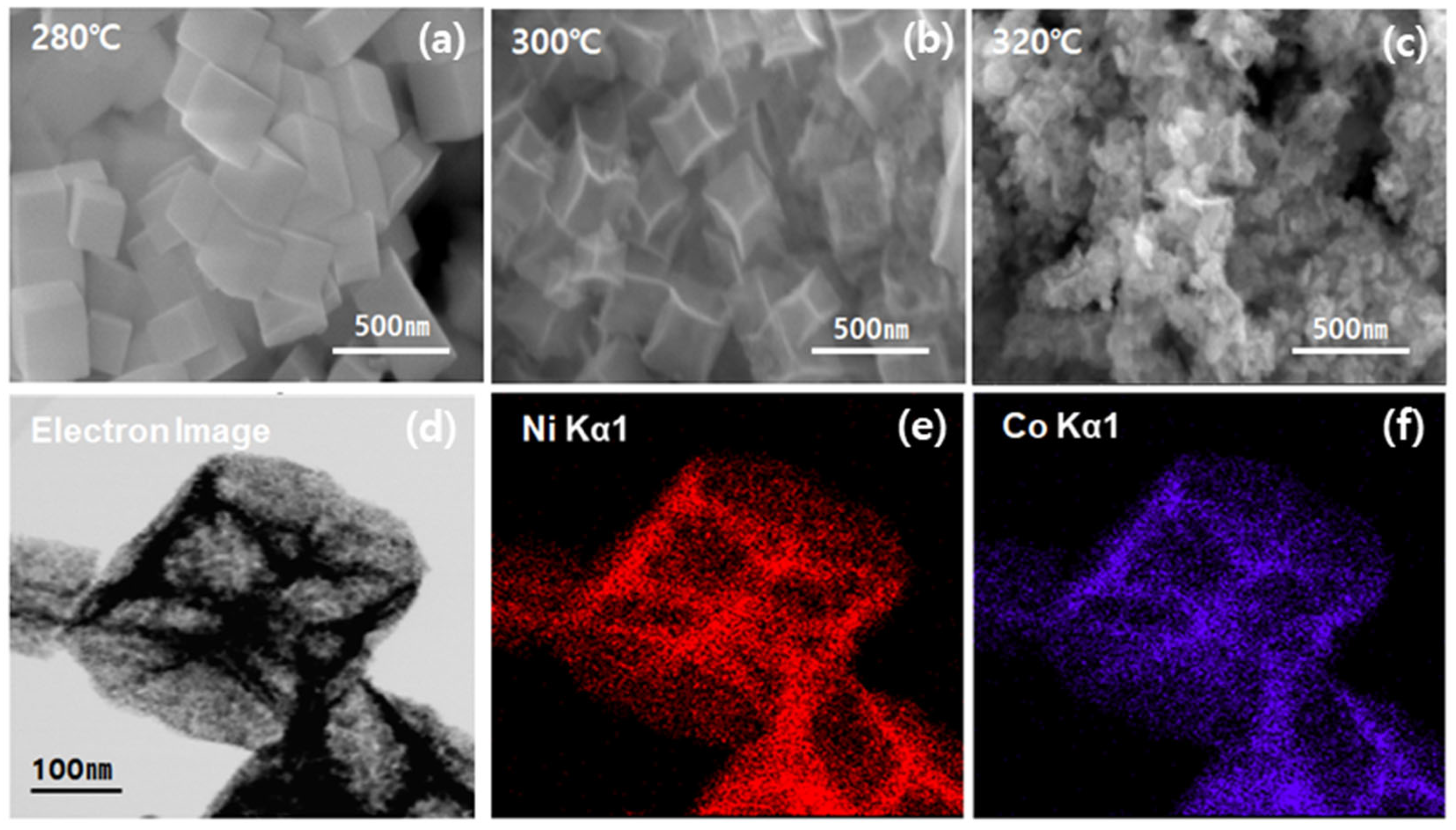
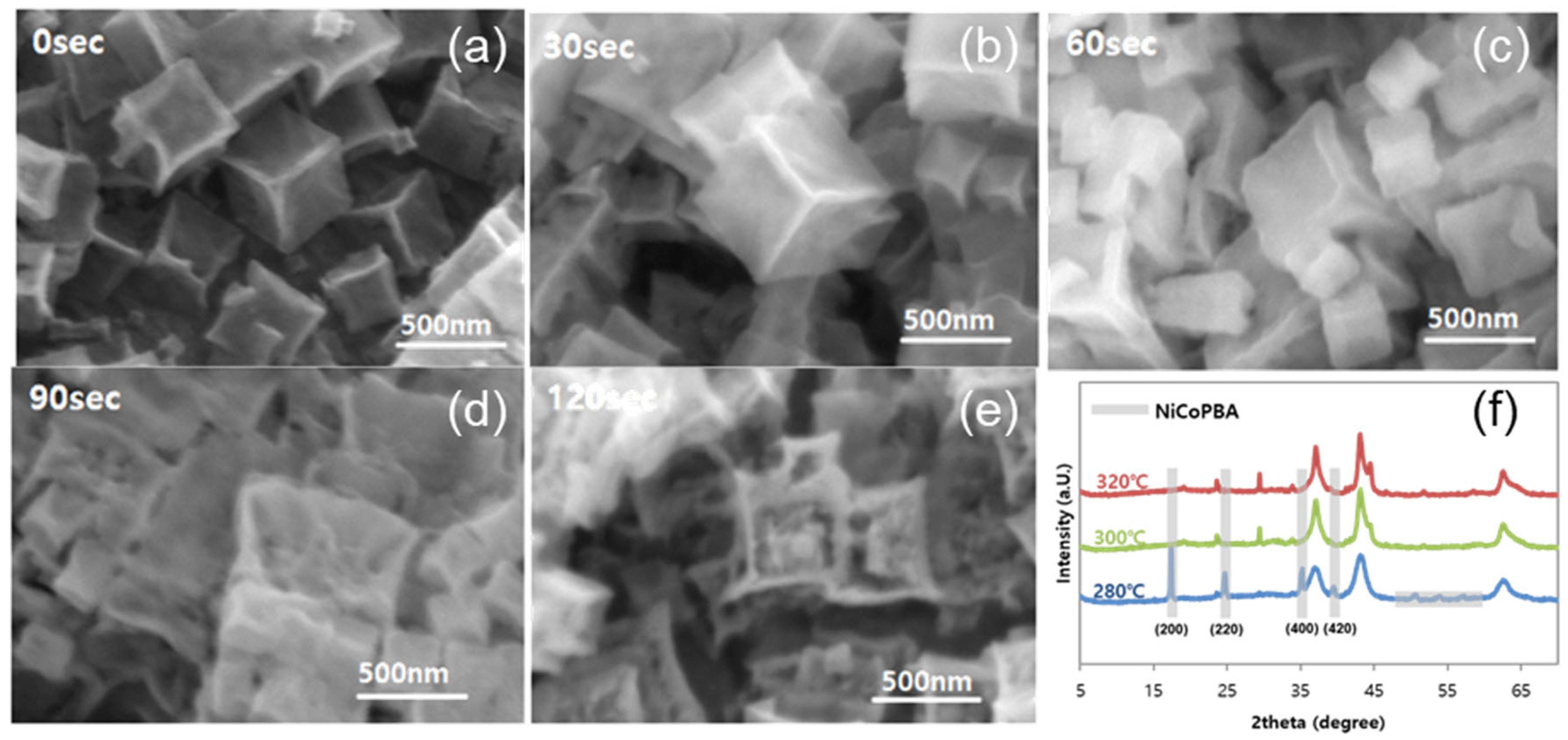
2.1. Structural Characterizations
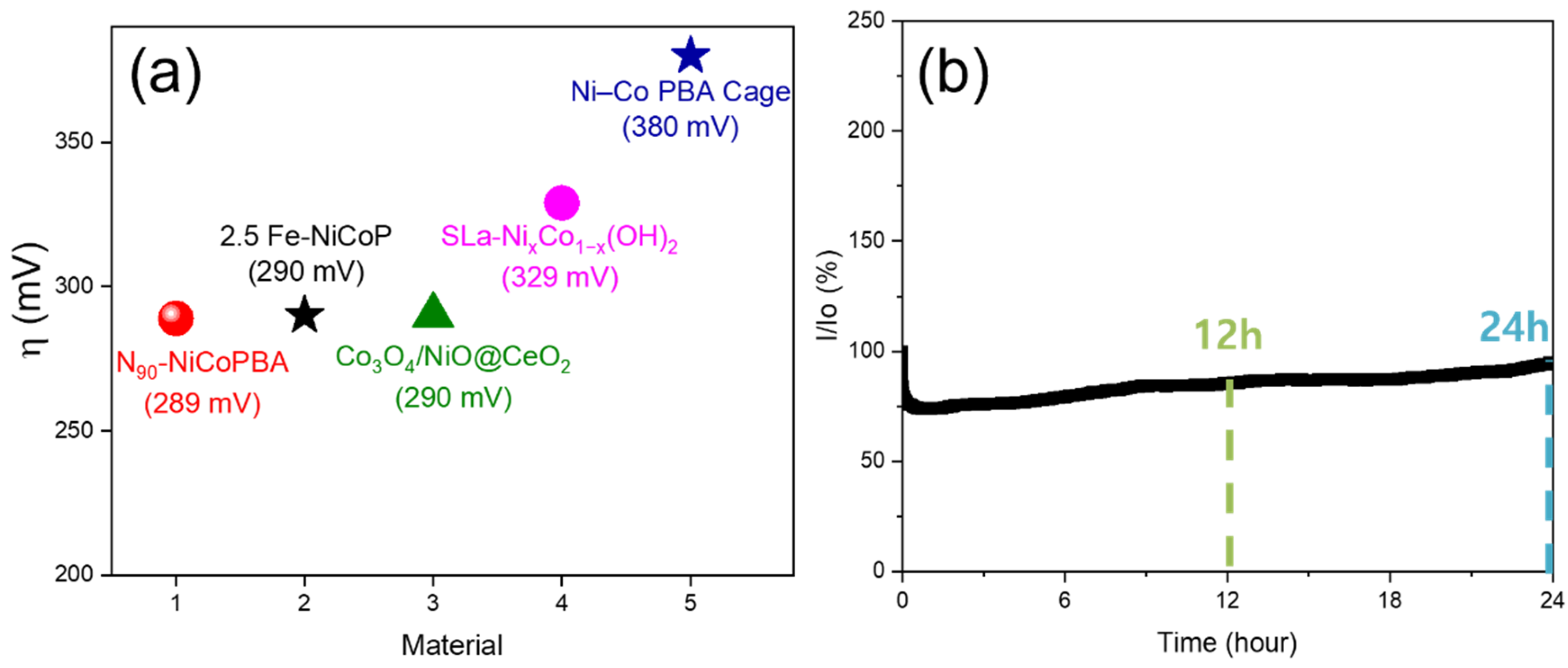
2.2. Catalyst Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of NiCoPBA/NF
3.3. Synthesis of Hollow NiCoPBA/NF (H–NiCoPBA/NF)
3.4. Synthesis of N-Doped Hollow NiCoPBA (N90–NiCoPBA/NF)
3.5. Material Characterizations
3.6. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Ma, J.; Wang, J.; Chen, S.; Wang, H.; Zhang, J. Interfacial Scaffolding Preparation of Hierarchical PBA-Based Derivative Electrocatalysts for Efficient Water Splitting. Adv. Energy Mater. 2019, 9, 1802939. [Google Scholar] [CrossRef]
- Bao, W.; Li, Y.; Zhang, J.; Ai, T.; Yang, C.; Feng, L. Interface engineering of the NiCo2O4@MoS2/TM heterostructure to realize the efficient alkaline oxygen evolution reaction. Int. J. Hydrog. Energy. 2023, 48, 12176–12184. [Google Scholar] [CrossRef]
- Fu, C.-F.; Wu, X.; Yang, J. Material Design for Photocatalytic Water Splitting from a Theoretical Perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Tran, D.T.; Nguyen, T.H.; Dinh, V.A.; Kim, N.H.; Lee, J.H. Single platinum atoms implanted 2D lateral anion-intercalated metal hydroxides of Ni2(OH)2(NO3)2 as efficient catalyst for high-yield water splitting. Appl. Catal. B Environ. 2022, 317, 121684. [Google Scholar] [CrossRef]
- Moi, C.T.; Sahu, A.; Qureshi, M. Tapping the Potential of High-Valent Mo and W Metal Centers for Dynamic Electronic Structures in Multimetallic FeVO(OH)/Ni(OH)2 for Ultrastable and Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2023, 15, 5336–5344. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Li, M.-Y.; Li, X.; Bao, W.-W.; Jin, C.-Q.; Feng, X.-H.; Liu, G.; Yang, C.-M.; Zhang, N.-N. Chromium-Modified Ultrathin CoFe LDH as High-Efficiency Electrode for Hydrogen Evolution Reaction. Nanomaterials 2022, 12, 1227. [Google Scholar] [CrossRef]
