Palbociclib-Induced Cellular Senescence Is Modulated by the mTOR Complex 1 and Autophagy
Abstract
1. Introduction
2. Results
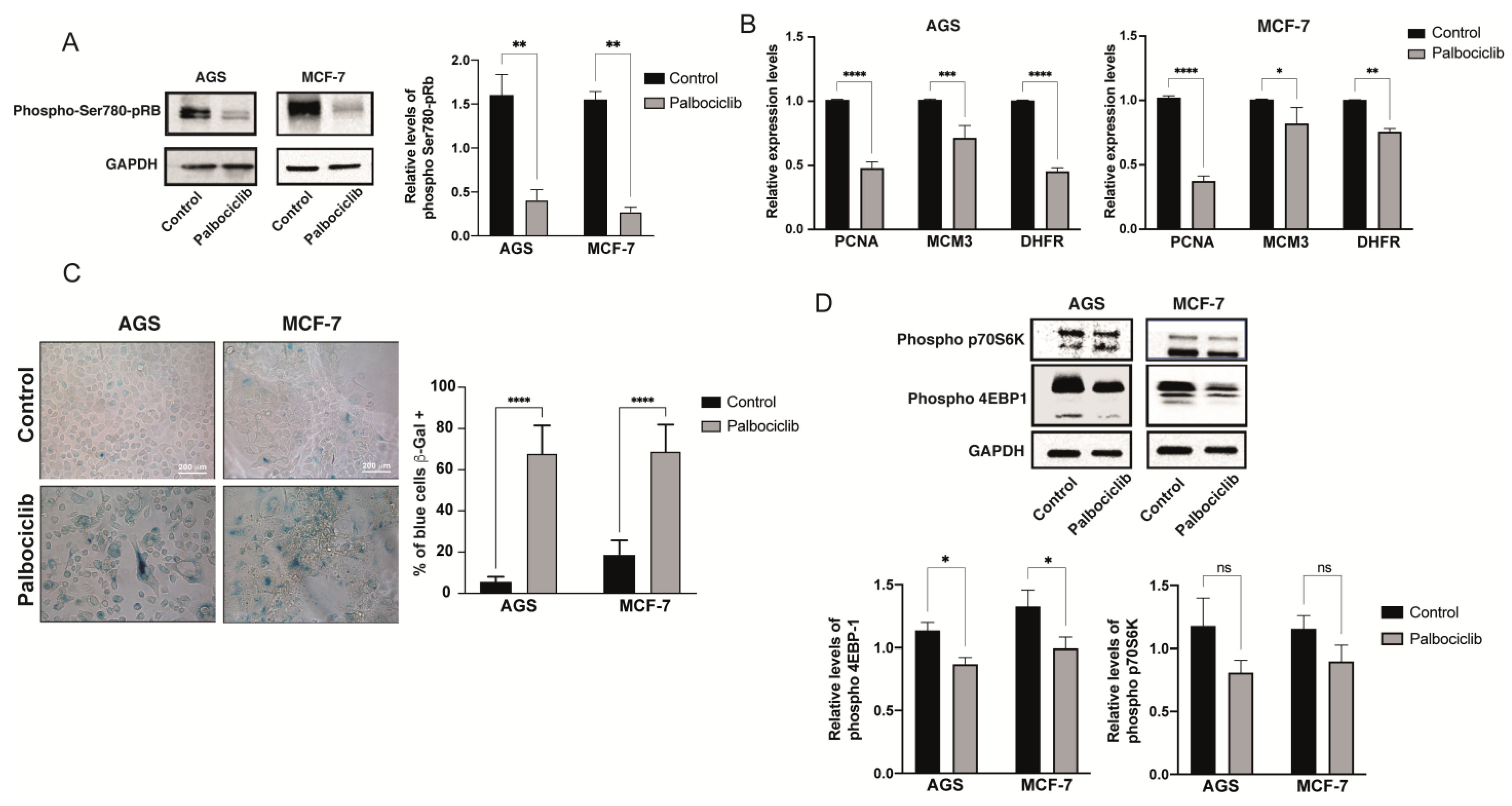
2.1. Palbociclib-Induced Senescence in AGS and MCF-7 Cell Lines
2.2. Palbociclib-Induced Cellular Senescence Results in Reduced Levels of mTORC1 Activity
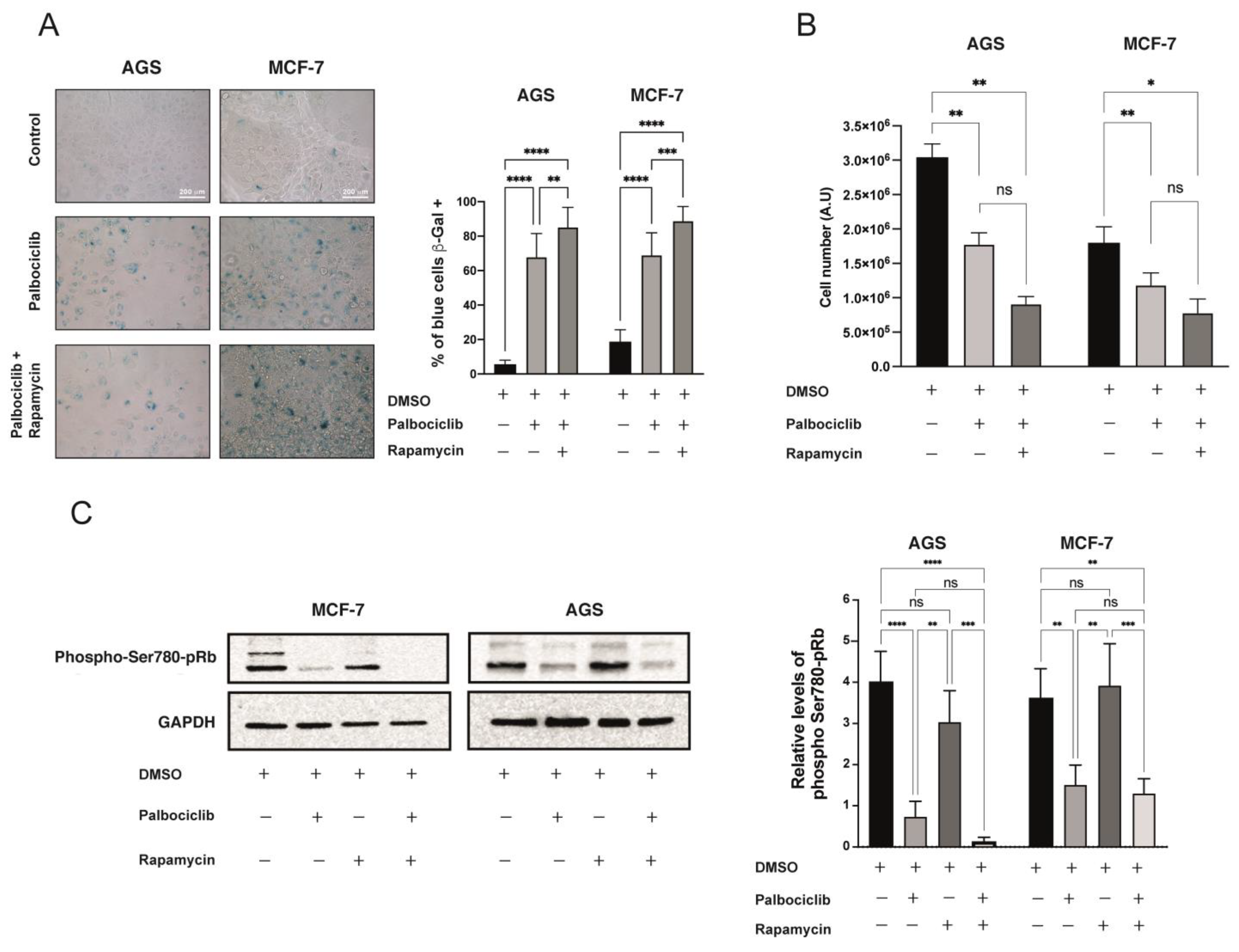
2.3. Further Inhibition of the mTORC1 Complex Exacerbates the Senescent Phenotype Induced by Palbociclib
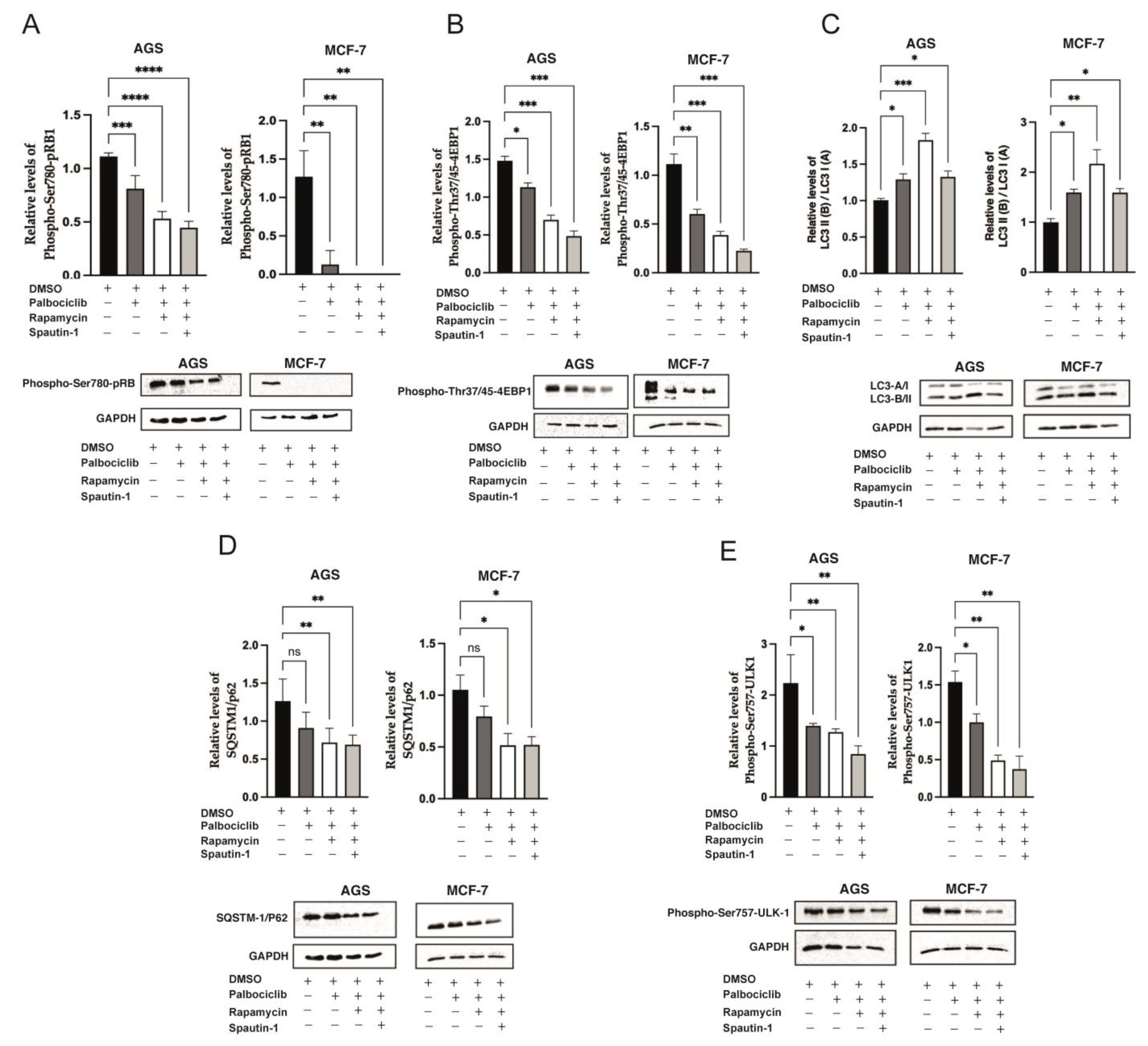
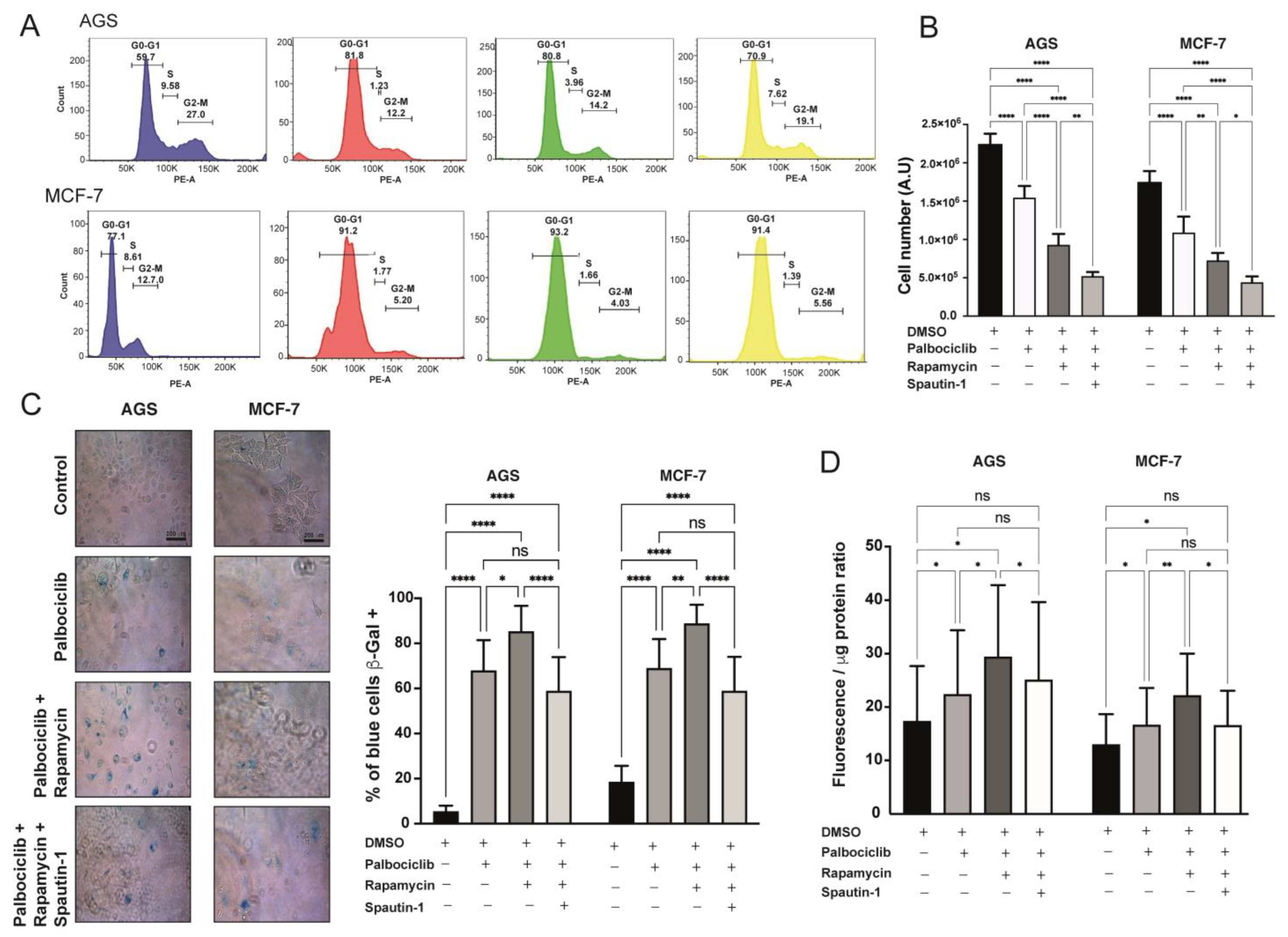
2.4. Blockade of Autophagy in Palbociclib-Driven Senescent Cells with Inhibition of mTORC1 Reverses the Senescent Phenotype
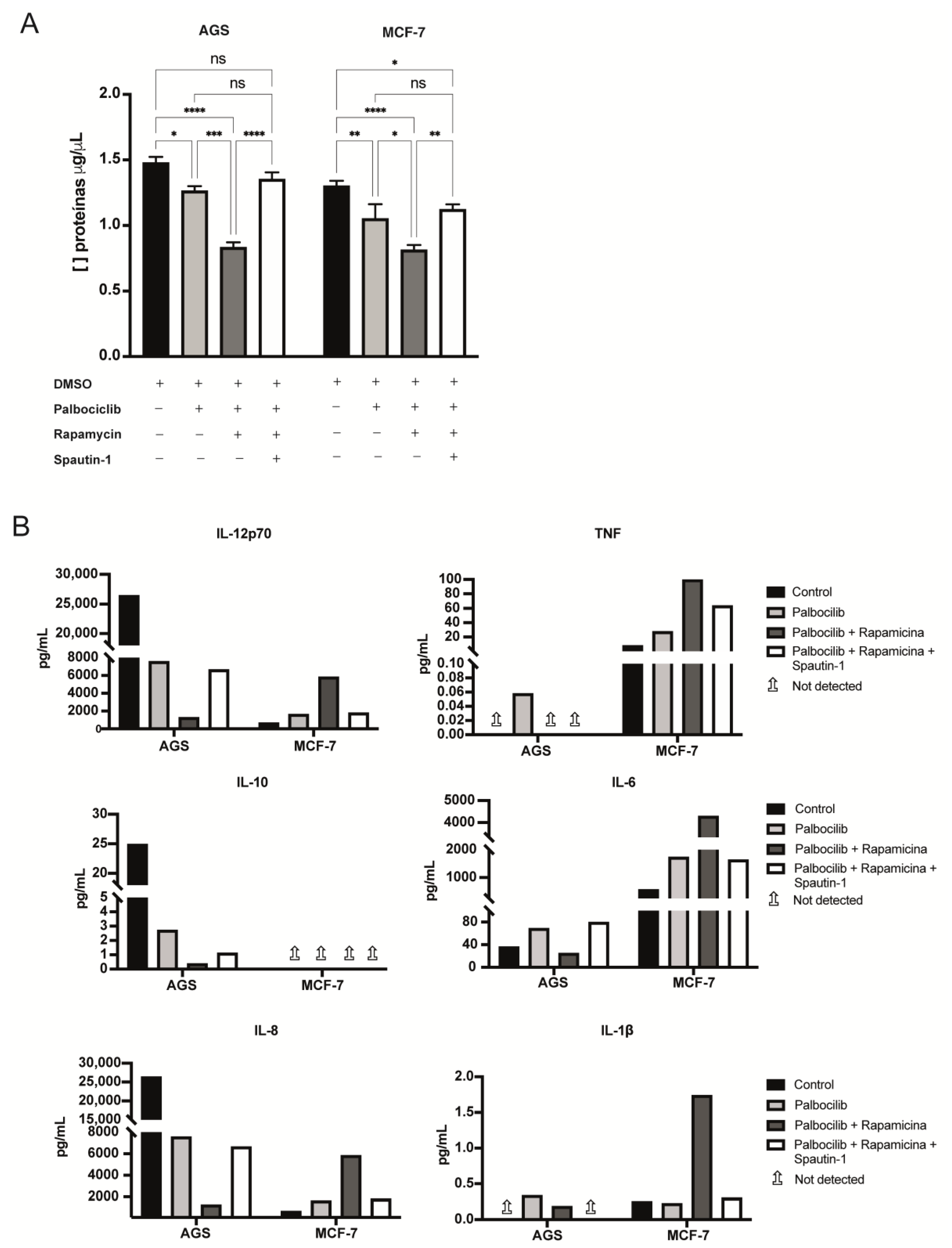
2.5. The Levels of Selected Factors Secreted by Senescent Cells Change upon Inhibition of mTORC1 and/or Autophagy
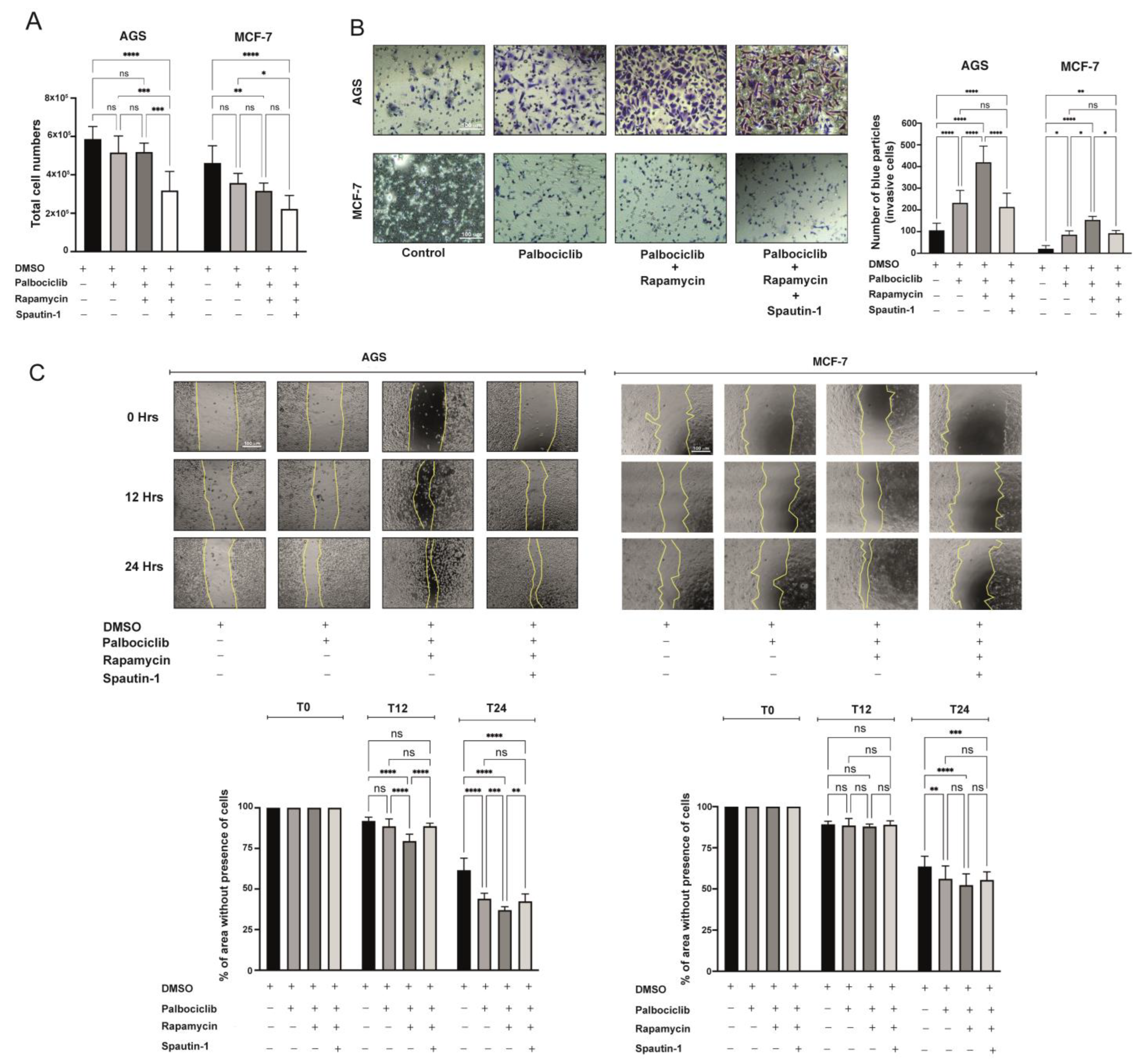
2.6. The SASP Derived from Palbociclib-Driven Senescent Cells with mTORC1 Inhibition Enhances the Pro-Invasive and Pro-Migratory Capabilities of Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Induction of Cellular Senescence
4.3. Senescence-Associated β-Galactosidase Assay
4.4. Fluorometry- and Flow Cytometry-Based Assays for the Detection of Senescent Cells
4.5. Flow Cytometry Analyses
4.6. Cell Counting
4.7. Pharmacological Inhibition of the mTOR Complex 1 (mTORC1)
4.8. Autophagy Inhibition
4.9. Protein Preparation and Quantification
4.10. Protein Electrophoresis in Polyacrylamide Gels, SDS-PAGE
4.11. Preparation of Total RNA and RT-qPCR
4.12. Harvesting of Conditioned Media
4.13. Cytokine Analysis of Conditioned Media
4.14. Wound Healing Assays
4.15. Invasion Assays
4.16. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Serrano, M. Senescence in tumours: Evidence from mice and humans. Nat. Rev. Cancer 2010, 10, 51–57. [Google Scholar] [CrossRef]
- Gal, H.; Majewska, J.; Krizhanovsky, V. The intricate nature of senescence in development and cell plasticity. Semin. Cancer Biol. 2021, 87, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Yun, M. Cellular senescence in tissue repair: Every cloud has a silver lining. Int. J. Dev. Biol. 2018, 62, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Rhinn, M.; Ritschka, B.; Keyes, W. Cellular senescence in development, regeneration and disease. Development 2019, 146, dev151837. [Google Scholar] [CrossRef]
- Regulski, M.J. Cellular Senescence: What, Why, and How. Wounds 2017, 29, 168–174. [Google Scholar]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Bianchi-Smiraglia, A.; Lipchick, B.; Nikiforov, M. The Immortal Senescence. Methods Mol. Biol. 2017, 1534, 1–15. [Google Scholar]
- Carnero, A.; Blanco-Aparicio, C.; Kondoh, H.; Lleonart, M.; Martinez-Leal, J.; Mondello, C.; Scovassi, A.I.; Bisson, W.H.; Amedei, A.; Roy, R.; et al. Disruptive chemicals, senescence and immortality. Carcinogenesis 2015, 36 (Suppl. S1), S19–S37. [Google Scholar] [CrossRef]
