Human Monocytes Are Suitable Carriers for the Delivery of Oncolytic Herpes Simplex Virus Type 1 In Vitro and in a Chicken Embryo Chorioallantoic Membrane Model of Cancer
Abstract
:1. Introduction
2. Results
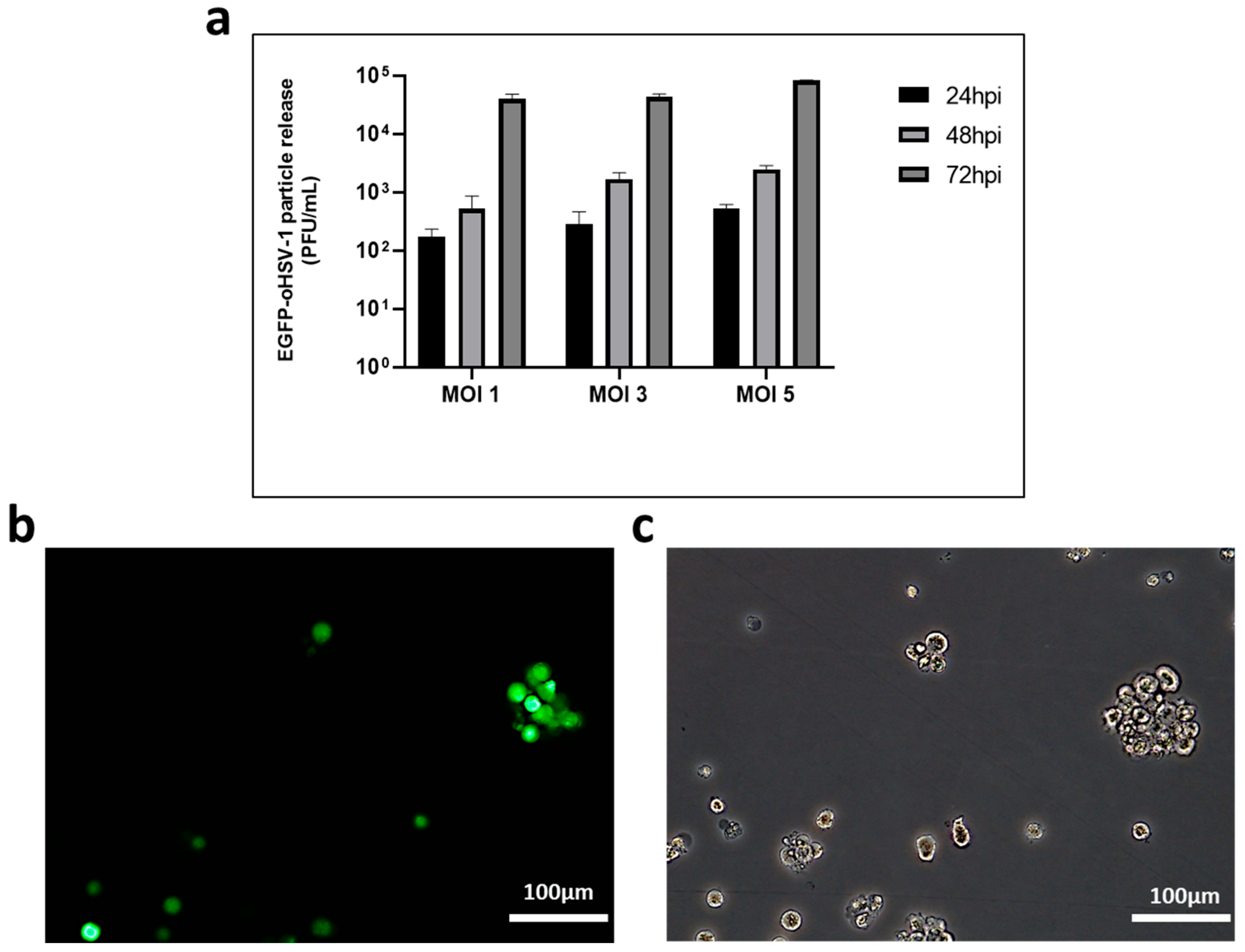
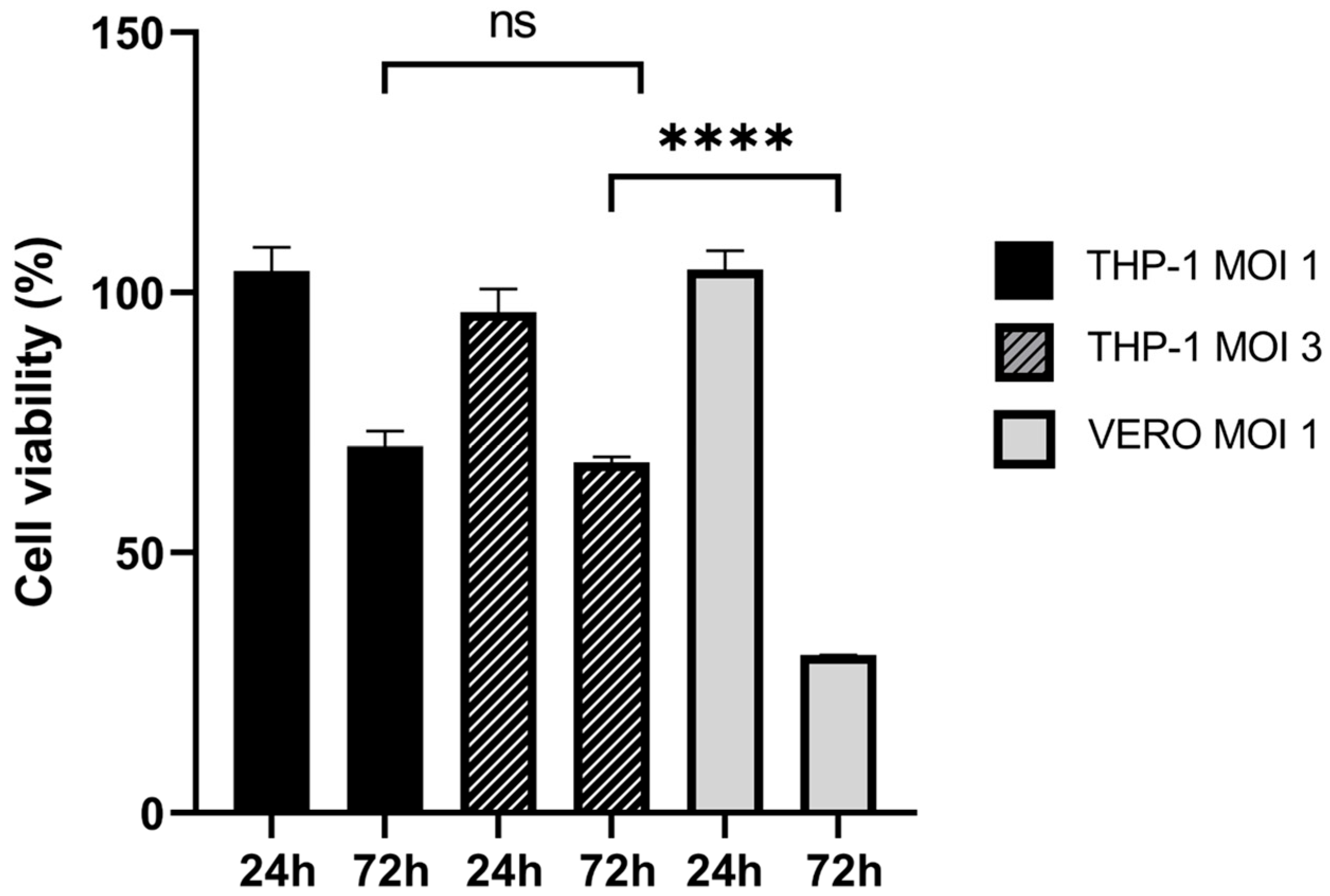
2.1. THP-1 Cells Can Be Infected by EGFP-oHSV-1
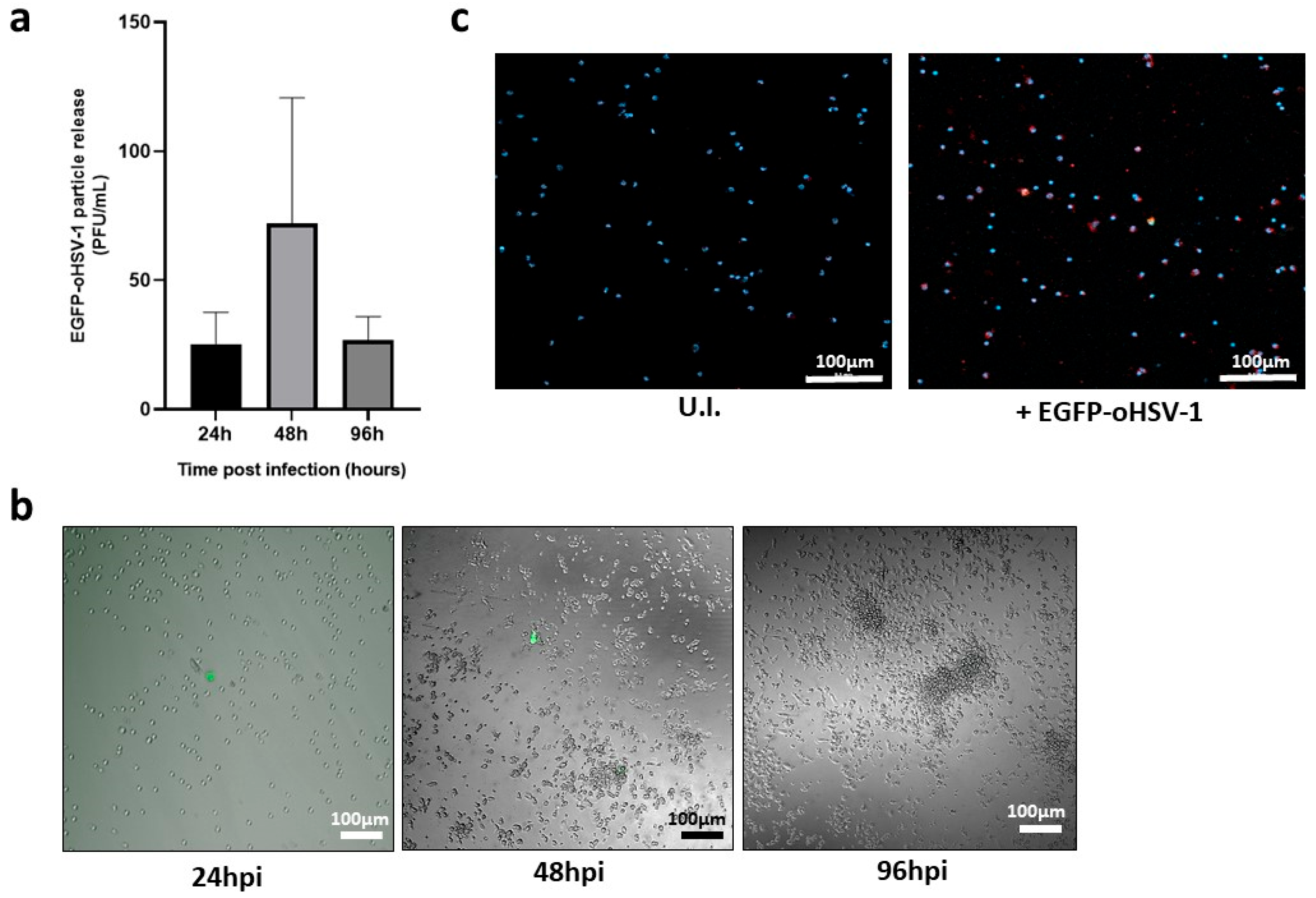
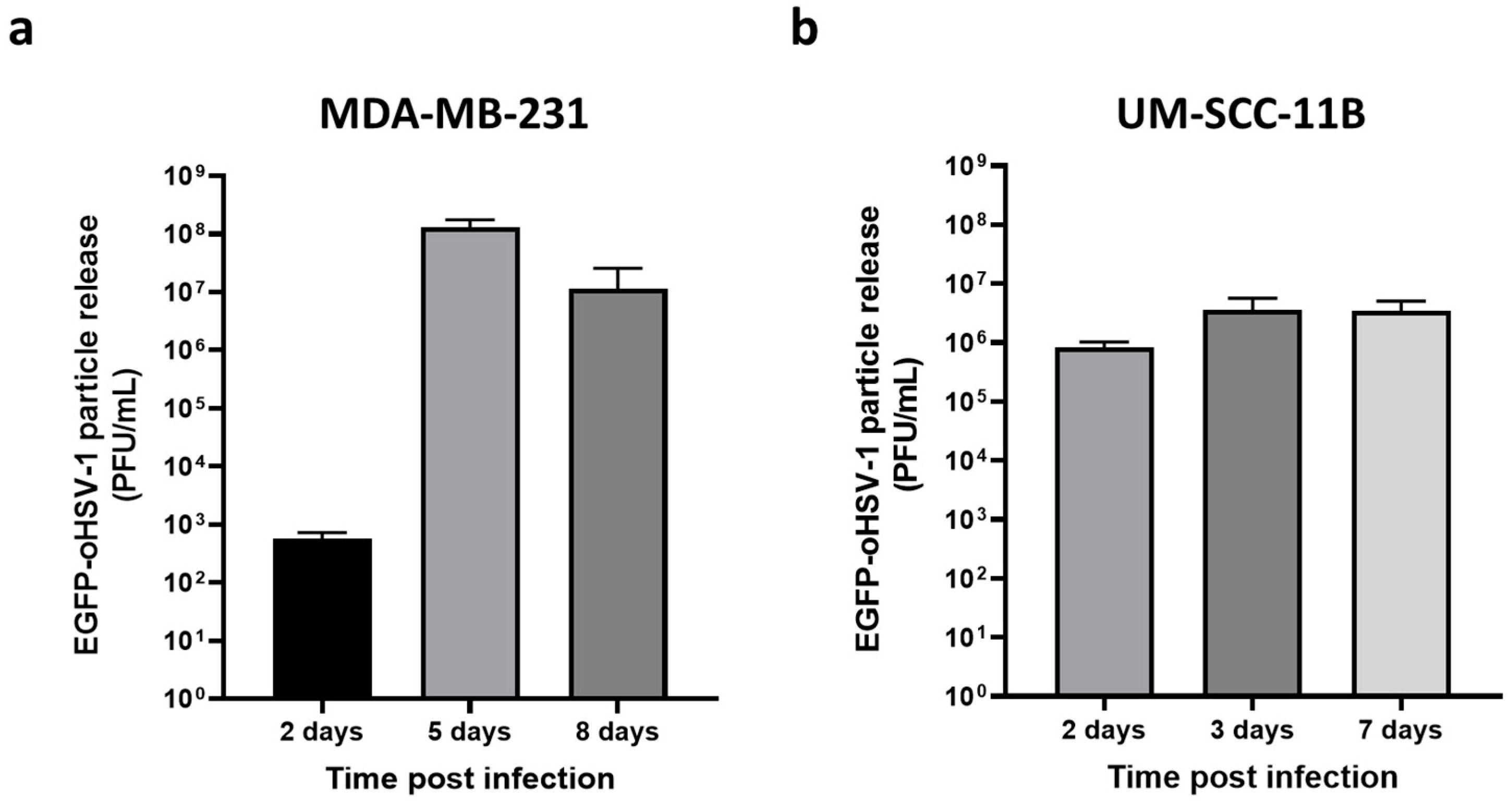
2.2. EGFP-oHSV-1 Infection Is Transmitted by Monocytes to MDA-MB-231 and UM-SCC-11B Cells in a Coculture Assay
2.3. EGFP-oHSV-1-Infected Monocytes Migrate towards Supernatants of Cancer Cells
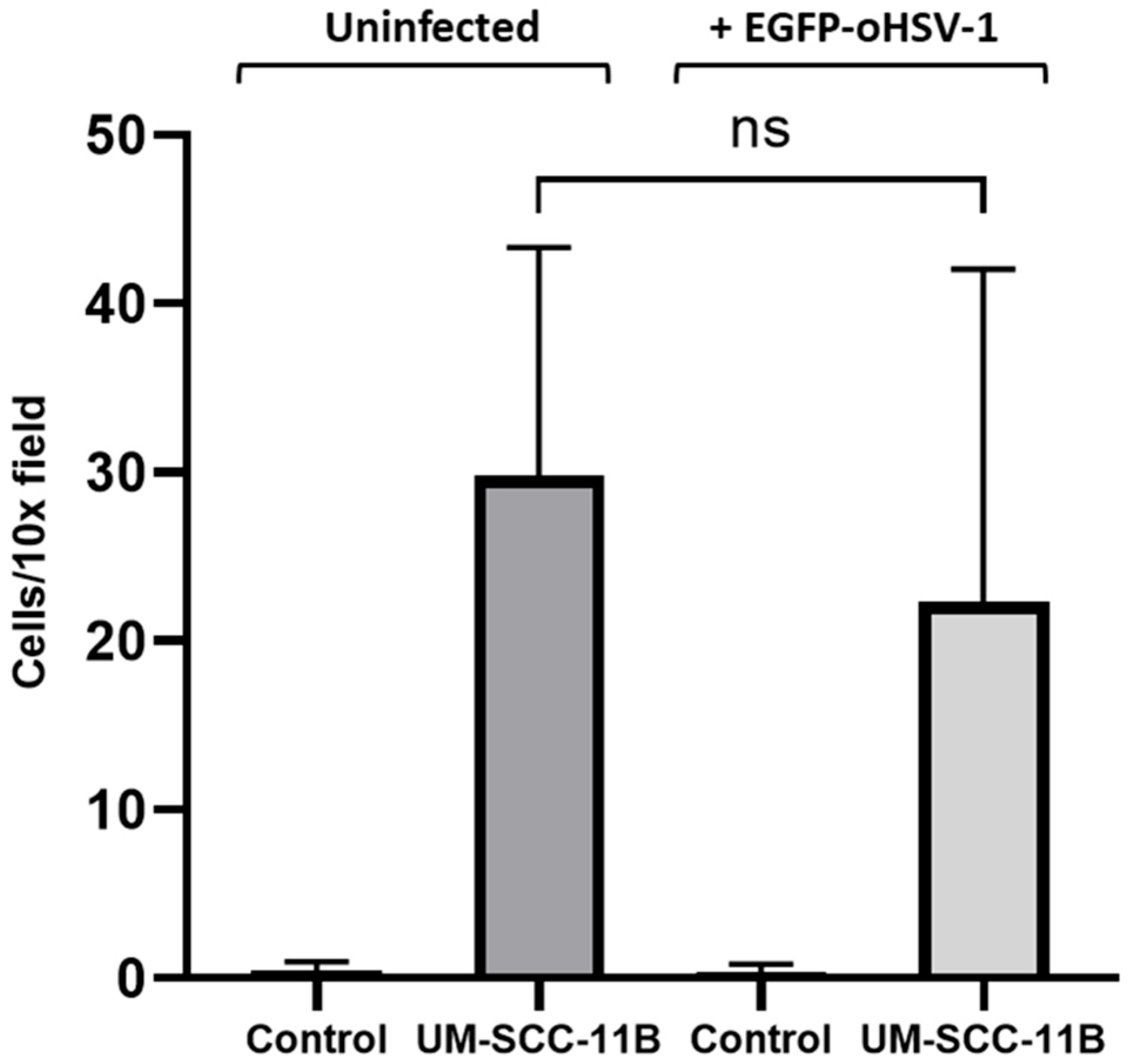
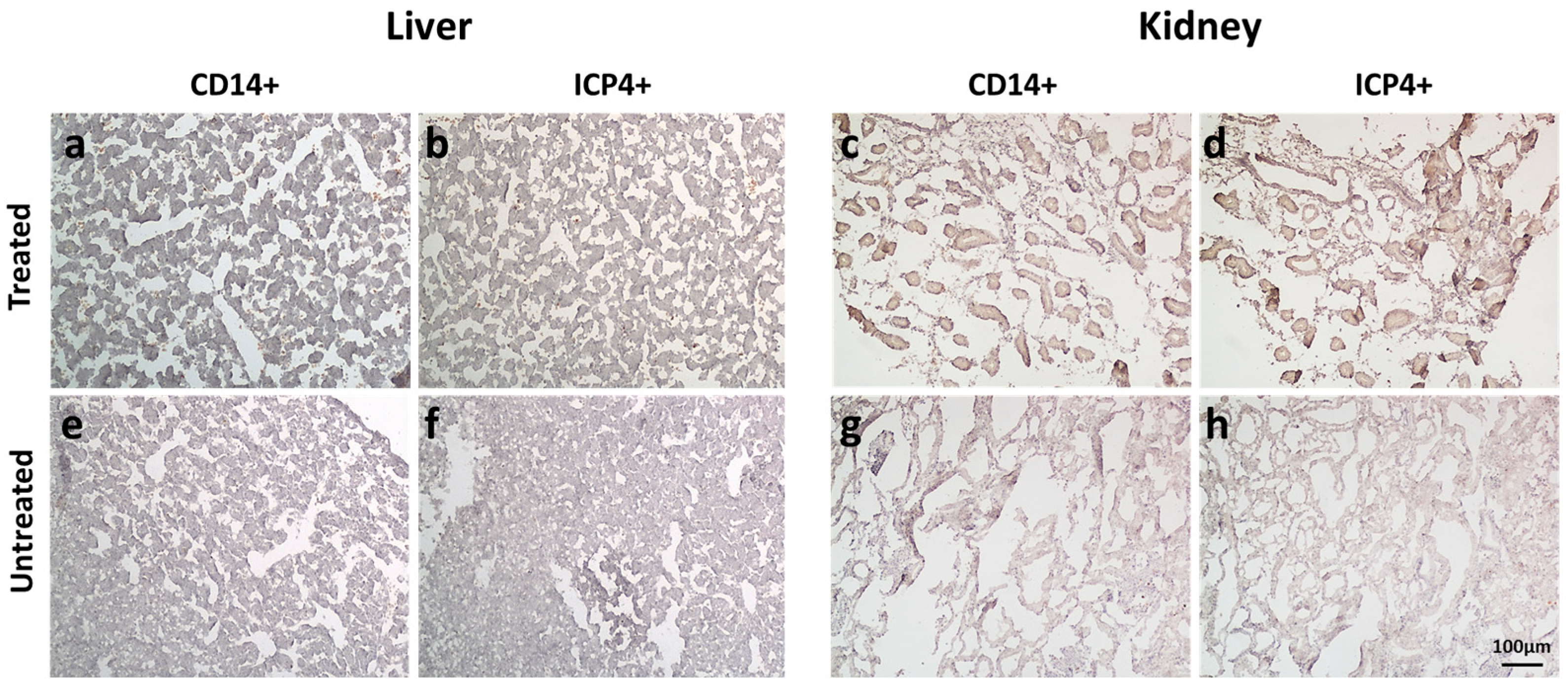
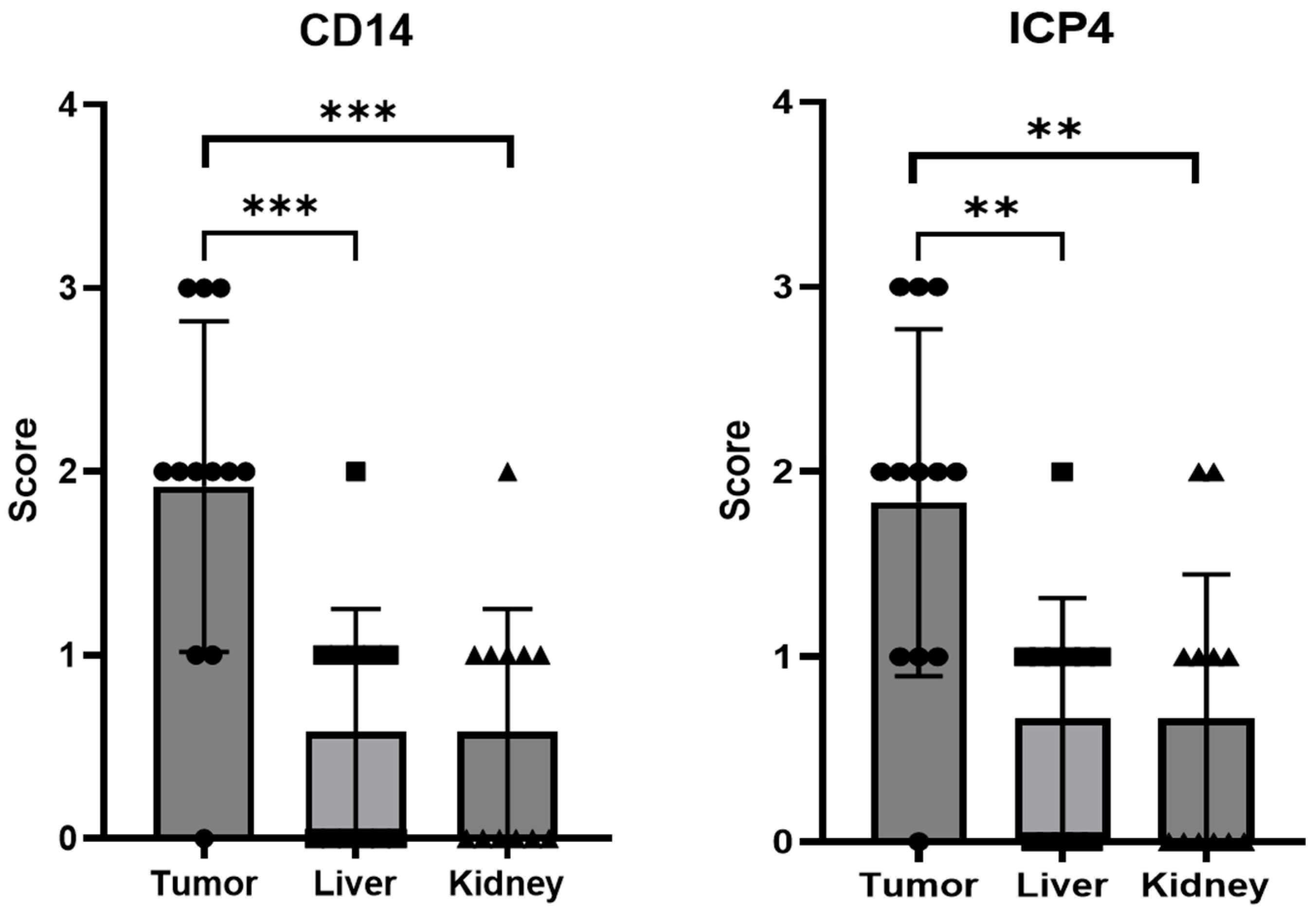
2.4. EGFP-oHSV-1-Infected THP-1 Cells Migrate Selectively towards UM-SCC-11B Tumors in a CAM Model of Head-and-Neck Cancer
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Migration Assays
4.3. Viruses
4.4. Cell Infection and Coculture Assays
4.5. Immunofluorescence of Primary Monocytes
4.6. THP-1 Cell Infection for CAM Assay
4.7. CAM Assay
4.8. Real-Time PCR Assay
4.9. Immunohistochemistry (IHC)
4.10. Immunofluorescence Staining of Tumor Section
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic Virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic Viruses: A New Class of Immunotherapy Drugs. Nat. Rev. Drug. Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Vitiello, A.; Conciatori, V.; Parolin, C.; Calistri, A.; Palù, G. Perspectives on Immunotherapy via Oncolytic Viruses. Infect. Agents Cancer 2019, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with oncolytic viruses: Progress and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Malhotra, J.; Kim, E.S. Oncolytic Viruses and Cancer Immunotherapy. Curr. Oncol. Rep. 2023, 25, 19–28. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Han, D.; Tang, B.; Ma, J. Delivery and Biosafety of Oncolytic Virotherapy. Front. Oncol. 2020, 16, 475. [Google Scholar] [CrossRef]
- Mohr, I.; Sternberg, D.; Ward, S.; Leib, D.; Mulvey, M.; Gluzman, Y. A Herpes Simplex Virus Type 1 Gamma34.5 Second-Site Suppressor Mutant That Exhibits Enhanced Growth in Cultured Glioblastoma Cells Is Severely Attenuated in Animals. J. Virol. 2001, 75, 5189–5196. [Google Scholar] [CrossRef]
- He, B.; Gross, M.; Roizman, B. The γ134.5 Protein of Herpes Simplex Virus 1 Complexes with Protein Phosphatase 1α to Dephosphorylate the α Subunit of the Eukaryotic Translation Initiation Factor 2 and Preclude the Shutoff of Protein Synthesis by Double-Stranded RNA-Activated Protein Kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 843. [Google Scholar]
- Verpooten, D.; Yijie, M.; Hou, S.; Yan, Z.; He, B. Control of TANK-Binding Kinase 1-Mediated Signaling by the γ34.5 Protein of Herpes Simplex Virus 1. J. Biol. Chem. 2009, 284, 1097. [Google Scholar] [CrossRef]
- Orvedahl, A.; Alexander, D.; Tallóczy, Z.; Sun, Q.; Wei, Y.; Zhang, W.; Burns, D.; Leib, D.A.; Levine, B. HSV-1 ICP34.5 Confers Neurovirulence by Targeting the Beclin 1 Autophagy Protein. Cell Host Microbe 2007, 1, 23–35. [Google Scholar] [CrossRef]
