Effects of Soluble Guanylate Cyclase Stimulators and Activators on Anti-Aggregatory Signalling in Patients with Coronary Artery Spasm
Abstract
:1. Introduction
2. Methods
2.1. Patients and Control Subjects: Selection
2.2. Platelet Aggregometry
2.3. Statistical Methodology
3. Results
3.1. Subject/Patient Characteristics
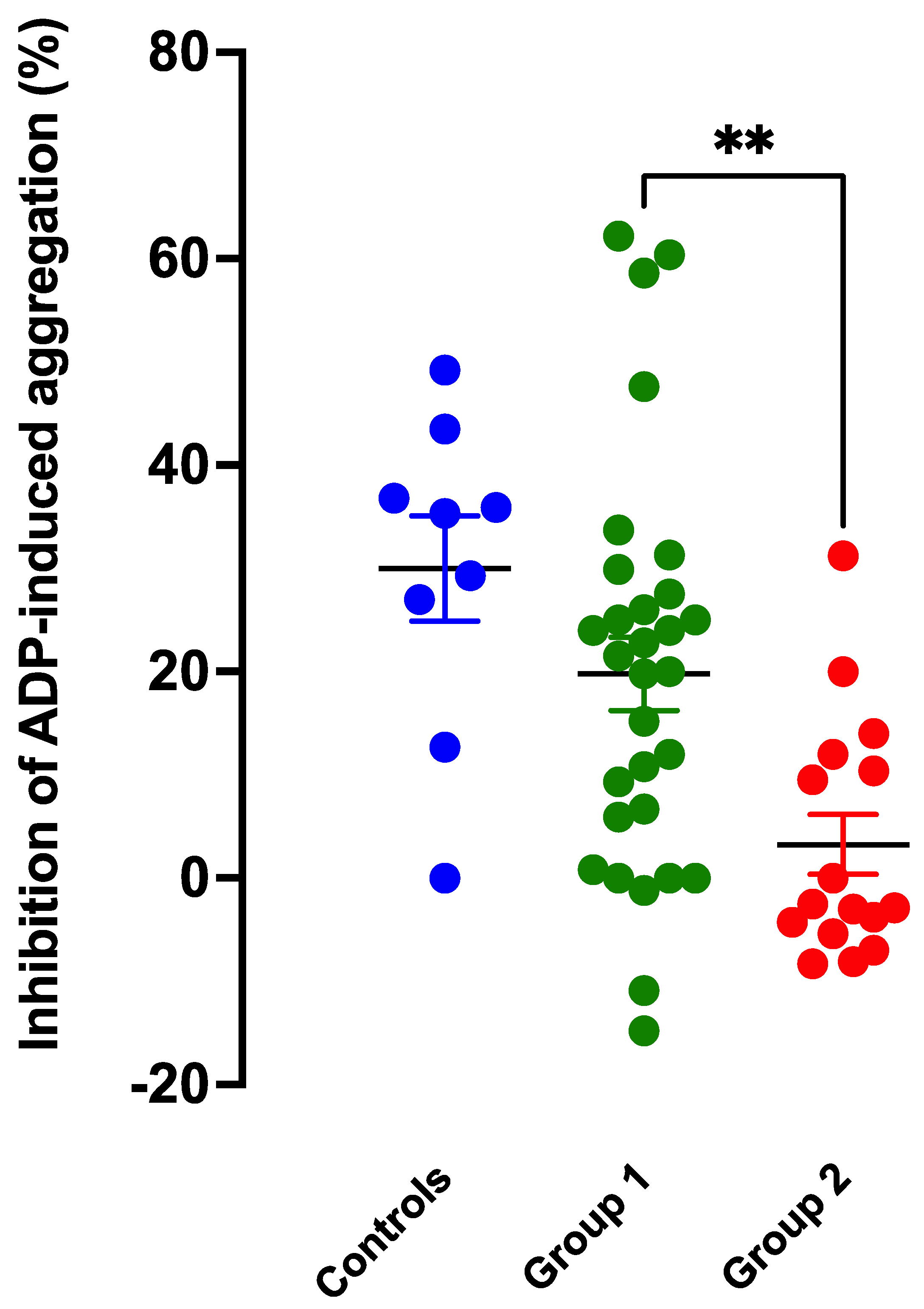
3.2. Anti-Aggregatory Effects of the NO Donor SNP
3.3. Intrinsic Anti-Aggregatory Effects of RIO and CINA
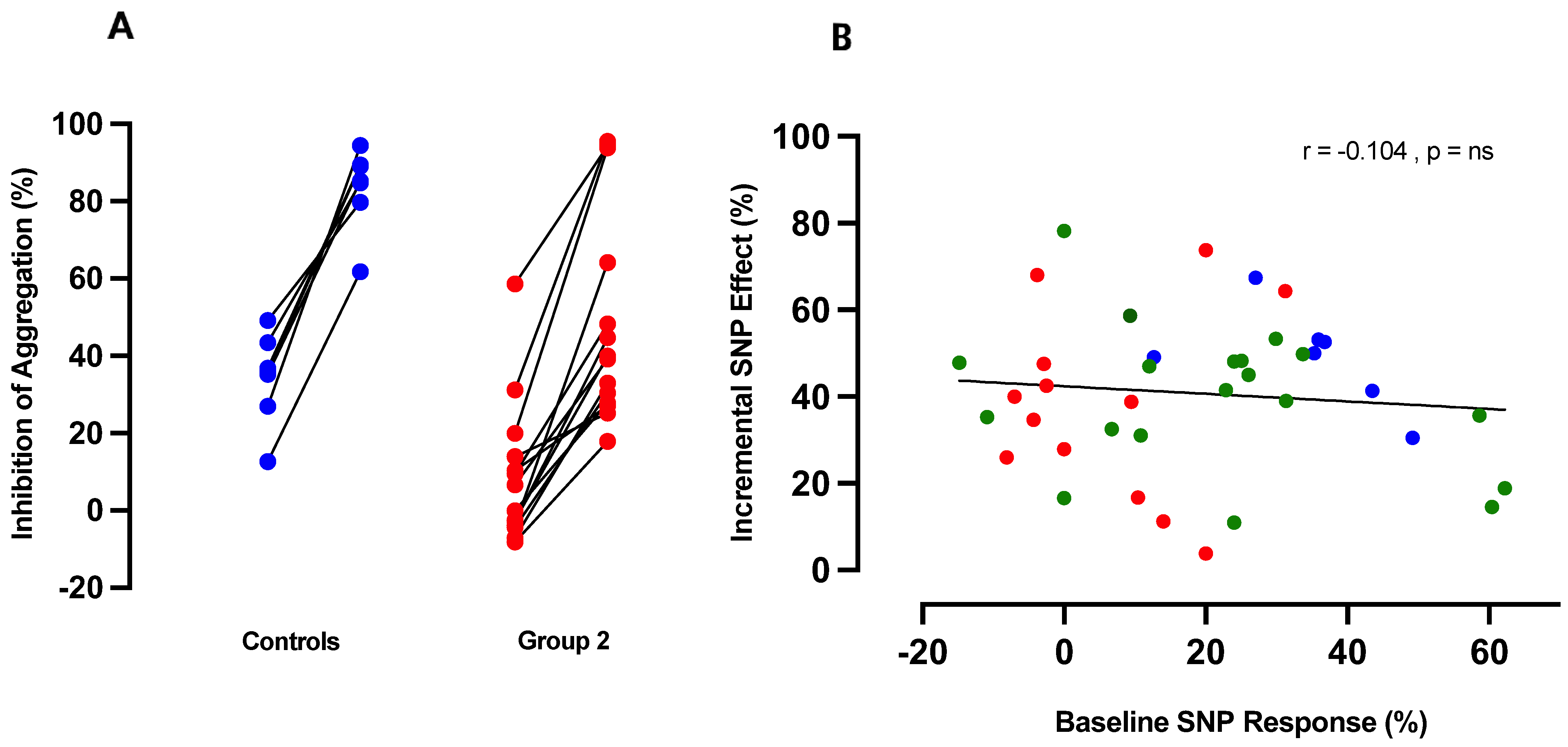
3.4. Potentiation of Anti-Aggregatory Responses to NO by RIO Is Independent of NO Resistance
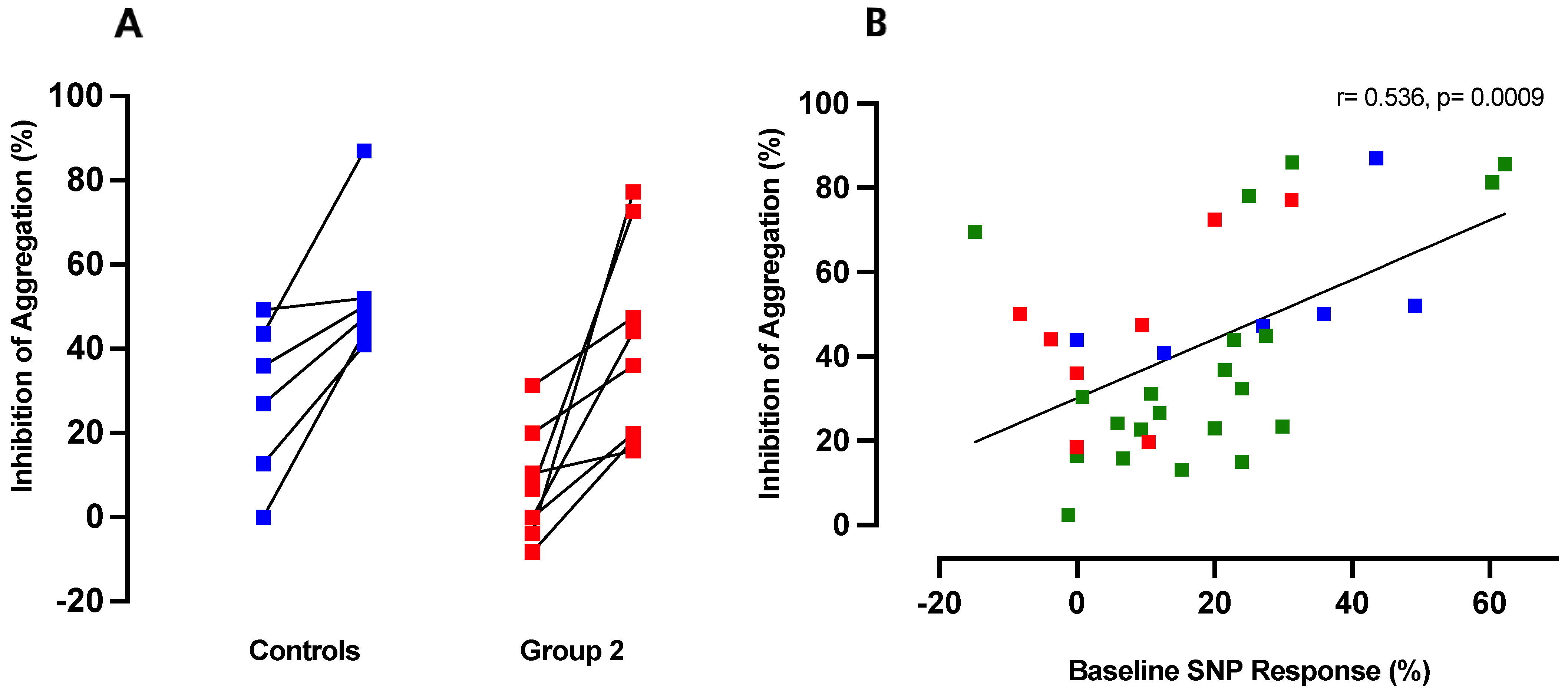
3.5. Inhibition of ADP-Induced Aggregation by CINA Is Directly Proportional to Platelet Responsiveness to NO
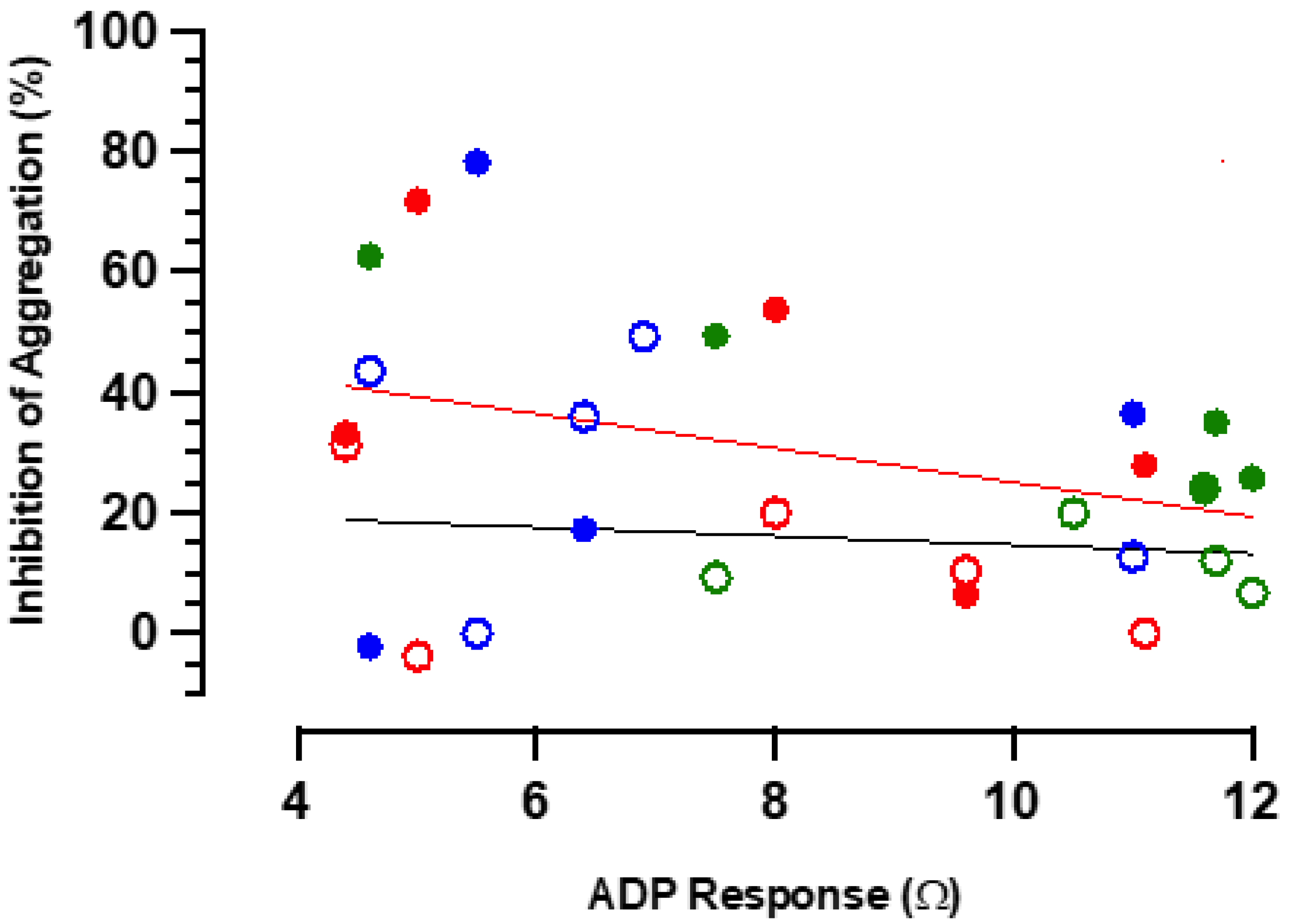
3.6. Anti-Aggregatory Responses to RIO Are Not Subject to Significant Physiological Antagonism
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godo, S.; Shimokawa, H. Endothelial Functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.F.; Martin, J.F.; Honey, A.C.; Hassall, D.G.; Beesley, J.E.; Moncada, S. Rapid development of atherosclerotic lesions in the rabbit carotid artery induced by perivascular manipulation. Atherosclerosis 1989, 76, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Gkaliagkousi, E.; Ritter, J.; Ferro, A. Platelet-derived nitric oxide signaling and regulation. Circ. Res. 2007, 101, 654–662. [Google Scholar] [CrossRef]
