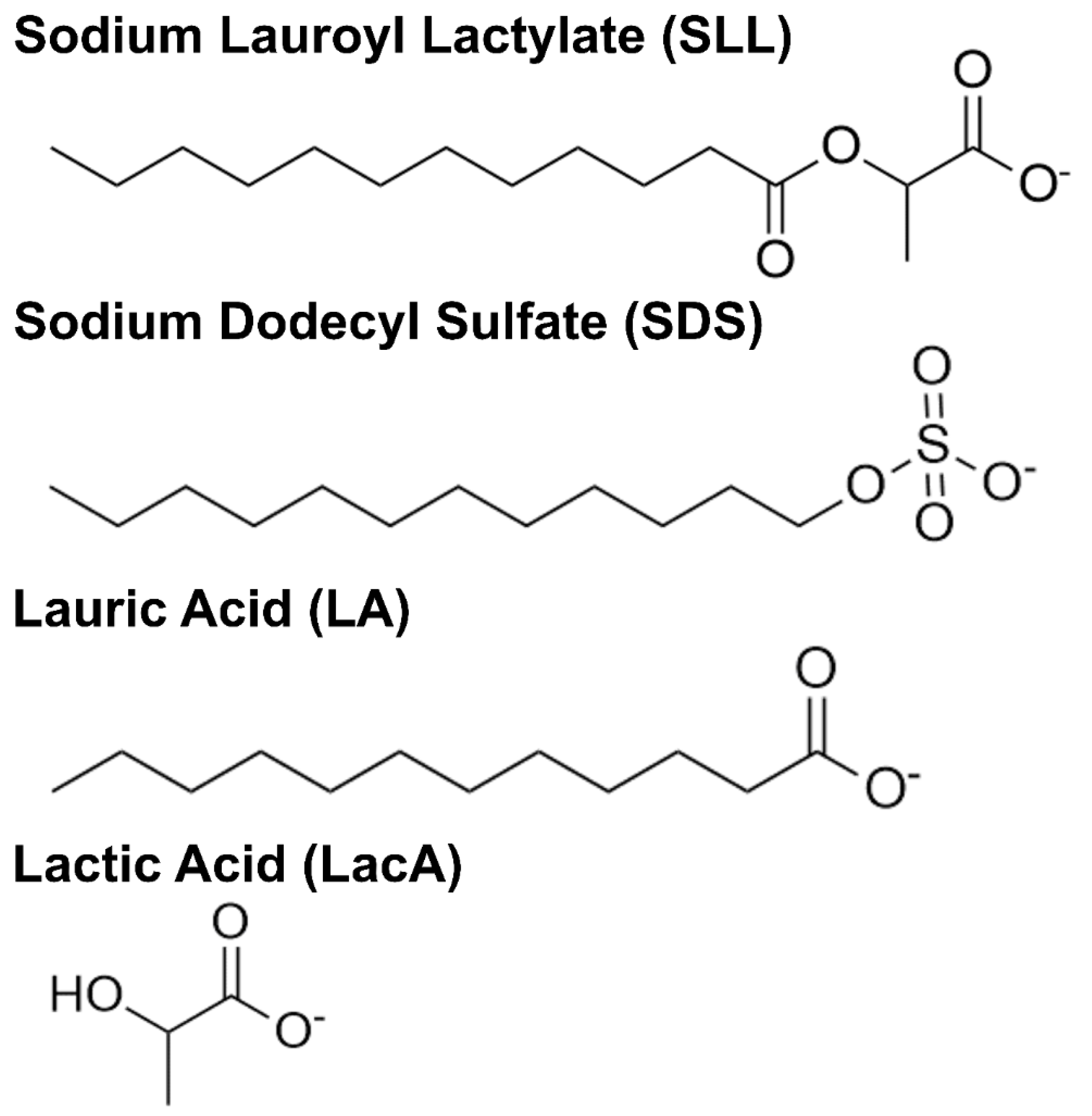
Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study
Abstract
:1. Introduction
2. Results and Discussion
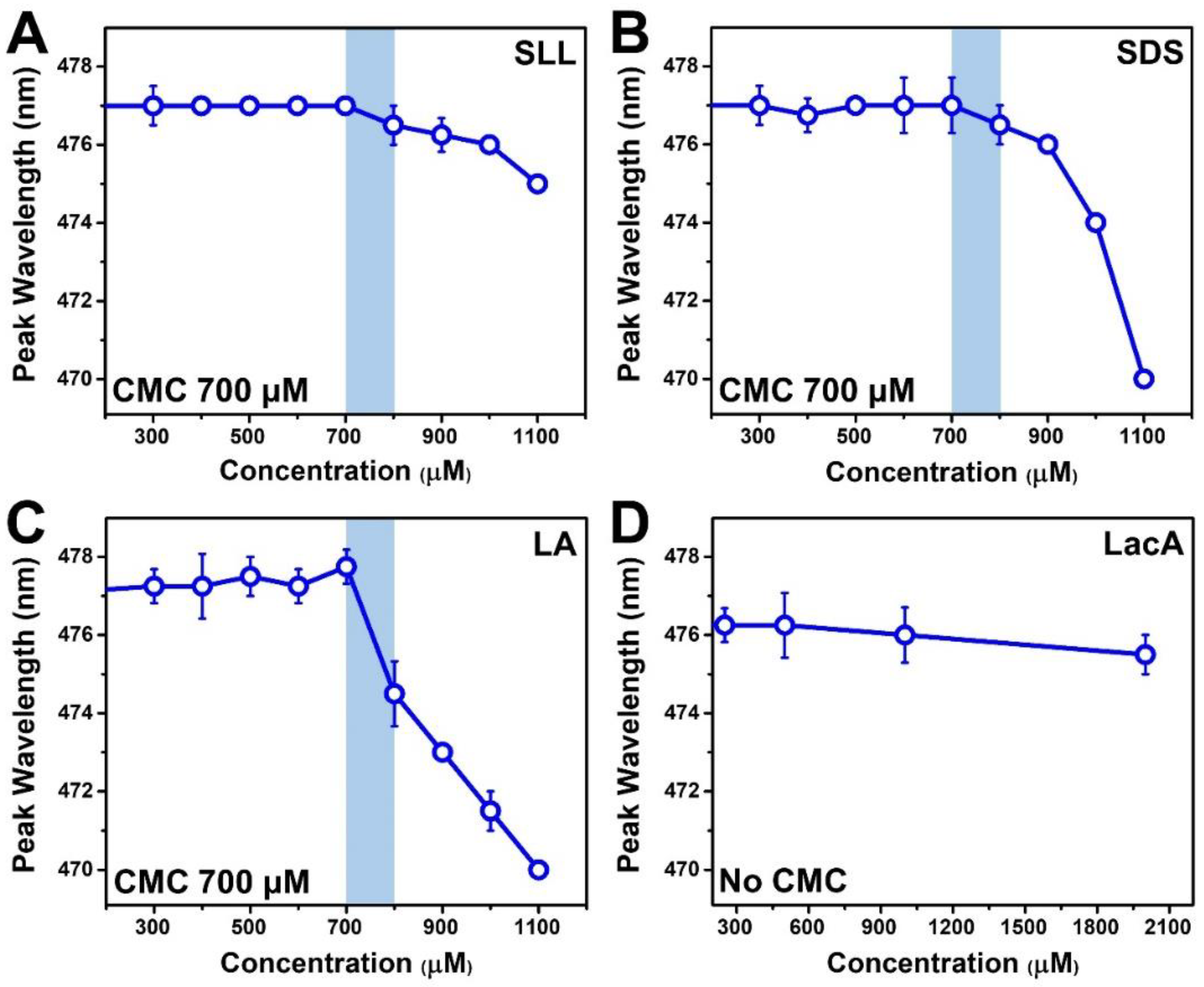
2.1. Critical Micelle Concentration Determination
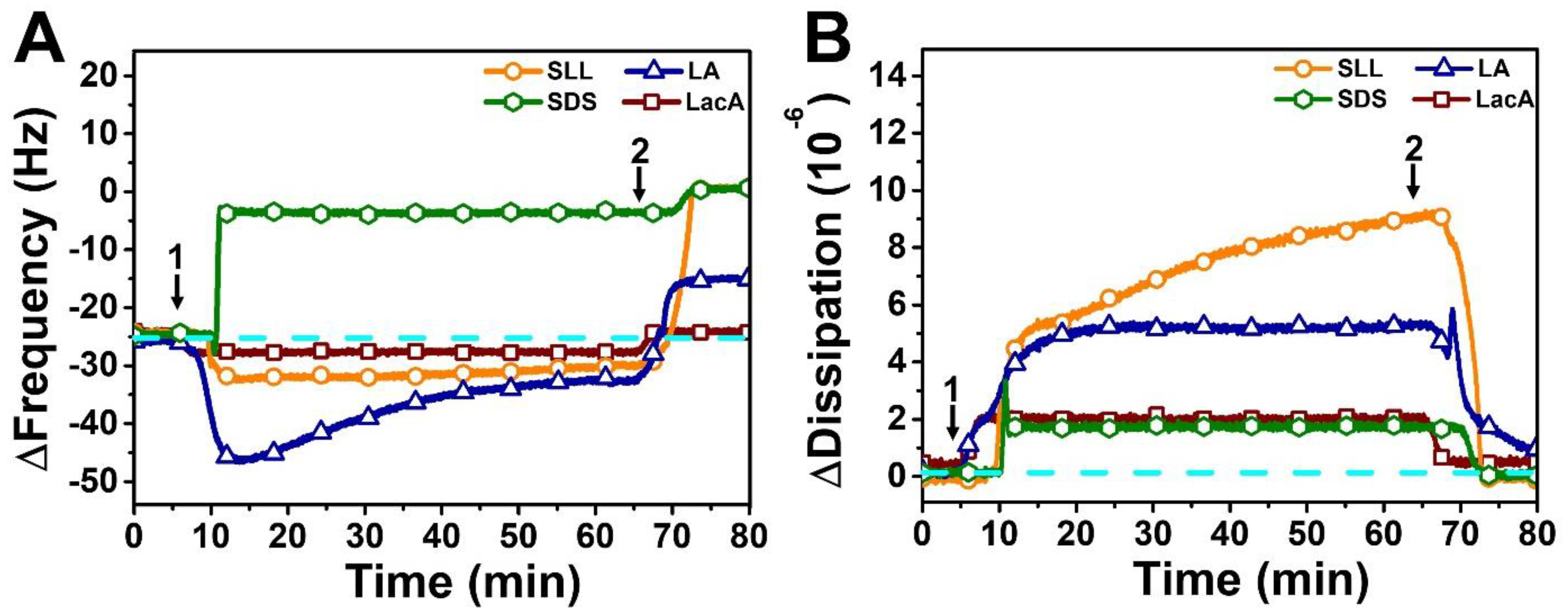
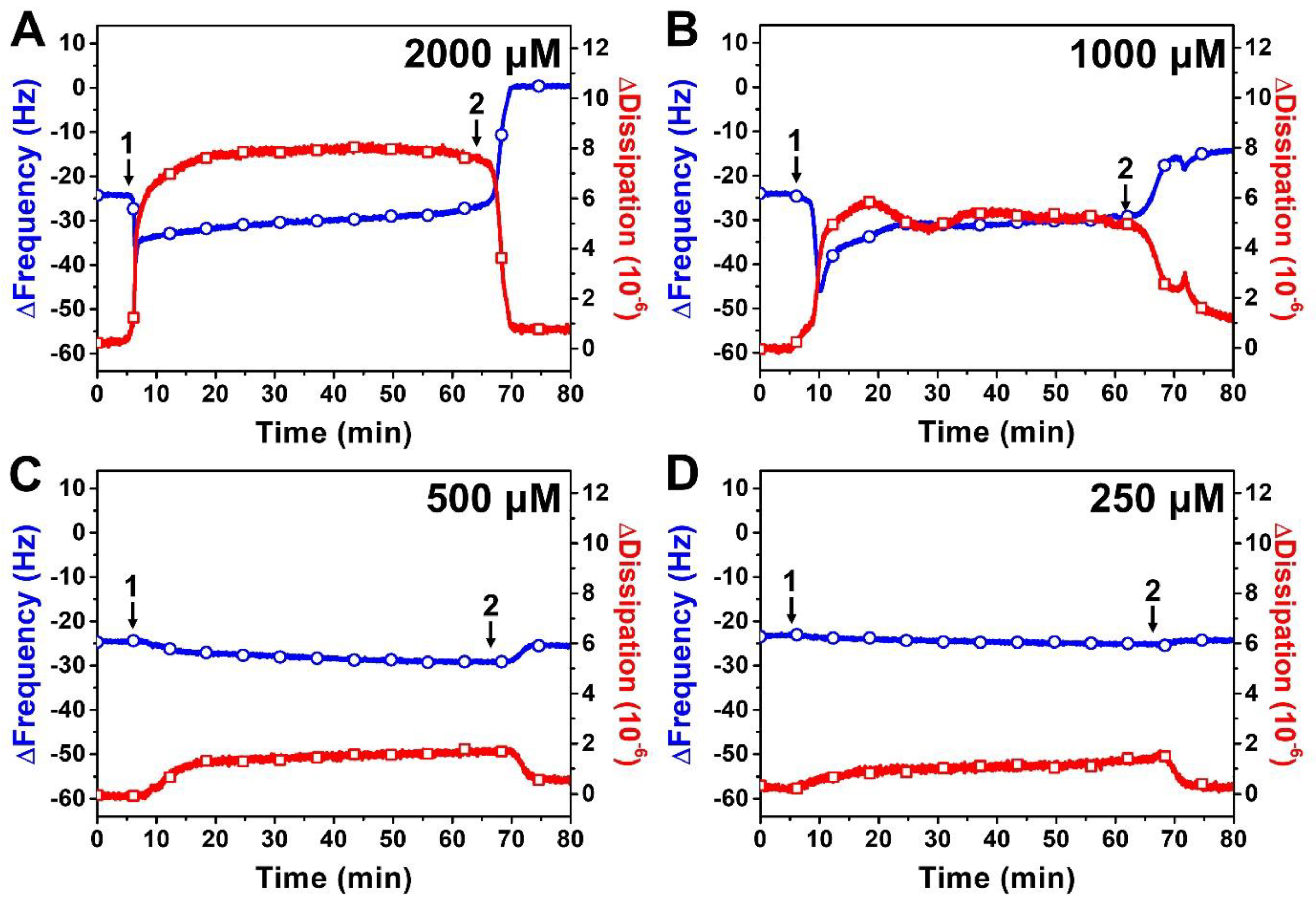
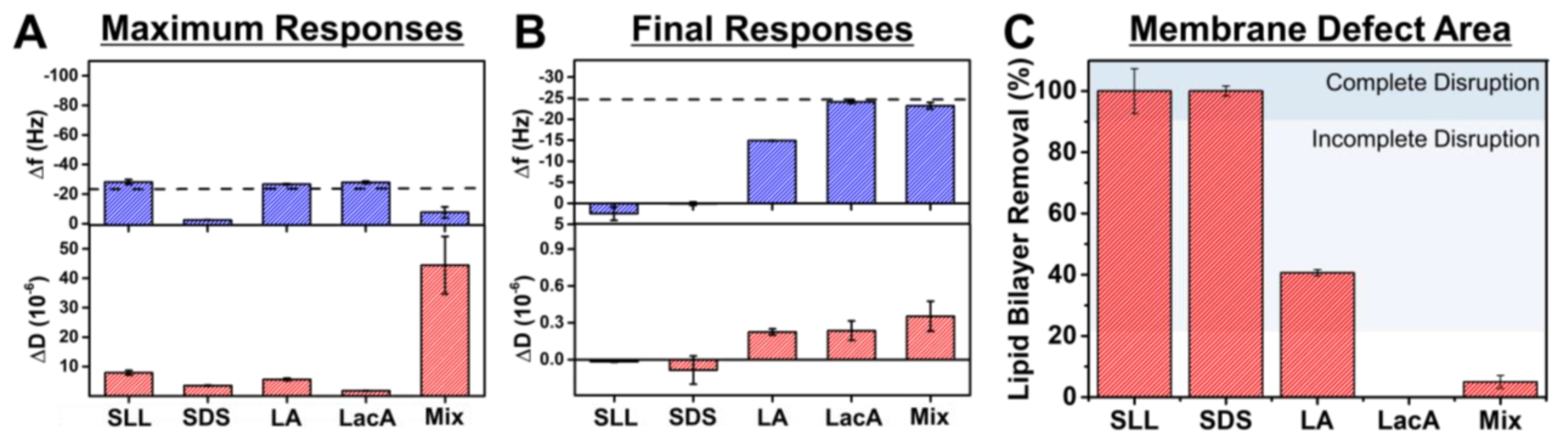
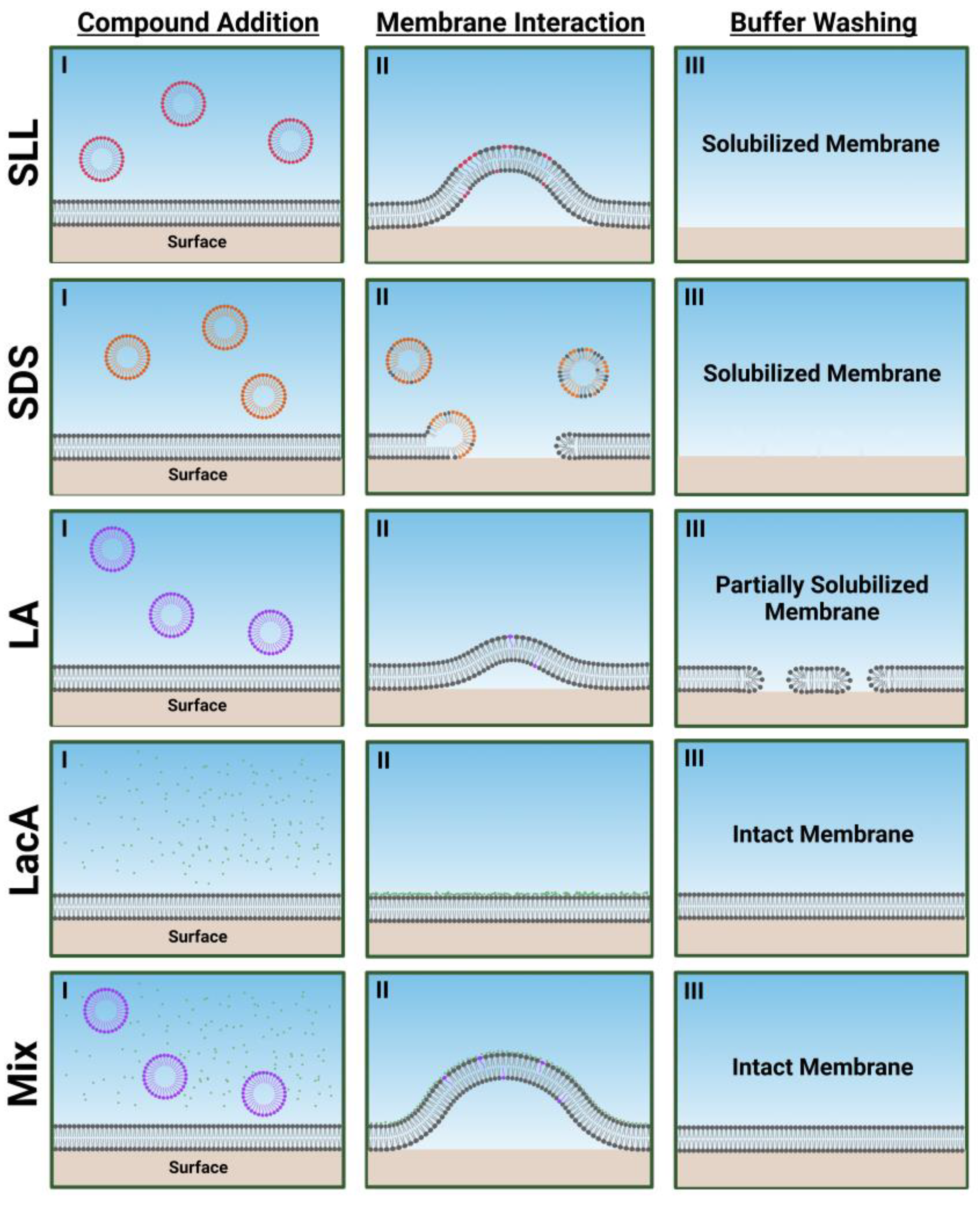
2.2. QCM-D Tracking of Phospholipid Membrane Interactions
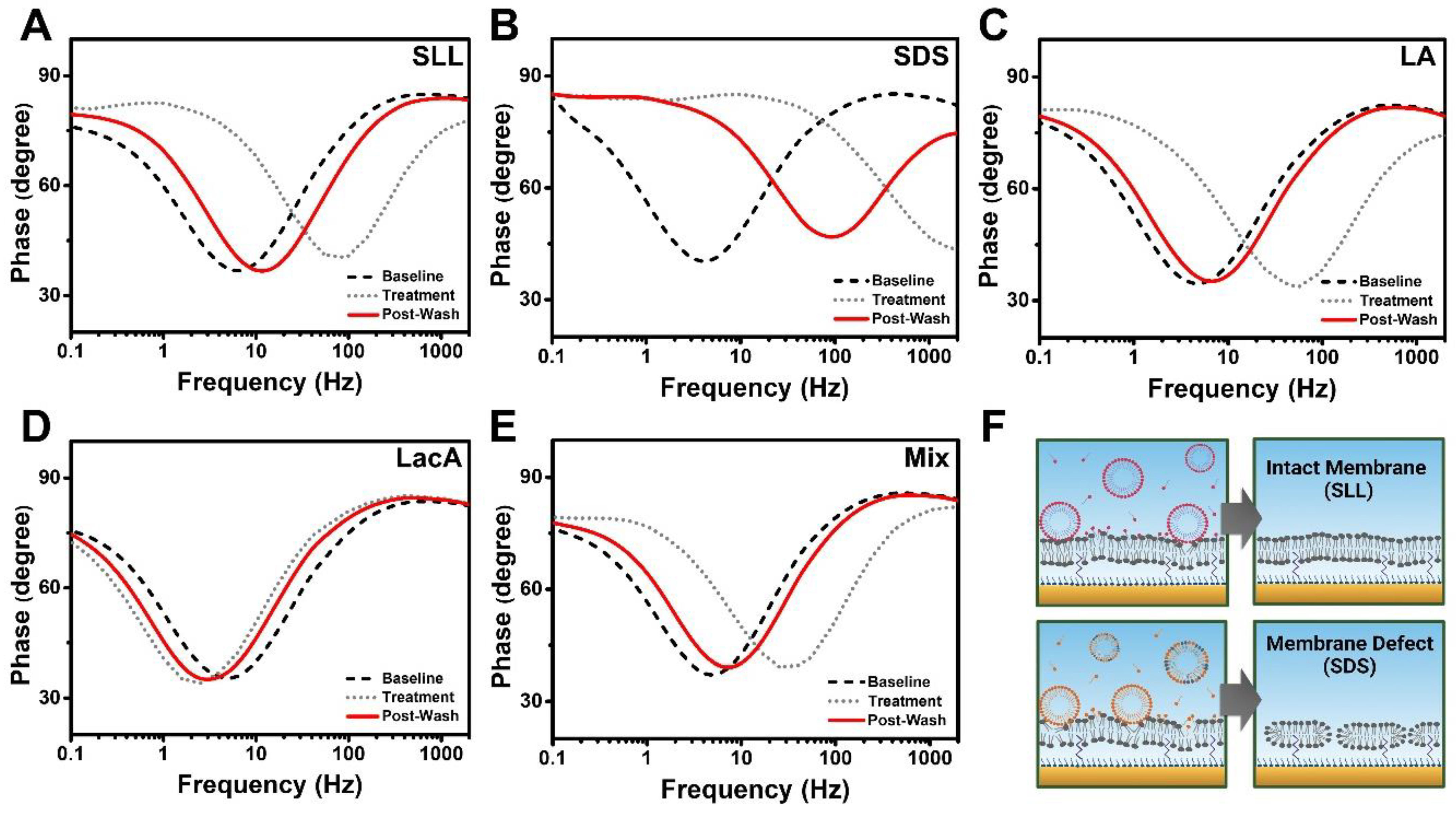
2.3. EIS Characterization of Membrane-Disruptive Interactions