- Wang, P.; Kan, Q.; Yu, Z.; Li, L.; Zhang, Z.; Pan, X.; Feng, T. Recombinant Adenovirus Expressing ICP47 Gene Suppresses the Ability of Dendritic Cells by Restricting Specific T Cell Responses. Cell. Immunol. 2013, 282, 129–135. [Google Scholar] [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 Deleted Herpes Simplex Virus with Enhanced Oncolytic, Immune Stimulating, and Anti-Tumour Properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene Laherparepvec: First in Class Oncolytic Virotherapy. Hum. Vaccines Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ferguson, M.S.; Lemoine, N.R.; Wang, Y. Systemic Delivery of Oncolytic Viruses: Hopes and Hurdles. Adv. Virol. 2012, 2012, 805629. [Google Scholar] [CrossRef]
- Bradley, H.; Markowitz, L.E.; Gibson, T.; McQuillan, G.M. Seroprevalence of Herpes Simplex Virus Types 1 and 2—United States, 1999–2010. J. Infect. Dis. 2014, 209, 325–333. [Google Scholar] [CrossRef]
- Hadryś, A.; Sochanik, A.; McFadden, G.; Jazowiecka-Rakus, J. Mesenchymal Stem Cells as Carriers for Systemic Delivery of Oncolytic Viruses. Eur. J. Pharmacol. 2020, 874, 172991. [Google Scholar] [CrossRef]
- Reale, A.; Calistri, A.; Altomonte, J. Giving Oncolytic Viruses a Free Ride: Carrier Cells for Oncolytic Virotherapy. Pharmaceutics 2021, 13, 2192. [Google Scholar] [CrossRef]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef]
- Laviron, M.; Boissonnas, A. Ontogeny of Tumor-Associated Macrophages. Front. Immunol. 2019, 10, 1799. [Google Scholar] [CrossRef]
- Stakheyeva, M.; Patysheva, M.; Kaigorodova, E.; Zavyalova, M.; Tarabanovskaya, N.; Choynzonov, E.; Cherdyntseva, N. Tumor Properties Mediate the Relationship Between Peripheral Blood Monocytes and Tumor-Associated Macrophages in Breast Cancer. Cancer Investig. 