- Vanhoutte, P.M.; Shimokawa, H.; Feletou, M.; Tang, E.H. Endothelial dysfunction and vascular disease—A 30th anniversary update. Acta Physiol. 2017, 219, 22–96. [Google Scholar] [CrossRef]
- Vallance, P.; Leone, A.; Calver, A.; Collier, J.; Moncada, S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet 1992, 339, 572–575. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef]
- Schächinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000, 101, 1899–1906. [Google Scholar] [CrossRef]
- Worthley, M.I.; Holmes, A.S.; Willoughby, S.R.; Kucia, A.M.; Heresztyn, T.; Stewart, S.; Chirkov, Y.Y.; Zeitz, C.J.; Horowitz, J.D. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J. Am. Coll. Cardiol. 2007, 49, 304–310. [Google Scholar] [CrossRef]
- Chirkov, Y.Y.; Horowitz, J.D. Impaired tissue responsiveness to organic nitrates and nitric oxide: A new therapeutic frontier? Pharmacol. Ther. 2007, 116, 287–305. [Google Scholar] [CrossRef]
- Loscalzo, J. Antiplatelet and antithrombotic effects of organic nitrates. Am. J. Cardiol. 1992, 70, 18b–22b. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E.; Loscalzo, J.; Barnard, M.R.; Alpert, C.; Keaney, J.F.; Michelson, A.D. Nitric oxide released from activated platelets inhibits platelet recruitment. J. Clin. Investig. 1997, 100, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Ong, G.J.; Girolamo, O.C.; De Menezes Caceres, V.; Muminovic, A.; Chirkov, Y.Y.; Horowitz, J.D. Angina due to coronary artery spasm (variant angina): Diagnosis and intervention strategies. Expert Rev. Cardiovasc. Ther. 2021, 19, 917–927. [Google Scholar] [CrossRef]