- Tran, D.T.; Le, H.T.; Luyen Doan, T.L.; Kim, N.H.; Lee, J.H. Pt nanodots monolayer modified mesoporous Cu@CuxO nanowires for improved overall water splitting reactivity. Nano Energy 2019, 59, 216–228. [Google Scholar] [CrossRef]
- Asefa, T. Metal-Free and Noble Metal-Free Heteroatom-Doped Nanostructured Carbons as Prospective Sustainable Electrocatalysts. Acc. Chem. Res. 2016, 49, 1873–1883. [Google Scholar] [CrossRef]
- Zhang, H.; Maijenburg, A.W.; Li, X.; Schweizer, S.L.; Wehrspohn, R.B. Bifunctional Heterostructured Transition Metal Phosphides for Efficient Electrochemical Water Splitting. Adv. Funct. Mater. 2020, 30, 2003261. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Feng, S.; Du, D.; Lin, Y. Single-Atom Catalysts for Electrochemical Water Splitting. ACS Energy Lett. 2018, 3, 1713–1721. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Ito, Y.; Cong, W.; Tan, Y.; Liu, P.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Nanoporous Graphene with Single-Atom Nickel Dopants: An Efficient and Stable Catalyst for Electrochemical Hydrogen Production. Angew. Chem. Int. Ed. 2015, 54, 14031–14035. [Google Scholar] [CrossRef]
- Wu, X.; Ru, Y.; Bai, Y.; Zhang, G.; Shi, Y.; Pang, H. PBA composites and their derivatives in energy and environmental applications. Coord. Chem. Rev. 2022, 451, 214260. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, G.; Xu, Y.; Zhang, H.; Guo, X.; Liu, Y.; Pang, H. A new strategy for the controllable growth of MOF@PBA architectures. J. Mater. Chem. A 2019, 7, 17266–17271. [Google Scholar] [CrossRef]
- Lu, K.; Zhu, X.-Y.; Li, Y.; Gu, N. Progress in the preparation of Prussian blue-based nanomaterials for biomedical applications. J. Mater. Chem. B 2023. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yu, L.; Wu, H.B.; Lou, X.W.D. Formation of Nickel Sulfide Nanoframes from Metal-Organic Frameworks with Enhanced Pseudocapacitive and Electrocatalytic Properties. Angew. Chem. Int. Ed. 2015, 54, 5331–5335. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Y.; Xiao, X.; Shan, Y.; Bai, Y.; Xue, H.-G.; Pang, H.; Tian, Z.; Xu, Q. In Situ Anchoring Polymetallic Phosphide Nanoparticles within Porous Prussian Blue Analogue Nanocages for Boosting Oxygen Evolution Catalysis. Nano Lett. 2021, 21, 3016–3025. [Google Scholar] [CrossRef]