2022, 40, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Zhou, W.; Bao, S. Reciprocal Supportive Interplay between Glioblastoma and Tumor-Associated Macrophages. Cancers 2014, 6, 723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.T.; Knops, A.; Swendseid, B.; Martinez-Outschoom, U.; Harshyne, L.; Philp, N.; Rodeck, U.; Luginbuhl, A.; Cognetti, D.; Johnson, J.; et al. Prognostic Significance of Tumor-Associated Macrophage Content in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Front. Oncol. 2019, 9, 656. [Google Scholar] [CrossRef]
- Hao, Q.; Vadgama, J.V.; Wang, P. CCL2/CCR2 Signaling in Cancer Pathogenesis. Cell Commun. Signal. 2020, 18, 82. [Google Scholar] [CrossRef]
- Kranjc, M.K.; Novak, M.; Pestell, R.G.; Lah, T.T. Cytokine CCL5 and Receptor CCR5 Axis in Glioblastoma Multiforme. Radiol. Oncol. 2019, 53, 397–406. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, M.; Zhang, Y.; Ge, S.; Zhong, F.; Xia, G.; Sun, C. Tumor-Associated Macrophages: A Potential Target for Cancer Therapy. Front. Oncol. 2021, 11, 693517. [Google Scholar] [CrossRef]
- Vonderheide, R.H.; Bear, A.S. Tumor-Derived Myeloid Cell Chemoattractants and T Cell Exclusion in Pancreatic Cancer. Front. Immunol. 2020, 11, 605619. [Google Scholar] [CrossRef]
- Bunuales, M.; Garcia-Aragoncillo, E.; Casado, R.; Quetglas, J.I.; Hervas-Stubbs, S.; Bortolanza, S.; Benavides-Vallve, C.; Ortiz-De-Solorzano, C.; Prieto, J.; Hernandez-Alcoceba, R. Evaluation of Monocytes as Carriers for Armed Oncolytic Adenoviruses in Murine and Syrian Hamster Models of Cancer. Hum. Gene Ther. 2012, 23, 1258–1268. [Google Scholar] [CrossRef]
- Iscaro, A.; Jones, C.; Forbes, N.; Mughal, A.; Howard, F.N.; Janabi, H.A.; Demiral, S.; Perrie, Y.; Essand, M.; Weglarz, A.; et al. Targeting circulating monocytes with CCL2-loaded liposomes armed with an oncolytic adenovirus. Nanomedicine 2022, 40, 102506. [Google Scholar] [CrossRef]