- Teragawa, H.; Oshita, C.; Ueda, T. Coronary spasm: It’s common, but it’s still unsolved. World J. Cardiol. 2018, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Imam, H.; Nguyen, T.H.; Stafford, I.; Liu, S.; Heresztyn, T.; Chirkov, Y.Y.; Horowitz, J.D. Impairment of platelet NO signalling in coronary artery spasm: Role of hydrogen sulphide. Br. J. Pharmacol. 2021, 178, 1639–1650. [Google Scholar] [CrossRef]
- Chirkov, Y.Y.; Holmes, A.S.; Chirkova, L.P.; Horowitz, J.D. Nitrate resistance in platelets from patients with stable angina pectoris. Circulation 1999, 100, 129–134. [Google Scholar] [CrossRef]
- Murad, F.; Waldman, S.; Molina, C.; Bennett, B.; Leitman, D. Regulation and role of guanylate cyclase-cyclic GMP in vascular relaxation. Prog. Clin. Biol. Res. 1987, 249, 65–76. [Google Scholar]
- Sandner, P.; Zimmer, D.P.; Milne, G.T.; Follmann, M.; Hobbs, A.; Stasch, J.P. Soluble Guanylate Cyclase Stimulators and Activators. Handb. Exp. Pharmacol. 2021, 264, 355–394. [Google Scholar] [CrossRef]
- Ghofrani, H.A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Limaye, S.B.; Horowitz, J.D. The coronary slow flow phenomenon—A new coronary microvascular disorder. Cardiology 2002, 97, 197–202. [Google Scholar] [CrossRef]