- Wong, R. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. CR 2011, 30, 87. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A. Targeted therapy in cancer. Cancer Chemother. Pharmacol. 2015, 76, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Ewald, J.; Desotelle, J.; Wilding, G.; Jarrard, D. Therapy-induced senescence in cancer. J. Natl. Cancer Inst. 2010, 102, 1536–1546. [Google Scholar] [CrossRef]
- Saleh, T.; Bloukh, S.; Carpenter, V.J.; Alwohoush, E.; Bakeer, J.; Darwish, S.; Azab, B.; Gewirtz, D.A. Therapy-Induced Senescence: An “Old” Friend Becomes the Enemy. Cancers 2020, 12, 822. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, A.; Henry, N. Palbociclib for the Treatment of Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3591–3596. [Google Scholar] [CrossRef]
- Mangini, N.; Wesolowski, R.; Ramaswamy, B.; Lustberg, M.; Berger, M. Palbociclib: A Novel Cyclin-Dependent Kinase Inhibitor for Hormone Receptor-Positive Advanced Breast Cancer. Ann. Pharmacother. 2015, 49, 1252–1260. [Google Scholar] [CrossRef]
- McCartney, A.; Moretti, E.; Sanna, G.; Pestrin, M.; Risi, E.; Malorni, L.; Biganzoli, L.; Di Leo, A. The role of abemaciclib in treatment of advanced breast cancer. Ther. Adv. Med. Oncol. 2018, 10, 1758835918776925. [Google Scholar] [CrossRef]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M.; et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Xue, W.; Zender, L.; Yon, M.; Hernando, E.; Lowe, S. Implications of cellular senescence in tissue damage response, tumor suppression, and stem cell biology. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 513–522. [Google Scholar] [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef] [PubMed]
- Cuollo, L.; Antonangeli, F.; Santoni, A.; Soriani, A. The Senescence-Associated Secretory Phenotype (SASP) in the Challenging Future of Cancer Therapy and Age-Related Diseases. Biology 2020, 9, 485. [Google Scholar] [CrossRef]
- Rentschler, M.; Braumüller, H.; Briquez, P.; Wieder, T. Cytokine-Induced Senescence in the Tumor Microenvironment and Its Effects on Anti-Tumor Immune Responses. Cancers 2022, 14, 1364. [Google Scholar] [CrossRef]
- Faheem, M.; Seligson, N.; Ahmad, S.; Rasool, R.; Gandhi, S.; Bhagat, M.; Goswami, A. Convergence of therapy-induced senescence (TIS) and EMT in multistep carcinogenesis: Current opinions and emerging perspectives. Cell Death Discov. 2020, 6, 51. [Google Scholar] [CrossRef]
- Laberge, R.; Awad, P.; Campisi, J.; Desprez, P. Epithelial-mesenchymal transition induced by senescent fibroblasts. Cancer Microenviron. Off. J. Int. Cancer Microenviron. Soc. 2011, 5, 39–44. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef]
