Research Progress in Improving Photosynthetic Efficiency
Abstract
1. Introduction
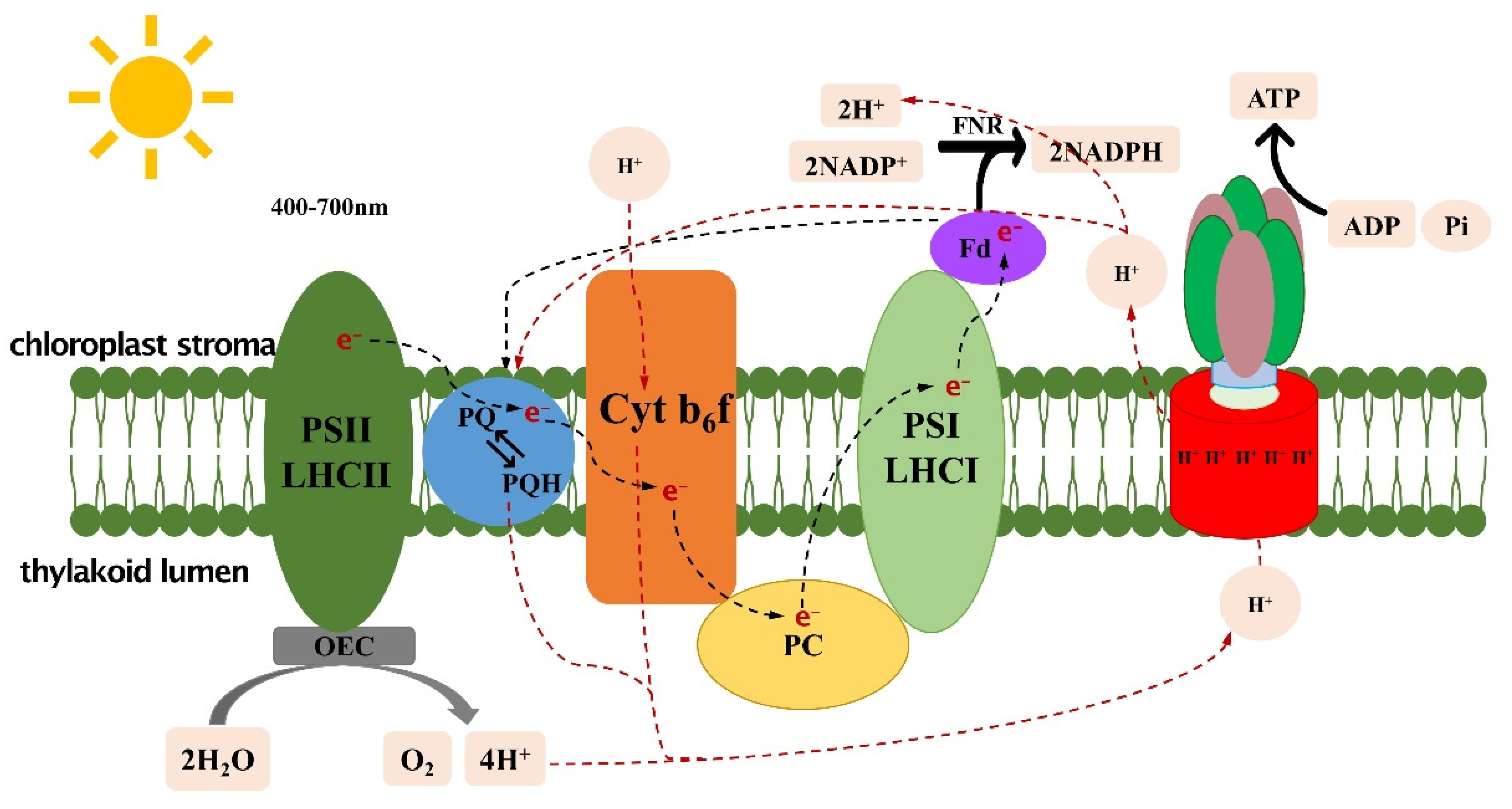
2. Light Reactions
3. Increased Light-Capture Capability and Optimized Light Absorption and Conversion
4. Accelerating Recovery of NPQ (Non-Photochemical Quenching)
5. Improving the Cytb6f Complex
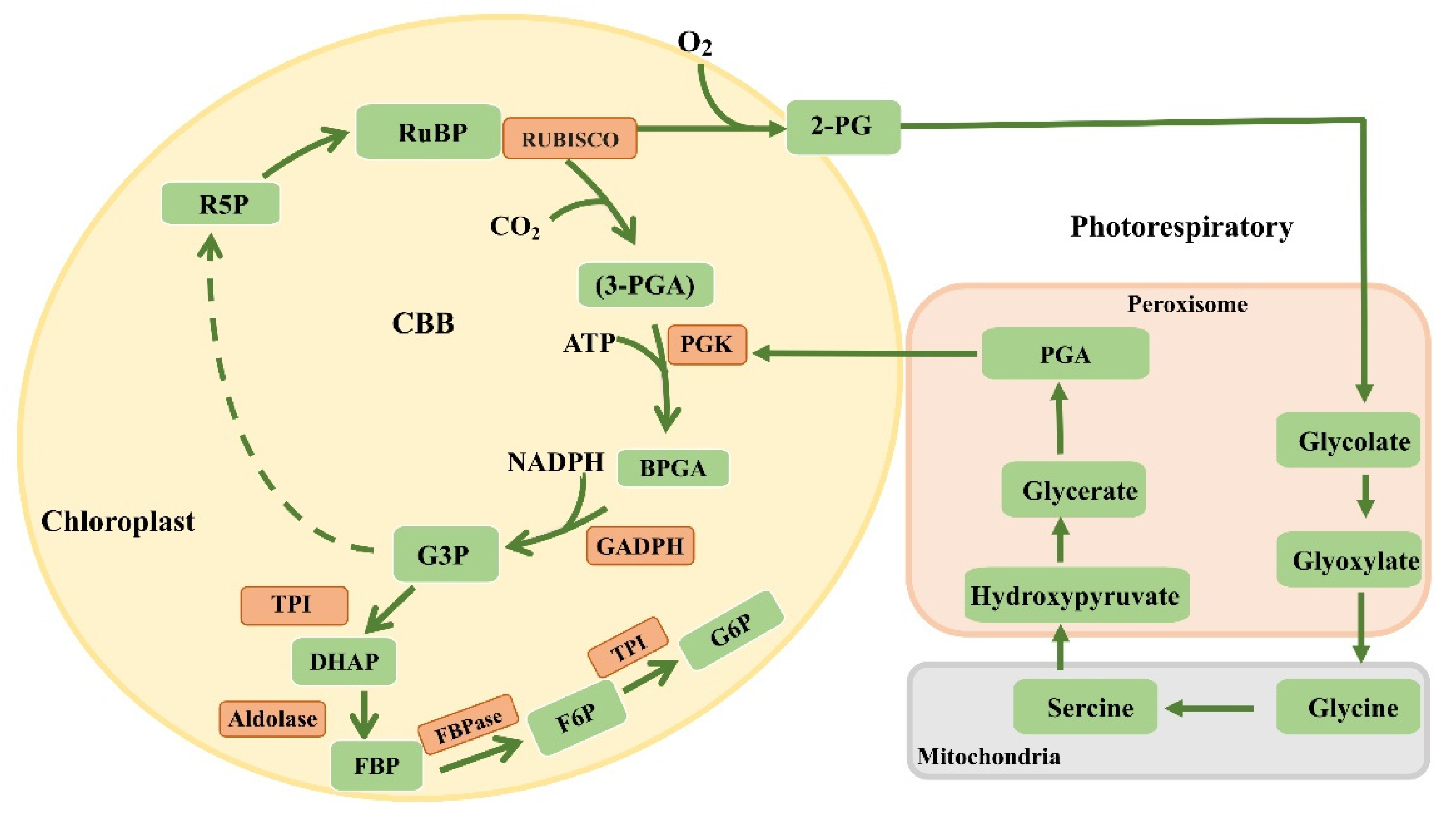
6. Dark Reactions
7. Modification of Rubisco
8. Optimization of Enzymes in the Calvin Cycle
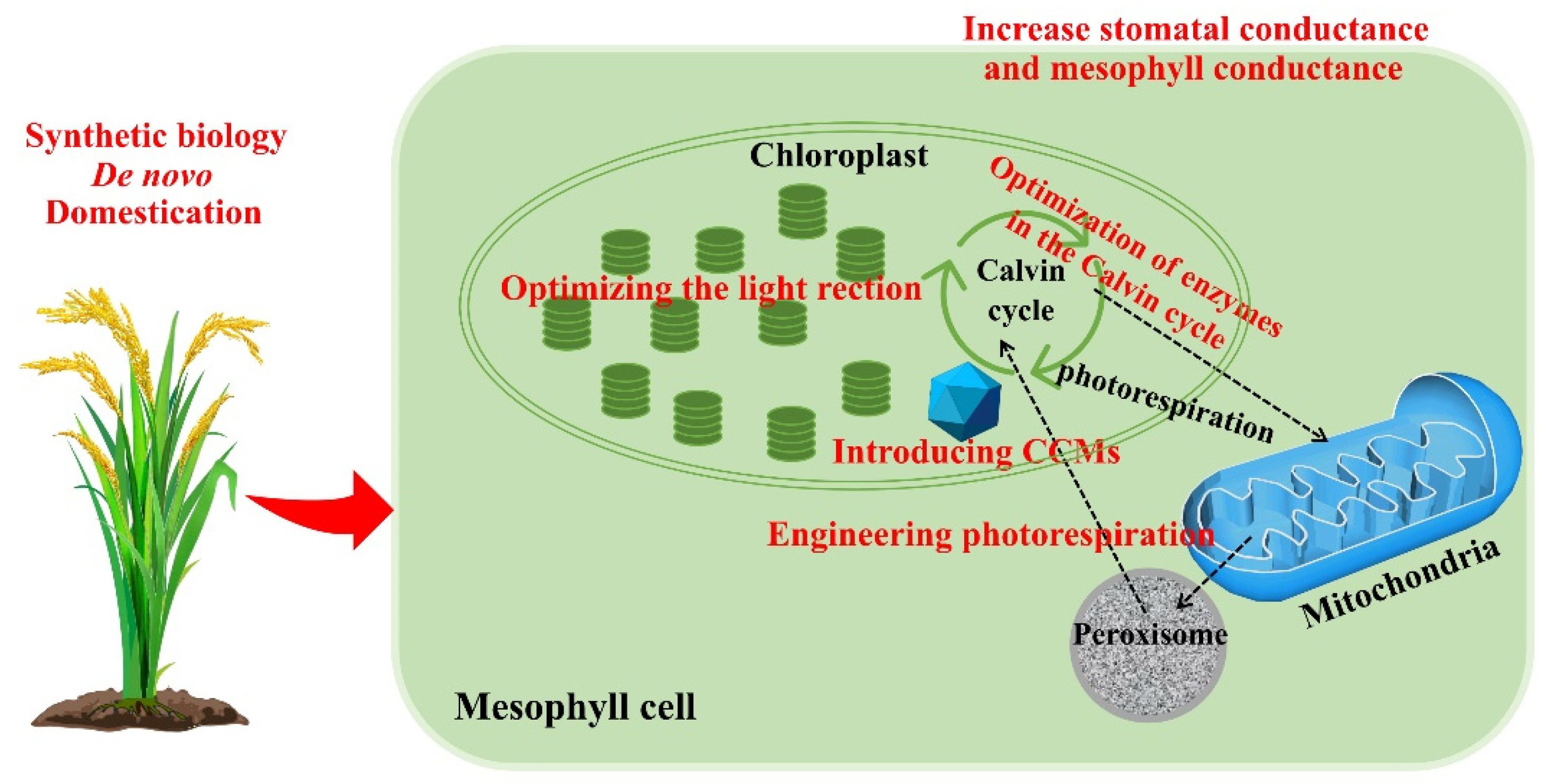
9. Introduction of Carbon Concentration Mechanisms into C3 Plants
10. Reconstructing the Photorespiratory Pathway
11. Redomestication/De Novo Domestication
12. Changes in Stomatal and Mesophyll Conductance
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BPGA | glycerate 1,3-bisphosphate |
| CBB | Calvin–Benson–Bassham |
| CCMs | carbon concentration mechanisms |
| CET | cyclic electron transport |
| Cytb6f | cytochrome b6f |
| DHAP | dihydroxyacetone phosphate |
| FBPase | fructose 1,6-bisphosphatase |
| Fd, | ferredoxin |
| F6-P | fructose 6-phosphate |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| gm | and mesophyll conductance |
| GPI | glucose phosphate isomerase |
| gs | stomatal conductance |
| G3P | glyceraldehyde 3-phosphate |
| LET | linear electron transport |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NPQ | Non-Photochemical Quenching |
| PC | plastocyanin |