- Ong, P.; Athanasiadis, A.; Borgulya, G.; Vokshi, I.; Bastiaenen, R.; Kubik, S.; Hill, S.; Schäufele, T.; Mahrholdt, H.; Kaski, J.C.; et al. Clinical usefulness, angiographic characteristics, and safety evaluation of intracoronary acetylcholine provocation testing among 921 consecutive white patients with unobstructed coronary arteries. Circulation 2014, 129, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.R.; Rajendran, S.; Chan, W.P.; Procter, N.; Leslie, S.; Liberts, E.A.; Heresztyn, T.; Chirkov, Y.Y.; Horowitz, J.D. Ramipril sensitizes platelets to nitric oxide: Implications for therapy in high-risk patients. J. Am. Coll. Cardiol. 2012, 60, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.R.; Stewart, S.; Chirkov, Y.Y.; Kennedy, J.A.; Holmes, A.S.; Horowitz, J.D. Beneficial clinical effects of perhexiline in patients with stable angina pectoris and acute coronary syndromes are associated with potentiation of platelet responsiveness to nitric oxide. Eur. Heart J. 2002, 23, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Stepien, J.M.; Prideaux, R.M.; Willoughby, S.R.; Chirkov, Y.Y.; Horowitz, J.D. Pilot study examining the effect of cholesterol lowering on platelet nitric oxide responsiveness and arterial stiffness in subjects with isolated mild hypercholesterolaemia. Clin. Exp. Pharmacol. Physiol. 2003, 30, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Procter, N.E.; Ball, J.; Ngo, D.T.; Isenberg, J.S.; Hylek, E.M.; Chirkov, Y.Y.; Stewart, S.; Horowitz, J.D. Gender and tachycardia: Independent modulation of platelet reactivity in patients with atrial fibrillation. J. Geriatr. Cardiol. 