- Dou, Z.; Berger, S. Senescence Elicits Stemness: A Surprising Mechanism for Cancer Relapse. Cell Metab. 2018, 27, 710–711. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.; Belenki, D.; Däbritz, J.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Burton, D.; Krizhanovsky, V. Physiological and pathological consequences of cellular senescence. Cell. Mol. Life Sci. CMLS 2014, 71, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Saleh, T.; Tyutynuk-Massey, L.; Cudjoe, E.K., Jr.; Idowu, M.O.; Landry, J.W.; Gewirtz, D.A. Non-Cell Autonomous Effects of the Senescence-Associated Secretory Phenotype in Cancer Therapy. Front. Oncol. 2018, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, N. The role of SASP in tumor microenvironment. Clin. Calcium 2017, 27, 835–843. [Google Scholar]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm. Regen. 2022, 42, 11. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Zhang, J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int. J. Mol. Sci. 2019, 20, 755. [Google Scholar] [CrossRef]
- Folkerts, H.; Hilgendorf, S.; Vellenga, E.; Bremer, E.; Wiersma, V. The multifaceted role of autophagy in cancer and the microenvironment. Med. Res. Rev. 2019, 39, 517–560. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Allam, A.; Aboulaghras, S.; Bakrim, S.; El Menyiy, N.; Alshahrani, M.; Al Awadh, A.A.; Benali, T.; Lee, L.-H.; El Omari, N.; et al. Targeting mTOR as a Cancer Therapy: Recent Advances in Natural Bioactive Compounds and Immunotherapy. Cancers 2022, 14, 5520. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Sabatini, D.M. mTOR and cancer: Insights into a complex relationship. Nat. Rev. Cancer 2006, 6, 729–734. [Google Scholar] [CrossRef]
- Shimobayashi, M.; Hall, M.N. Making new contacts: The mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell Biol. 2014, 15, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Bar-Peled, L.; Efeyan, A.; Wang, S.; Sancak, Y.; Sabatini, D.M. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011, 334, 678–683. [Google Scholar] [CrossRef]
- Beugnet, A.; Tee, A.R.; Taylor, P.M.; Proud, C.G. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J. 2003, 372, 555–566. [Google Scholar] [CrossRef]
- Johnson, C.E.; Tee, A.R. Exploiting cancer vulnerabilities: mTOR, autophagy, and homeostatic imbalance. Essays Biochem. 2017, 61, 699–710. [Google Scholar] [PubMed]
- Papadopoli, D.; Boulay, K.; Kazak, L.; Pollak, M.; Mallette, F.; Topisirovic, I.; Hulea, L. mTOR as a central regulator of lifespan and aging. F1000Research 2019, 8, 998. [Google Scholar] [CrossRef] [PubMed]
- Laberge, R.M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Orjalo, A.; Bhaumik, D.; Gengler, B.; Scott, G.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106. [Google Scholar] [CrossRef]