| PET | photosynthetic electron transport |
| PEP | phosphoenolpyruvate |
| PGK | phosphoglycerate kinase |
| PGA | phosphoglycerate |
| PQ | plastoquinone |
| PQH | semi-plastoquinone |
| PSI | photosystem I |
| PSI-LHCI | photosystem I light-harvesting complex I |
| PSII | photosystem II |
| PSII-LHCII | photosystem II light-harvesting complex II |
| RuBP | ribulose 1,5-bisphosphonate |
| Rubisco | ribulose 1,5-bisphosphate carboxylase/oxygenase |
| Ru5P | ribulose 5-phosphate |
| SBPase | Sedoheptulose-1,7-bisphosphatase |
| TPI | triose phosphate isomerase |
| 2PG | 2-phosphoglycerate |
| 3-PGA | 3-phosphoglycerate |
References
- Schluter, U.; Weber, A.P. The road to C4 photosynthesis: Evolution of a complex trait via intermediary states. Plant Cell Physiol. 2016, 57, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, M.; Maoka, T.; Morita, R.; Takaichi, S. A novel carotenoid derivative, lutein 3-acetate, accumulates in senescent leaves of rice. Plant Cell Physiol. 2009, 50, 1573–1577. [Google Scholar] [CrossRef]
- Baker, N.R.; Harbinson, J.; Kramer, D.M. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ. 2007, 30, 1107–1125. [Google Scholar] [CrossRef]
- Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 1961, 191, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I.; Tsujimoto, H.Y.; McSwain, B.D. Photosynthetic phosphorylation and electron transport. Nature 1965, 207, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P. Metabolic regulation of photosynthetic membrane structure tunes electron transfer function. Biochem. J. 2018, 475, 1225–1233. [Google Scholar] [CrossRef]