- Peng, K.W.; Dogan, A.; Vrana, J.; Liu, C.; Ong, H.T.; Kumar, S.; Dispenzieri, A.; Dietz, A.B.; Russell, S.J. Tumor-Associated Macrophages Infiltrate Plasmacytomas and Can Serve as Cell Carriers for Oncolytic Measles Virotherapy of Disseminated Myeloma. Am. J. Hematol. 2009, 84, 401–407. [Google Scholar] [CrossRef]
- Krutzke, L.; Allmendinger, E.; Hirt, K.; Kochanek, S. Chorioallantoic Membrane Tumor Model for Evaluating Oncolytic Viruses. Hum. Gene Ther. 2020, 31, 1100–1113. [Google Scholar] [CrossRef]
- Durupt, F.; Koppers-Lalic, D.; Balme, B.; Budel, L.; Terrier, O.; Lina, B.; Thomas, L.; Hoeben, R.C.; Rosa-Calatrava, M. The Chicken Chorioallantoic Membrane Tumor Assay as Model for Qualitative Testing of Oncolytic Adenoviruses. Cancer Gene Ther. 2012, 19, 58–68. [Google Scholar] [CrossRef]
- Ribatti, D. The Chick Embryo Chorioallantoic Membrane (CAM). A Multifaceted Experimental Model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Garcia, P.; Wang, Y.; Viallet, J.; Macek Jilkova, Z. The Chicken Embryo Model: A Novel and Relevant Model for Immune-Based Studies. Front. Immunol. 2021, 12, 791081. [Google Scholar] [CrossRef]
- Lester, J.T.; DeLuca, N.A. Herpes Simplex Virus 1 ICP4 Forms Complexes with TFIID and Mediator in Virus-Infected Cells. J. Virol. 2011, 85, 5733–5744. [Google Scholar] [CrossRef]
- Morahan, P.S.; Mama, S.; Anaraki, F.; Leary, K. Molecular Localization of Abortive Infection of Resident Peritoneal Macrophages by Herpes Simplex Virus Type 1. J. Virol. 1989, 63, 2300–2307. [Google Scholar] [CrossRef]