- Kolesnichenko, M.; Hong, L.; Liao, R.; Vogt, P.K.; Sun, P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle 2012, 11, 2391–2401. [Google Scholar] [CrossRef]
- Romero-Pozuelo, J.; Figlia, G.; Kaya, O.; Martin-Villalba, A.; Teleman, A. Cdk4 and Cdk6 Couple the Cell-Cycle Machinery to Cell Growth via mTORC1. Cell Rep. 2020, 31, 107504. [Google Scholar] [CrossRef]
- Hale, A.; Ledbetter, D.; Gawriluk, T.; Rucker, E. Autophagy: Regulation and role in development. Autophagy 2013, 9, 951–972. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Kim, J.W.; Jeoung, J.A.; Kim, M.S.; Kang, C. Autophagy Is Pro-Senescence When Seen in Close-Up, but Anti-Senescence in Long-Shot. Mol. Cells 2017, 40, 607–612. [Google Scholar] [PubMed]
- Gewirtz, D.A. Autophagy and senescence: A partnership in search of definition. Autophagy 2013, 9, 808–812. [Google Scholar] [CrossRef]
- Capparelli, C.; Chiavarina, B.; Whitaker-Menezes, D.; Pestell, T.G.; Pestell, R.G.; Hulit, J.; Andò, S.; Howell, A.; Martinez-Outschoorn, U.E.; Sotgia, F.; et al. CDK inhibitors (p16/p19/p21) induce senescence and autophagy in cancer-associated fibroblasts, “fueling” tumor growth via paracrine interactions, without an increase in neo-angiogenesis. Cell Cycle 2012, 11, 3599–3610. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.E.; Jeselsohn, R.; Bihani, T.; Hu, M.G.; Foltopoulou, P.; Kuperwasser, C.; Hinds, P.W. Cyclin D1 activity regulates autophagy and senescence in the mammary epithelium. Cancer Res 2012, 72, 6477–6489. [Google Scholar] [CrossRef]
- Young, A.R.; Narita, M.; Ferreira, M.; Kirschner, K.; Sadaie, M.; Darot, J.F.; Tavaré, S.; Arakawa, S.; Shimizu, S.; Watt, F.M.; et al. Autophagy mediates the mitotic senescence transition. Genes Dev. 2009, 23, 798–803. [Google Scholar] [CrossRef]
- Carroll, B.; Dunlop, E. The lysosome: A crucial hub for AMPK and mTORC1 signalling. Biochem. J. 2017, 474, 1453–1466. [Google Scholar] [CrossRef]
- Carroll, B.; Nelson, G.; Rabanal-Ruiz, Y.; Kucheryavenko, O.; Dunhill-Turner, N.; Chesterman, C.; Zahari, Q.; Zhang, T.; Conduit, S.E.; Mitchell, C.A.; et al. Persistent mTORC1 signaling in cell senescence results from defects in amino acid and growth factor sensing. J. Cell Biol. 2017, 216, 1949–1957. [Google Scholar] [CrossRef]
- Carroll, B.; Korolchuk, V. Nutrient sensing, growth and senescence. FEBS J. 2018, 285, 1948–1958. [Google Scholar] [CrossRef]
- Bojko, A.; Czarnecka-Herok, J.; Charzynska, A.; Dabrowski, M.; Sikora, E. Diversity of the Senescence Phenotype of Cancer Cells Treated with Chemotherapeutic Agents. Cells 2019, 8, 1501. [Google Scholar] [CrossRef] [PubMed]