- Goda, E.S.; Hong, S.E.; Yoon, K.R. Facile synthesis of Cu-PBA nanocubes/graphene oxide composite as binder-free electrodes for supercapacitor. J. Alloys Compd. 2021, 859, 157868. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, X.; Wei, X.-L.; Bao, W.-W.; Feng, X.-H.; Zhang, J.-J. Coupling Interface Construction of Ni(OH)2/MoS2 Composite Electrode for Efficient Alkaline Oxygen Evolution Reaction. Catalysts 2022, 12, 966. [Google Scholar] [CrossRef]
- Herrera, V.; Palmillas, Z.; Pirri, R.; Reyes, Y.; Leiza, J.R.; Asua, J.M. Morphology of Three-Phase PS/PBA Composite Latex Particles Containing in Situ Produced Block Copolymers. Macromolecules 2010, 43, 1356–1363. [Google Scholar] [CrossRef]
- Dong, X.; Liu, X.; Cheng, M.; Huang, D.; Zhang, G.; Wang, W.; Du, L.; Wang, G.; Liu, H. Prussian blue and its analogues: Reborn as emerging catalysts for a Fenton-like process in water purification. Coord. Chem. Rev. 2023, 482, 215067. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Yang, T.; Chen, X.; Cheng, Z.; Hou, Q.; Lin, X.; Liu, S.; Wei, S.; Wang, Z. Co3+-rich CoFe-PBA encapsulated in ultrathin MoS2 sheath as integrated core-shell architectures for highly efficient OER. J. Alloys Compd. 2023, 942, 169004. [Google Scholar] [CrossRef]
- Kang, L.; Li, J.; Wang, Y.; Gao, W.; Hao, P.; Lei, F.; Xie, J.; Tang, B. Dual-oxidation-induced lattice disordering in a Prussian blue analog for ultrastable oxygen evolution reaction performance. J. Colloid Interface Sci. 2023, 630, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ning, G.; Shi, K.; Zheng, M.; Sun, Y.; Gao, Y.; Zhang, Y.; Wang, H. N-doped hollow porous carbon spheres@CoCuFe alloy nanospheres as novel non-precious metal electrocatalysts for HER and OER. Int. J. Hydrog. Energy. 