2016, 13, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Henry, P.J.; Lulich, K.M.; Paterson, J.W. Experimental testing of Mackay’s model for functional antagonism in the isolated costo-uterus of the rat. Br. J. Pharmacol. 1985, 86, 131–139. [Google Scholar] [CrossRef]
- Stasch, J.P.; Evgenov, O.V. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb. Exp. Pharmacol. 2013, 218, 279–313. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Leung, S.W.; Vanhoutte, P.M. Hypoxic Vasospasm Mediated by cIMP: When Soluble Guanylyl Cyclase Turns Bad. J. Cardiovasc. Pharmacol. 2015, 65, 545–548. [Google Scholar] [CrossRef]
- Kaski, J.-C.; Crea, F.; Gersh, B.J.; Camici, P.G. Reappraisal of Ischemic Heart Disease. Circulation 2018, 138, 1463–1480. [Google Scholar] [CrossRef]
- Hill, N.S.; Rahaghi, F.F.; Sood, N.; Frey, R.; Ghofrani, H.A. Individual dose adjustment of riociguat in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir. Med. 2017, 129, 124–129. [Google Scholar] [CrossRef]
- Paulus, W.J.; Tschöpe, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Pieske, B.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; Butler, J.; Lam, C.S.P.; Ponikowski, P.; Voors, A.A.; Jia, G.; et al. Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2020, 382, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Cullivan, S.; Murphy, C.A.; Weiss, L.; Comer, S.P.; Kevane, B.; McCullagh, B.; Maguire, P.B.; Ní Ainle, F.; Gaine, S.P. Platelets, extracellular vesicles and coagulation in pulmonary arterial hypertension. Pulm. Circ. 2021, 11, 204589402. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga-Costa, K.; Roque, C.R.; Vasconcelos-Silva, A.A.; Sousa-Brito, H.L.; Martins, C.S.; Caetano-Souza, M.M.; Duarte, G.P.; da Silva, J.K.R.; Borges, R.S.; dos Santos, A.A.; et al. The soluble guanylate cyclase stimulator, 1-nitro-2-phenylethane, reverses monocrotaline-induced pulmonary arterial hypertension in rats. Life Sci. 2021, 275, 119334. [Google Scholar] [CrossRef] [PubMed]
- Martínez Pereyra, V.; Seitz, A.; Hubert, A.; Beck, S.; Hofmann, U.; Schwab, M.; Bekeredjian, R.; Sechtem, U.; Ong, P. Repurposing Riociguat for Treatment of Refractory Angina Resulting From Coronary Spasm. JACC Case Rep. 2021, 3, 392–396. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Controls (n = 9) | Patients: Group 1 (n = 30) | Patients: Group 2 (n = 16) | p |
|---|---|---|---|---|
| Men/Women, n | 4 vs. 5 | 18 vs. 10 | 4 vs. 14 | 0.007 * |
| Age (mean/SEM) | 56 ± 8 | 74 ± 2 | 64 ± 3 | 0.006 # |
| Coronary Stenoses, n (%) | 0 | 10 (33) | 0 | 0.008 * |
| Heart Failure, n (%) | 0 | 7 (23) | 0 | 0.071 |
| AF, n (%) | 1 (11%) | 11 (36) | 1 (6) | 0.035 * |
| Diabetes, n (%) | 0 | 14 (46) | 2 (12) | 0.026 * |
| Hypertension, n (%) | 2 * | 13 (43) | 2 (12) | 0.049 * |
| Medication | ||||
| Aspirin, n (%) | 0 | 2 (6) | 1 (6) | 1.000 |