- Cairney, C.; Bilsland, A.; Evans, T.; Roffey, J.; Bennett, D.; Narita, M.; Torrance, C.J.; Keith, W.N. Cancer cell senescence: A new frontier in drug development. Drug Discov. Today 2012, 17, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Dossou, A.; Basu, A. The Emerging Roles of mTORC1 in Macromanaging Autophagy. Cancers 2019, 11, 1422. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.; Tee, A. mTOR and autophagy: A dynamic relationship governed by nutrients and energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Bernard, M.; Yang, B.; Migneault, F.; Turgeon, J.; Dieude, M.; Olivier, M.A.; Cardin, G.B.; El-Diwany, M.; Underwood, K.; Rodier, F.; et al. Autophagy drives fibroblast senescence through MTORC2 regulation. Autophagy 2020, 16, 2004–2016. [Google Scholar] [CrossRef]
- Payea, M.; Anerillas, C.; Tharakan, R.; Gorospe, M. Translational Control during Cellular Senescence. Mol. Cell. Biol. 2021, 41, e00512-20. [Google Scholar] [CrossRef]
- Rizzolio, F.; Lucchetti, C.; Caligiuri, I.; Marchesi, I.; Caputo, M.; Klein-Szanto, A.; Bagella, L.; Castronovo, M.; Giordano, A. Retinoblastoma tumor-suppressor protein phosphorylation and inactivation depend on direct interaction with Pin1. Cell Death Differ. 2012, 19, 1152–1161. [Google Scholar] [CrossRef]
- Kim, S.; Leong, A.; Kim, M.; Yang, H. CDK4/6 initiates Rb inactivation and CDK2 activity coordinates cell-cycle commitment and G1/S transition. Sci. Rep. 2022, 12, 16810. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.; Pruitt, S.; Hershberger, P.; Witkiewicz, A.; Goodrich, D. Cell Cycle and Beyond: Exploiting New RB1 Controlled Mechanisms for Cancer Therapy. Trends Cancer 2019, 5, 308–324. [Google Scholar] [CrossRef]
- Kwapisz, D. Cyclin-dependent kinase 4/6 inhibitors in breast cancer: Palbociclib, ribociclib, and abemaciclib. Breast Cancer Res. Treat. 2017, 166, 41–54. [Google Scholar] [CrossRef]
- Xu, S.; Cai, Y.; Wei, Y. mTOR Signaling from Cellular Senescence to Organismal Aging. Aging Dis. 2014, 5, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Cayo, A.; Segovia, R.; Venturini, W.; Moore-Carrasco, R.; Valenzuela, C.; Brown, N. mTOR Activity and Autophagy in Senescent Cells, a Complex Partnership. Int. J. Mol. Sci. 2021, 22, 8149. [Google Scholar] [CrossRef]
- Fingar, D.; Richardson, C.; Tee, A.; Cheatham, L.; Tsou, C.; Blenis, J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 2004, 24, 200–216. [Google Scholar] [CrossRef]
- Hara, K.; Yonezawa, K.; Weng, Q.; Kozlowski, M.; Belham, C.; Avruch, J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J. Biol. Chem. 1998, 273, 14484–14494. [Google Scholar] [CrossRef]