2022, 47, 5947–5960. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Q.; Luo, J.; Fu, H.C.; Chen, X.H.; Wu, L.L.; Luo, H.Q.; Li, N.B. Fabrication of 2D/3D hierarchical PBA and derivative electrocatalysts for overall water splitting. Appl. Surf. Sci. 2021, 551, 149360. [Google Scholar] [CrossRef]
- Yan, D.; Chen, R.; Xiao, Z.; Wang, S. Engineering the electronic structure of Co3O4 by carbon-doping for efficient overall water splitting. Electrochim. Acta 2019, 303, 316–322. [Google Scholar] [CrossRef]
- Qin, C.; Tian, S.; Jiang, Z.-J.; Thandavarayan, M.; Jiang, Z. Low temperature plasma-assisted synthesis and modification of water splitting electrocatalysts. Electrochim. Acta 2023, 449, 142179. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, J.; Xia, W.; Peng, Z.; Wu, Z.; Lei, W.; Xia, K.; Xin, H.L.; Wang, D. Porous Structured Ni–Fe–P Nanocubes Derived from a Prussian Blue Analogue as an Electrocatalyst for Efficient Overall Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 26134–26142. [Google Scholar] [CrossRef]
- Liu, M.; Yu, F.; Ma, C.; Xue, X.; Fu, H.; Yuan, H.; Yang, S.; Wang, G.; Guo, X.; Zhang, L. Effective Oxygen Reduction Reaction Performance of FeCo Alloys In Situ Anchored on Nitrogen-Doped Carbon by the Microwave-Assistant Carbon Bath Method and Subsequent Plasma Etching. Nanomaterials 2019, 9, 1284. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Chen, S.; Xie, F.; Riley, D.J. Anodic Transformation of a Core-Shell Prussian Blue Analogue to a Bifunctional Electrocatalyst for Water Splitting. Adv. Funct. Mater. 2021, 31, 2106835. [Google Scholar] [CrossRef]
- Parajuli, D.; Tanaka, H.; Sakurai, K.; Hakuta, Y.; Kawamoto, T. Thermal Decomposition Behavior of Prussian Blue in Various Conditions. Materials 2021, 14, 1151. [Google Scholar] [CrossRef]
- Han, L.; Yu, X.-Y.; Lou, X.W.D. Formation of Prussian-Blue-Analog Nanocages via a Direct Etching Method and their Conversion into Ni-Co-Mixed Oxide for Enhanced Oxygen Evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef]