- Munir, M.T.; Kay, M.K.; Kang, M.H.; Rahman, M.M.; Al-Harrasi, A.; Choudhury, M.; Moustaid-Moussa, N.; Hussain, F.; Rahman, S.M. Tumor-Associated Macrophages as Multifaceted Regulators of Breast Tumor Growth. Int. J. Mol. Sci. 2021, 22, 6526. [Google Scholar] [CrossRef]
- Lee, D.H.; Ghiasi, H. Roles of M1 and M2 Macrophages in Herpes Simplex Virus 1 Infectivity. J. Virol. 2017, 91, e00578-17. [Google Scholar] [CrossRef]
- Nowak, M.; Klink, M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells 2020, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, F.; Lonardi, S.; Bernardini, G.; Telfer, B.; Mandelli, G.E.; Santucci, A.; Vermi, W.; Giurisato, E. Tumor-Associated Macrophages in Osteosarcoma: From Mechanisms to Therapy. Int. J. Mol. Sci. 2020, 21, 5207. [Google Scholar] [CrossRef] [PubMed]
- Cencini, E.; Fabbri, A.; Sicuranza, A.; Gozzetti, A.; Bocchia, M. The Role of Tumor-Associated Macrophages in Hematologic Malignancies. Cancers 2021, 13, 3597. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A.; et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res. 2017, 77, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of Tumour-Infiltrating Macrophages via CCL2/CCR2 Signalling as a Therapeutic Strategy against Hepatocellular Carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef]
- Moon, E.K.; Carpenito, C.; Sun, J.; Wang, L.C.S.; Kapoor, V.; Predina, J.; Powell, D.J.; Riley, J.L.; June, C.H.; Albelda, S.M. Expression of a Functional CCR2 Receptor Enhances Tumor Localization and Tumor Eradication by Retargeted Human T Cells Expressing a Mesothelin-Specific Chimeric Antibody Receptor. Clin. Cancer Res. 2011, 17, 4719–4730. [Google Scholar] [CrossRef]