3. Conclusions and Outlook
4. Materials and Methods
4.1. Reagents
4.2. Sample Preparation
4.3. Critical Micelle Concentration (CMC) Assay
4.4. Quartz Crystal Microbalance–Dissipation (QCM-D)
4.5. Electrochemical Impedance Spectroscopy (EIS)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Decuypere, J.; Dierick, N. The combined use of triacylglycerols containing medium-chain fatty acids and exogenous lipolytic enzymes as an alternative to in-feed antibiotics in piglets: Concept, possibilities and limitations. An overview. Nutr. Res. Rev. 2003, 16, 193–210. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Bacterial membrane lipids in the action of antimicrobial agents. J. Pept. Sci. 2011, 17, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B. Rationalizing the membrane interactions of cationic amphipathic antimicrobial peptides by their molecular shape. Curr. Opin. Colloid. Interface Sci. 2009, 14, 349–355. [Google Scholar] [CrossRef]
- Quinn, P. Membranes as targets of antimicrobial lipids. In Lipid and Essential Oils as Antimicrobial Agents, 1st ed.; Thormar, H., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 1–24. [Google Scholar]
- Jackman, J.A.; Hakobyan, A.; Zakaryan, H.; Elrod, C.C. Inhibition of African swine fever virus in liquid and feed by medium-chain fatty acids and glycerol monolaurate. J. Anim. Sci. Biotechnol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Valle-González, E.R.; Cho, N.-J. Antibacterial free fatty acids and monoglycerides: Biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef]
- Churchward, C.P.; Alany, R.G.; Snyder, L.A. Alternative antimicrobials: The properties of fatty acids and monoglycerides. Crit. Rev. Microbiol. 2018, 44, 561–570. [Google Scholar] [CrossRef]
- Gahan, C.G.; Patel, S.J.; Chen, L.M.; Manson, D.E.; Ehmer, Z.J.; Blackwell, H.E.; Van Lehn, R.C.; Lynn, D.M.J.L. Bacterial quorum sensing signals promote large-scale remodeling of lipid membranes. Langmuir 2021, 37, 9120–9136. [Google Scholar] [CrossRef]
- Gooran, N.; Yoon, B.K.; Jackman, J.A. Supported lipid bilayer platform for characterizing the membrane-disruptive behaviors of Triton X-100 and potential detergent replacements. Int. J. Mol. Sci. 2022, 23, 869. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, J.; Yang, X.; Bai, L.; Zhou, Y.; Wu, Z.; Qin, Z. Synthesis and properties of sodium fatty acyl lactylates. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 129946. [Google Scholar] [CrossRef]
- Sidling, D.R.; Anunson, P.N.; Kanningem, K.T. Antimicrobial Composition Containing Acyl Lactylate and Glycol and Methods of Microbial Growth Suppression through Application Thereof. (Russia Patent No. RU 2749675 C2) The Federal Service for Intellectual Property. 2021. Available online: https://www.elibrary.ru/item.asp?id=46315856 (accessed on 15 January 2023).