- Marco, J.F.; Gancedo, J.R.; Gracia, M.; Gautier, J.L.; Ríos, E.; Berry, F.J. Characterization of the Nickel Cobaltite, NiCo2O4, Prepared by Several Methods: An XRD, XANES, EXAFS, and XPS Study. J. Solid State Chem. 2000, 153, 74–81. [Google Scholar] [CrossRef]
- Kim, J.-G.; Pugmire, D.L.; Battaglia, D.; Langell, M.A. Analysis of the NiCo2O4 spinel surface with Auger and X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2000, 165, 70–84. [Google Scholar] [CrossRef]
- BRIGGS, D. Practical Surface Analysis, Auger X-ray Photoelect. Spectroscory 1990, 1, 151–152. Available online: https://cir.nii.ac.jp/crid/1570009749188908928 (accessed on 1 March 2023).
- Jiménez, V.M.; Fernández, A.; Espinós, J.P.; González-Elipe, A.R. The state of the oxygen at the surface of polycrystalline cobalt oxide. J. Electron Spectros. Relat. Phenom. 1995, 71, 61–71. [Google Scholar] [CrossRef]
- Marcus-Saubat, B.; Beaufils, J.P.; Barbaux, Y. Effect of the surface structure on the departure from stoichiometry of Co3O4. J. Chim. Phys. 1986, 83, 317–321. [Google Scholar] [CrossRef]
- Singh, T.I.; Rajeshkhanna, G.; Pan, U.N.; Kshetri, T.; Lin, H.; Kim, N.H.; Lee, J.H. Alkaline Water Splitting Enhancement by MOF-Derived Fe–Co–Oxide/Co@NC-mNS Heterostructure: Boosting OER and HER through Defect Engineering and In Situ Oxidation. Small 2021, 17, 2101312. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Q.; Dong, L.; Wu, H.B.; Yu, X.-Y. Activating the hydrogen evolution and overall water splitting performance of NiFe LDH by cation doping and plasma reduction. Appl. Catal. B Environ. 2020, 266, 118627. [Google Scholar] [CrossRef]