- Bruun, T.; Kristoffersen, A.K.; Rollag, H.; Degré, M. Interaction of herpes simplex virus with mononuclear phagocytes is dependent on the differentiation stage of the cells. APMIS 1998, 10, 305–314. [Google Scholar] [CrossRef]
- Sartori, E.; Calistri, A.; Salata, C.; Del Vecchio, C.; Palù, G.; Parolin, C. Herpes simplex virus type 2 infection increases human immunodeficiency virus type 1 entry into human primary macrophages. Virol. J. 2011, 8, 166. [Google Scholar] [CrossRef]
- Nielsen, L.N.; Forchhammer, J.; Dabelsteen, E.; Jepsen, A.; Teglbjaerg, C.S.; Norrild, B. Herpes simplex virus-induced changes of the keratin type intermediate filament in rat epithelial cells. J. Gen. Virol. 1987, 68, 737–748. [Google Scholar] [CrossRef]
- Murata, T.; Goshima, F.; Nishizawa, Y.; Daikoku, T.; Takakuwa, H.; Ohtsuka, K.; Yoshikawa, T.; Nishiyama, Y. Phosphorylation of cytokeratin 17 by herpes simplex virus type 2 US3 protein kinase. Microbiol. Immunol. 2002, 46, 707–719. [Google Scholar] [CrossRef]
- Campos, P.F.; Gilbert, T.M.P. DNA Extraction from Formalin-Fixed Material. Methods Mol. Biol. 2012, 840, 81–85. [Google Scholar]
- Tischer, B.K.; von Einem, J.; Kaufer, B.; Osterrieder, N. Two-Step Red-Mediated Recombination for Versatile High-Efficiency Markerless DNA Manipulation in Escherichia Coli. Biotechniques 2006, 40, 191–197. [Google Scholar]
- Nygårdas, M.; Paavilainen, H.; Müther, N.; Nagel, C.H.; Röyttä, M.; Sodeik, B.; Hukkanen, V. A Herpes Simplex Virus-Derived Replicative Vector Expressing LIF Limits Experimental Demyelinating Disease and Modulates Autoimmunity. PLoS ONE 2013, 8, e64200. [Google Scholar] [CrossRef]