- Khor, E.S.; Wong, P.F. The roles of MTOR and miRNAs in endothelial cell senescence. Biogerontology 2020, 21, 517–530. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Guan, K. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Schmeisser, K.; Parker, J. Pleiotropic Effects of mTOR and Autophagy During Development and Aging. Front. Cell Dev. Biol. 2019, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, C.A.; Vargas, L.; Martinez, V.; Bravo, S.; Brown, N.E. Palbociclib-induced autophagy and senescence in gastric cancer cells. Exp. Cell Res. 2017, 360, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, H.; Kim, M.; Xu, L.; Li, Y.; Zhang, L.; Cai, Y.; Norberg, H.V.; Zhang, T.; Furuya, T.; et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 2011, 147, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.; Mowers, E.; Drake, L.; Macleod, K. Measuring autophagy in stressed cells. Methods Mol. Biol. 2015, 1292, 129–150. [Google Scholar]
- Rodríguez-Arribas, M.; Yakhine-Diop, S.; González-Polo, R.; Niso-Santano, M.; Fuentes, J. Turnover of Lipidated LC3 and Autophagic Cargoes in Mammalian Cells. Methods Enzymol. 2017, 587, 55–70. [Google Scholar]
- Mizushima, N.; Yoshimori, T. How to interpret LC3 immunoblotting. Autophagy 2007, 3, 542–545. [Google Scholar] [CrossRef]
- Lippai, M.; Lőw, P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. BioMed Res. Int. 2014, 2014, 832704. [Google Scholar] [CrossRef]
- Kim, J.; Guan, K. Regulation of the autophagy initiating kinase ULK1 by nutrients: Roles of mTORC1 and AMPK. Cell Cycle 2011, 10, 1337–1338. [Google Scholar] [CrossRef]
- Dunlop, E.; Tee, A. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem. Soc. Trans. 2013, 41, 939–943. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr. Drug Discov. Technol. 2016, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Bielak-Zmijewska, A.; Mosieniak, G. A common signature of cellular senescence; does it exist? Ageing Res. Rev. 2021, 71, 101458. [Google Scholar] [CrossRef]
- Weinberg, R. The retinoblastoma protein and cell cycle control. Cell 1995, 81, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Shang, J.; Zou, X.; Xu, J.; Han, Y. Palbociclib induces cell senescence and apoptosis of gastric cancer cells by inhibiting the Notch pathway. Oncol. Lett. 2021, 22, 603. [Google Scholar] [CrossRef] [PubMed]
- Bollard, J.; Miguela, V.; Ruiz de Galarreta, M.; Venkatesh, A.; Bian, C.B.; Roberto, M.P.; Tovar, V.; Sia, D.; Molina-Sánchez, P.; Nguyen, C.B.; et al. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut 2017, 66, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.; Martin, M.; Rugo, H.; Jones, S.; Im, S.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Liu, M.; Liu, H.; Chen, J. Mechanisms of the CDK4/6 inhibitor palbociclib (PD 0332991) and its future application in cancer treatment (Review). Oncol. Rep. 2018, 39, 901–911. [Google Scholar] [CrossRef]
- Flaherty, K.; Lorusso, P.; Demichele, A.; Abramson, V.; Courtney, R.; Randolph, S.; Shaik, M.N.; Wilner, K.D.; O’Dwyer, P.J.; Schwartz, G.K. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 568–576. [Google Scholar] [CrossRef]