| Calcium Channel Antagonists, n (%) | 0 | 5 (16) | 9 (56) | 0.008 * |
| NAC, n (%) | 0 | 0 (0) | 4 (25) | 0.011 * |
| ACE inhibitors, n (%) | 2 (22) | 6 (20) | 2 (12) | 0.694 |
| Perhexiline, n (%) | 0 | 9 (30) | 1 (6) | 0.130 |
| Statins, n (%) | 1 | 8 (26) | 2 (12) | 0.455 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muminovic, A.; Chirkov, Y.Y.; Horowitz, J.D. Effects of Soluble Guanylate Cyclase Stimulators and Activators on Anti-Aggregatory Signalling in Patients with Coronary Artery Spasm. Int. J. Mol. Sci. 2023, 24, 9273. https://doi.org/10.3390/ijms24119273
Muminovic A, Chirkov YY, Horowitz JD. Effects of Soluble Guanylate Cyclase Stimulators and Activators on Anti-Aggregatory Signalling in Patients with Coronary Artery Spasm. International Journal of Molecular Sciences. 2023; 24(11):9273. https://doi.org/10.3390/ijms24119273
Chicago/Turabian StyleMuminovic, Armin, Yuliy Y. Chirkov, and John D. Horowitz. 2023. "Effects of Soluble Guanylate Cyclase Stimulators and Activators on Anti-Aggregatory Signalling in Patients with Coronary Artery Spasm" International Journal of Molecular Sciences 24, no. 11: 9273. https://doi.org/10.3390/ijms24119273
APA StyleMuminovic, A., Chirkov, Y. Y., & Horowitz, J. D. (2023). Effects of Soluble Guanylate Cyclase Stimulators and Activators on Anti-Aggregatory Signalling in Patients with Coronary Artery Spasm. International Journal of Molecular Sciences, 24(11), 9273. https://doi.org/10.3390/ijms24119273