- Burrows, P.A.; Sazanov, L.A.; Svab, Z.; Maliga, P.; Nixon, P.J. Identification of a functional respiratory complex in chloroplasts through analysis of tobacco mutants containing disrupted plastid ndh genes. EMBO J. 1998, 17, 868–876. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schunemann, D.; Finazzi, G.; Joliot, P.; Barbato, R.; Leister, D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Perspectives on improving light distribution and light use efficiency in crop canopies. Plant Physiol. 2021, 185, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Biotechnol. Adv. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef]
- Kirst, H.; Gabilly, S.T.; Niyogi, K.K.; Lemaux, P.G.; Melis, A. Photosynthetic antenna engineering to improve crop yields. Planta 2017, 245, 1009–1020. [Google Scholar] [CrossRef]
- Ort, D.R.; Melis, A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar] [CrossRef]
- Kirst, H.; Formighieri, C.; Melis, A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim. Biophys. Acta 2014, 1837, 1653–1664. [Google Scholar] [CrossRef]
- Bielczynski, L.W.; Schansker, G.; Croce, R. Consequences of the reduction of the photosystem II antenna size on the light acclimation capacity of Arabidopsis thaliana. Plant Cell Environ. 2020, 43, 866–879. [Google Scholar] [CrossRef]