- Wang, X.; Tsai, T.; Wei, X.; Zuo, B.; Davis, E.; Rehberger, T.; Hernandez, S.; Jochems, E.J.; Maxwell, C.V.; Zhao, J. Effect of lactylate and Bacillus subtilis on growth performance, peripheral blood cell profile, and gut microbiota of nursery pigs. Microorganisms 2021, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- Lensing, M.; Van der Klis, J.; Fabri, T.; Cazemier, A.; Else, A.J. Efficacy of a lactylate on production performance and intestinal health of broilers during a subclinical Clostridium perfringens infection. Poult. Sci. 2010, 89, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Boutte, T.; Skogerson, L. Stearoyl-2-lactylates and oleoyl lactylates. In Emulsifiers in Food Technology, 1st ed.; Norn, V., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 251–270. [Google Scholar]
- Qin, Z.; Zhang, J.; Zhou, Y.; Yang, X. Study on the properties of sodium lauroyl lactate anionic surfactant and cationic surfactant in a mixed system. J. Mol. Liq. 2022, 352, 118544. [Google Scholar] [CrossRef]
- Sguizzato, M.; Pula, W.; Bordin, A.; Pagnoni, A.; Drechsler, M.; Marvelli, L.; Cortesi, R. Manganese in diagnostics: A preformulatory study. Pharmaceutics 2022, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Topp, C.; Gangolli, S. Studies on the metabolism of calcium stearoyl-2-lactylate in the rat, mouse, guinea-pig and man. Food. Cosmet. Toxicol. 1981, 19, 7–11. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Park, S.; Mokrzecka, N.; Cho, N.-J. Characterizing the membrane-disruptive behavior of dodecylglycerol using supported lipid bilayers. Langmuir 2019, 35, 3568–3575. [Google Scholar] [CrossRef]
- Jackman, J.A.; Cho, N.-J.J.L. Supported lipid bilayer formation: Beyond vesicle fusion. Langmuir 2020, 36, 1387–1400. [Google Scholar] [CrossRef]
- Andersson, J.; Köper, I.; Knoll, W. Tethered membrane architectures—Design and applications. Front. Mater. 2018, 5, 55. [Google Scholar] [CrossRef]
- Fatma, N.; Panda, M.; Beg, M. Ester-bonded cationic gemini surfactants: Assessment of their cytotoxicity and antimicrobial activity. J. Mol. Liq. 2016, 222, 390–394. [Google Scholar] [CrossRef]
- Henriksen, J.R.; Andresen, T.L.; Feldborg, L.N.; Duelund, L.; Ipsen, J.H. Understanding detergent effects on lipid membranes: A model study of lysolipids. Biophys. J. 2010, 98, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, T.; Tschapek, B.; Infed, N.; Smits, S.H.; Ernst, R.; Schmitt, L. High-throughput evaluation of the critical micelle concentration of detergents. Anal. Biochem. 2011, 408, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsson, M.V.; Richter, J.; Lee, A.L.; DePhillips, P. 5-dodecanoylaminofluorescein as a probe for the determination of critical micelle concentration of detergents using fluorescence anisotropy. Anal. Biochem. 2005, 340, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Goddard, E.; Turro, N.; Kuo, P.; Ananthapadmanabhan, K. Fluorescence probes for critical micelle concentration determination. Langmuir 1985, 1, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Hudecz, F.; Ross, H.; Price, M.R.; Baldwin, R.W. Immunoconjugate design: A predictive approach for coupling of daunomycin to monoclonal antibodies. Bioconjug. Chem. 1990, 1, 197–204. [Google Scholar] [CrossRef]
- Nakahara, Y.; Kida, T.; Nakatsuji, Y.; Akashi, M. New fluorescence method for the determination of the critical micelle concentration by photosensitive monoazacryptand derivatives. Langmuir 2005, 21, 6688–6695. [Google Scholar] [CrossRef]
- Yoon, B.K.; Park, S.; Ma, G.J.; Kolahdouzan, K.; Zhdanov, V.P.; Jackman, J.A.; Cho, N.-J. Competing interactions of fatty acids and monoglycerides trigger synergistic phospholipid membrane remodeling. J. Phys. Chem. Lett. 2020, 11, 4951–4957. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Sut, T.N.; Cho, N.-J. Correlating membrane morphological responses with micellar aggregation behavior of capric acid and monocaprin. Langmuir 2017, 33, 2750–2759. [Google Scholar] [CrossRef]
- Dutkiewicz, E.; Jakubowska, A. Effect of electrolytes on the physicochemical behaviour of sodium dodecyl sulphate micelles. Colloid Polym. Sci. 2002, 280, 1009–1014. [Google Scholar]
- Thongngam, M.; McClements, D.J. Influence of pH, ionic strength, and temperature on self-association and interactions of sodium dodecyl sulfate in the absence and presence of chitosan. Langmuir 2005, 21, 79–86. [Google Scholar] [CrossRef]
- Palladino, P.; Ragone, R. Ionic strength effects on the critical micellar concentration of ionic and nonionic surfactants: The binding model. Langmuir 2011, 27, 14065–14070. [Google Scholar] [CrossRef]
- Kolahdouzan, K.; Jackman, J.A.; Yoon, B.K.; Kim, M.C.; Johal, M.S.; Cho, N.-J. Optimizing the formation of supported lipid bilayers from bicellar mixtures. Langmuir 2017, 33, 5052–5064. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Use of quartz vibrator for weighting thin films on a microbalance. Z. Phys. 1959, 155, 206–212. [Google Scholar] [CrossRef]