- Karasic, T.; O’Hara, M.; Teitelbaum, U.; Damjanov, N.; Giantonio, B.; d’Entremont, T.; Gallagher, M.; Zhang, P.J.; O’Dwyer, P.J. Phase II Trial of Palbociclib in Patients with Advanced Esophageal or Gastric Cancer. Oncologist 2020, 25, e1864–e1868. [Google Scholar] [CrossRef]
- Sikora, E.; Arendt, T.; Bennett, M.; Narita, M. Impact of cellular senescence signature on ageing research. Ageing Res. Rev. 2011, 10, 146–152. [Google Scholar] [CrossRef]
- Malaquin, N.; Tu, V.; Rodier, F. Assessing Functional Roles of the Senescence-Associated Secretory Phenotype (SASP). Methods Mol. Biol. 2019, 1896, 45–55. [Google Scholar]
- Ghosh, K.; Capell, B.C. The Senescence-Associated Secretory Phenotype: Critical Effector in Skin Cancer and Aging. J. Investig. Dermatol. 2016, 136, 2133–2139. [Google Scholar] [CrossRef]
- Lau, L.; David, G. Pro- and anti-tumorigenic functions of the senescence-associated secretory phenotype. Expert Opin. Ther. Targets 2019, 23, 1041–1051. [Google Scholar] [CrossRef]
- Schosserer, M.; Grillari, J.; Breitenbach, M. The Dual Role of Cellular Senescence in Developing Tumors and Their Response to Cancer Therapy. Front. Oncol. 2017, 7, 278. [Google Scholar] [CrossRef]
- Gewirtz, D.A. Autophagy, senescence and tumor dormancy in cancer therapy. Autophagy 2009, 5, 1232–1234. [Google Scholar] [CrossRef]
- Ata, R.; Antonescu, C.N. Integrins and Cell Metabolism: An Intimate Relationship Impacting Cancer. Int. J. Mol. Sci. 2017, 18, 189. [Google Scholar] [CrossRef]
- Chagin, A.S. Effectors of mTOR-autophagy pathway: Targeting cancer, affecting the skeleton. Curr. Opin. Pharmacol. 2016, 28, 1–7. [Google Scholar] [CrossRef]
- Al-Bari, M.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrezaei, F.; Movaghar, A.; Gharghabi, M. The Effect of Caffeine and chk2 Inhibitor on Doxorubicin-Induced Cellular Senescence in MCF-7 Cells. Drug Res. 2016, 66, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Montero, P.; Londoño-Vallejo, A.; Vernot, J. Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line. Cell Commun. Signal. CCS 2017, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Pospelova, T.V.; Bykova, T.V.; Zubova, S.G.; Katolikova, N.V.; Yartzeva, N.M.; Pospelov, V.A. Rapamycin induces pluripotent genes associated with avoidance of replicative senescence. Cell Cycle 2013, 12, 3841–3851. [Google Scholar] [CrossRef]
- Averous, J.; Fonseca, B.; Proud, C. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene 2008, 27, 1106–1113. [Google Scholar] [CrossRef]