- Ranjbari, A.; Demeestere, K.; Kim, K.-H.; Heynderickx, P.M. Oxygen vacancy modification of commercial ZnO by hydrogen reduction for the removal of thiabendazole: Characterization and kinetic study. Appl. Catal. B Environ. 2023, 324, 122265. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, P.; Wu, R.; Zhang, X.; Sun, C.; Li, H. Oxygen vacancies for promoting the electrochemical nitrogen reduction reaction. J. Mater. Chem. A 2021, 9, 6694–6709. [Google Scholar] [CrossRef]
- Kotnala, R.K.; Shah, J. Ferrite Materials. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2015; pp. 291–379. [Google Scholar] [CrossRef]
- Xiao, A.; Liu, Y.; Yang, T.; Jia, W.; Song, X.; Qian, Y.; Liang, C.; Ma, T. In-situ investigation on the oxygen vacancy-driven topotactic phase transition in charge-orbital ordered Nd0.5Sr0.5MnO3 films. Acta Mater. 2023, 245, 118616. [Google Scholar] [CrossRef]
- Dupin, J.-C.; Gonbeau, D.; Vinatier, P.; Levasseur, A. Systematic XPS studies of metal oxides, hydroxides and peroxides. Phys. Chem. Chem. Phys. 2000, 2, 1319–1324. [Google Scholar] [CrossRef]
- Wu, J.-B.; Lin, Y.-F.; Wang, J.; Chang, P.-J.; Tasi, C.-P.; Lu, C.-C.; Chiu, H.-T.; Yang, Y.-W. Correlation between N 1s XPS Binding Energy and Bond Distance in Metal Amido, Imido, and Nitrido Complexes. Inorg. Chem. 2003, 42, 4516–4518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Han, Z.; Wang, X.; Gai, H.; Chen, Z.; Guo, T.; Hou, X.; Xu, L.; Hu, X.; Huang, M.; et al. Discovery of Quantitative Electronic Structure-OER Activity Relationship in Metal-Organic Framework Electrocatalysts Using an Integrated Theoretical-Experimental Approach. Adv. Funct. Mater. 2021, 31, 2102066. [Google Scholar] [CrossRef]
- Zhao, Y.; Xi, M.; Qi, Y.; Sheng, X.; Tian, P.; Zhu, Y.; Yang, X.; Li, C.; Jiang, H. Redirecting dynamic structural evolution of nickel-contained RuO2 catalyst during electrochemical oxygen evolution reaction. J. Energy Chem. 2022, 69, 330–337. [Google Scholar] [CrossRef]
- Stoerzinger, K.A.; Qiao, L.; Biegalski, M.D.; Shao-Horn, Y. Orientation-Dependent Oxygen Evolution Activities of Rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.; Ma, W.; Xu, S.; Lu, Y.; Wei, W.; Zhu, J.; Jiang, D. Fe-doped NiCoP/Prussian blue analog hollow nanocubes as an efficient electrocatalyst for oxygen evolution reaction. Electrochim. Acta 2021, 367, 137492. [Google Scholar] [CrossRef]