- Yoon, B.K.; Jackman, J.A.; Kim, M.C.; Cho, N.-J. Spectrum of membrane morphological responses to antibacterial fatty acids and related surfactants. Langmuir 2015, 31, 10223–10232. [Google Scholar] [CrossRef]
- Yoon, B.K.; Park, H.; Zhdanov, V.P.; Jackman, J.A.; Cho, N.-J. Real-time nanoplasmonic sensing of three-dimensional morphological changes in a supported lipid bilayer and antimicrobial testing applications. Biosens. Bioelectron. 2021, 174, 112768. [Google Scholar] [CrossRef]
- Moon, S.; Yoon, B.K.; Jackman, J.A. Effect of membrane curvature nanoarchitectonics on membrane-disruptive interactions of antimicrobial lipids and surfactants. Langmuir 2022, 38, 4606–4616. [Google Scholar] [CrossRef]
- Valle-González, E.R.; Jackman, J.A.; Yoon, B.K.; Park, S.; Sut, T.N.; Cho, N.-J. Characterizing how acidic ph conditions affect the membrane-disruptive activities of lauric acid and glycerol monolaurate. Langmuir 2018, 34, 13745–13753. [Google Scholar] [CrossRef]
- Valle-González, E.R.; Jackman, J.A.; Yoon, B.K.; Mokrzecka, N.; Cho, N.-J. pH-dependent antibacterial activity of glycolic acid: Implications for anti-acne Formulations. Sci. Rep. 2020, 10, 7491. [Google Scholar] [CrossRef]
- Stenbæk, J.; Löf, D.; Falkman, P.; Jensen, B.; Cárdenas, M. An alternative anionic bio-sustainable anti-fungal agent: Investigation of its mode of action on the fungal cell membrane. J. Colloid. Interface. Sci. 2017, 497, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, C.G.; Cornell, B.A.; Grage, S.L.; Duckworth, P.; Carne, S.; Ulrich, A.S.; Martinac, B. Transient potential gradients and impedance measures of tethered bilayer lipid membranes: Pore-forming peptide insertion and the effect of electroporation. Biophys. J. 2014, 106, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Alghalayini, A.; Garcia, A.; Berry, T.; Cranfield, C.G. The use of tethered bilayer lipid membranes to identify the mechanisms of antimicrobial peptide interactions with lipid bilayers. Antibiotics 2019, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.W.; Jeon, W.-Y.; Yoon, B.K.; Jackman, J.A. Mechanistic evaluation of antimicrobial lipid interactions with tethered lipid bilayers by electrochemical impedance spectroscopy. Sensors 2022, 22, 3712. [Google Scholar] [CrossRef]
- Junghans, A.; Koper, I. Structural analysis of tethered bilayer lipid membranes. Langmuir 2010, 26, 11035–11040. [Google Scholar] [CrossRef]
- Koo, D.J.; Sut, T.N.; Tan, S.W.; Yoon, B.K.; Jackman, J.A. Biophysical characterization of LTX-315 anticancer peptide interactions with model membrane platforms: Effect of membrane surface charge. Int. J. Mol. Sci. 2022, 23, 10558. [Google Scholar] [CrossRef]
- Lu, Z.; van Niekerk, D.; Savva, A.; Kallitsis, K.; Thiburce, Q.; Salleo, A.; Pappa, A.-M.; Owens, R.M. Understanding electrochemical properties of supported lipid bilayers interfaced with organic electronic devices. J. Mater. Chem. C 2022, 10, 8050–8060. [Google Scholar] [CrossRef]
- Cseresnyés, I.; Rajkai, K.; Vozáry, E. Role of phase angle measurement in electical impedance spetroscopy. Int. Agrophys. 2013, 27, 377–383. [Google Scholar] [CrossRef]
- Cranfield, C.G.; Henriques, S.T.; Martinac, B.; Duckworth, P.; Craik, D.J.; Cornell, B. Kalata b1 and kalata b2 have a surfactant-like activity in phosphatidylethanolomine-containing lipid membranes. Langmuir 2017, 33, 6630–6637. [Google Scholar] [CrossRef]
- Keller, S.; Heetklotz, H.; Jahnke, N.; Blume, A. Thermodynamics of lipid membrane solubilization by sodium dodecyl sulfate. Biophys. J. 2006, 90, 4509–4521. [Google Scholar] [CrossRef]
- Tan, J.Y.B.; Yoon, B.K.; Cho, N.-J.; Lovrić, J.; Jug, M.; Jackman, J.A. Lipid nanoparticle technology for delivering biologically active fatty acids and monoglycerides. Int. J. Mol. Sci. 2021, 22, 9664. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gooran, N.; Tan, S.W.; Yoon, B.K.; Jackman, J.A. Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study. Int. J. Mol. Sci. 2023, 24, 9283. https://doi.org/10.3390/ijms24119283
Gooran N, Tan SW, Yoon BK, Jackman JA. Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study. International Journal of Molecular Sciences. 2023; 24(11):9283. https://doi.org/10.3390/ijms24119283
Chicago/Turabian StyleGooran, Negin, Sue Woon Tan, Bo Kyeong Yoon, and Joshua A. Jackman. 2023. "Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study" International Journal of Molecular Sciences 24, no. 11: 9283. https://doi.org/10.3390/ijms24119283
APA StyleGooran, N., Tan, S. W., Yoon, B. K., & Jackman, J. A. (2023). Unraveling Membrane-Disruptive Properties of Sodium Lauroyl Lactylate and Its Hydrolytic Products: A QCM-D and EIS Study. International Journal of Molecular Sciences, 24(11), 9283. https://doi.org/10.3390/ijms24119283