- Slobodnyuk, K.; Radic, N.; Ivanova, S.; Llado, A.; Trempolec, N.; Zorzano, A.; Nebreda, A.R. Autophagy-induced senescence is regulated by p38α signaling. Cell Death Dis. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Young, A.R.; Arakawa, S.; Samarajiwa, S.A.; Nakashima, T.; Yoshida, S.; Hong, S.; Berry, L.S.; Reichelt, S.; Ferreira, M.; et al. Spatial coupling of mTOR and autophagy augments secretory phenotypes. Science 2011, 332, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.; Wu, X.; Guan, J. Wound-healing assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [PubMed]
| RPL19 | F: 5′-CAT CCG CAA GCC TGT GAC G-3′ | R: 5′-TGT GAC CTT CTC TGG CAT TCG-3′ |
| PCNA | F: 5′-GGA TAT TAG CTC CAG CGG TGT AAA-3′ | R: 5′-TCT TCG GCC CTT AGT GTA ATG ATA-3′ |
| MCM3 | F: 5′-CCT TTC CCT CCA GCT CTG TCT AT-3′ | R: 5′-GTG ATG GTC TGG TGA TCC TTG TAG-3′ |
| DHFR | F: 5′-AAA CAA GGG GAA AGG GTT GGT TAG-3′ | R: 5′-CCT CCC ATA TTG TCC CAG AGT AGT-3′ |
| IL-8 | F: 5′-AGG CAC AAA CTT TCA GAG ACA GCA G-3′ | R: 5′-TGT TTA CAC ACA GTG AGA TGG TTC C-3′ |
| BECLIN1 | F: 5′-GGT GTC TCT CGC AGA TTC ATC-3′ | R: 5′-TCA GTC TTC GGC TGA GGT TCT-3′ |
| ULK-1 | F: 5′-GGC AAG TTC GAG TTC TCC CG-3′ | R: 5′-CGA CCT CCA AAT CGT GCT TCT-3′ |
| PGC1a | F: AAC AGC AGC AGA GAC AAA TGC ACC-3′ | R: 5′-TGC AGT TCC AGA GAG TTC CAC ACT-3′ |
| DEC1 | F: 5′-CCT TGA AGC ATG TGA AAG CA-3′ | R: 5′-CAT GTC TGG AAA CCT GAG CA-3′ |
| IL-6 | F: 5′-GGC ACC TCA GAT TGT TGT TGT T-3′ | R: 5′-GTG TCC TAA CGC TCA TAC TTT TAG T-3′ |
| IL-1a | F: 5′-AGA TGC CTG AGA TAC CCA AAA CC-3′ | R: 5′-CCA AGC ACA CCC AGT AGT CT-3′ |
| IL-1b | F: 5′-ATG ATG GCT TAT TAC AGT GGC AA-3′ | R: 5′-GTC GGA GAT TCG TAG CTG GA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cayo, A.; Venturini, W.; Rebolledo-Mira, D.; Moore-Carrasco, R.; Herrada, A.A.; Nova-Lamperti, E.; Valenzuela, C.; Brown, N.E. Palbociclib-Induced Cellular Senescence Is Modulated by the mTOR Complex 1 and Autophagy. Int. J. Mol. Sci. 2023, 24, 9284. https://doi.org/10.3390/ijms24119284
Cayo A, Venturini W, Rebolledo-Mira D, Moore-Carrasco R, Herrada AA, Nova-Lamperti E, Valenzuela C, Brown NE. Palbociclib-Induced Cellular Senescence Is Modulated by the mTOR Complex 1 and Autophagy. International Journal of Molecular Sciences. 2023; 24(11):9284. https://doi.org/10.3390/ijms24119284
Chicago/Turabian StyleCayo, Angel, Whitney Venturini, Danitza Rebolledo-Mira, Rodrigo Moore-Carrasco, Andrés A. Herrada, Estefanía Nova-Lamperti, Claudio Valenzuela, and Nelson E. Brown. 2023. "Palbociclib-Induced Cellular Senescence Is Modulated by the mTOR Complex 1 and Autophagy" International Journal of Molecular Sciences 24, no. 11: 9284. https://doi.org/10.3390/ijms24119284
APA StyleCayo, A., Venturini, W., Rebolledo-Mira, D., Moore-Carrasco, R., Herrada, A. A., Nova-Lamperti, E., Valenzuela, C., & Brown, N. E. (2023). Palbociclib-Induced Cellular Senescence Is Modulated by the mTOR Complex 1 and Autophagy. International Journal of Molecular Sciences, 24(11), 9284. https://doi.org/10.3390/ijms24119284