- Agarwalla, P.K.; Aghi, M.K. Oncolytic Herpes Simplex Virus Engineering and Preparation. Methods Mol. Biol. 2012, 797, 1–19. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reale, A.; Krutzke, L.; Cadamuro, M.; Vitiello, A.; von Einem, J.; Kochanek, S.; Palù, G.; Parolin, C.; Calistri, A. Human Monocytes Are Suitable Carriers for the Delivery of Oncolytic Herpes Simplex Virus Type 1 In Vitro and in a Chicken Embryo Chorioallantoic Membrane Model of Cancer. Int. J. Mol. Sci. 2023, 24, 9255. https://doi.org/10.3390/ijms24119255
Reale A, Krutzke L, Cadamuro M, Vitiello A, von Einem J, Kochanek S, Palù G, Parolin C, Calistri A. Human Monocytes Are Suitable Carriers for the Delivery of Oncolytic Herpes Simplex Virus Type 1 In Vitro and in a Chicken Embryo Chorioallantoic Membrane Model of Cancer. International Journal of Molecular Sciences. 2023; 24(11):9255. https://doi.org/10.3390/ijms24119255
Chicago/Turabian StyleReale, Alberto, Lea Krutzke, Massimiliano Cadamuro, Adriana Vitiello, Jens von Einem, Stefan Kochanek, Giorgio Palù, Cristina Parolin, and Arianna Calistri. 2023. "Human Monocytes Are Suitable Carriers for the Delivery of Oncolytic Herpes Simplex Virus Type 1 In Vitro and in a Chicken Embryo Chorioallantoic Membrane Model of Cancer" International Journal of Molecular Sciences 24, no. 11: 9255. https://doi.org/10.3390/ijms24119255
APA StyleReale, A., Krutzke, L., Cadamuro, M., Vitiello, A., von Einem, J., Kochanek, S., Palù, G., Parolin, C., & Calistri, A. (2023). Human Monocytes Are Suitable Carriers for the Delivery of Oncolytic Herpes Simplex Virus Type 1 In Vitro and in a Chicken Embryo Chorioallantoic Membrane Model of Cancer. International Journal of Molecular Sciences, 24(11), 9255. https://doi.org/10.3390/ijms24119255