- Yu, Z.; Lin, Y.-H.; Gao, X.-T.; Guo, B.; Ma, J.-L.; Zhang, Y.; Bai, F.-Y.; Dong, Y.-W.; Zhao, Z. Prussian-blue-analog derived hollow Co3O4/NiO decorated CeO2 nanoparticles for boosting oxygen evolution reaction. J. Alloys Compd. 2022, 914, 165344. [Google Scholar] [CrossRef]
- Wang, L.; Lin, C.; Huang, D.; Zhang, F.; Wang, M.; Jin, J. A Comparative Study of Composition and Morphology Effect of NixCo1–x(OH)2 on Oxygen Evolution/Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 10172–10180. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.T.; Lee, J.E.; Yun, S.Y.; Kwon, O.; Park, J.K.; Jeong, Y.K. Plasma-Induced Oxygen Vacancies in N-Doped Hollow NiCoPBA Nanocages Derived from Prussian Blue Analogue for Efficient OER in Alkaline Media. Int. J. Mol. Sci. 2023, 24, 9246. https://doi.org/10.3390/ijms24119246
Le HT, Lee JE, Yun SY, Kwon O, Park JK, Jeong YK. Plasma-Induced Oxygen Vacancies in N-Doped Hollow NiCoPBA Nanocages Derived from Prussian Blue Analogue for Efficient OER in Alkaline Media. International Journal of Molecular Sciences. 2023; 24(11):9246. https://doi.org/10.3390/ijms24119246
Chicago/Turabian StyleLe, Huu Tuan, Ji Eon Lee, So Yeon Yun, Ohyung Kwon, Jin Kuen Park, and Young Kyu Jeong. 2023. "Plasma-Induced Oxygen Vacancies in N-Doped Hollow NiCoPBA Nanocages Derived from Prussian Blue Analogue for Efficient OER in Alkaline Media" International Journal of Molecular Sciences 24, no. 11: 9246. https://doi.org/10.3390/ijms24119246
APA StyleLe, H. T., Lee, J. E., Yun, S. Y., Kwon, O., Park, J. K., & Jeong, Y. K. (2023). Plasma-Induced Oxygen Vacancies in N-Doped Hollow NiCoPBA Nanocages Derived from Prussian Blue Analogue for Efficient OER in Alkaline Media. International Journal of Molecular Sciences, 24(11), 9246. https://doi.org/10.3390/ijms24119246