- Bujaldon, S.; Kodama, N.; Rappaport, F.; Subramanyam, R.; Catherine, D.V.; Takahashi, Y.; Wollman, F.A. Functional accumulation of antenna proteins in chlorophyll b-Less mutants of Chlamydomonas reinhardtii. Mol. Plant 2017, 10, 115–130. [Google Scholar] [CrossRef]
- Negi, S.; Perrine, Z.; Friedland, N.; Kumar, A.; Tokutsu, R.; Minagawa, J.; Berg, H.; Barry, A.N.; Govindjee, G.; Sayre, R. Light regulation of light-harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae (dagger). Plant J. 2020, 103, 584–603. [Google Scholar] [CrossRef]
- Kirst, H.; Melis, A. The chloroplast signal recognition particle (CpSRP) pathway as a tool to minimize chlorophyll antenna size and maximize photosynthetic productivity. Biotechnol. Adv. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.X.; Song, Q.S.; Li, M.; Liu, X.Y.; Shi, Z.; Chen, F.M.; Chen, G.Y.; Zheng, H.Q.; Zhu, X.G. Decreasing photosystem antenna size by inhibiting chlorophyll synthesis: A double-edged sword for photosynthetic efficiency. Crop Environ. 2023, 2, 46–58. [Google Scholar] [CrossRef]
- Ghosh, D.; Mohapatra, S.; Dogra, V. Improving photosynthetic efficiency by modulating non-photochemical quenching. Trends Plant Sci. 2023, 28, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Johnson, M.P.; Perez-Bueno, M.L.; Kiss, A.Z.; Ruban, A.V. Photosynthetic acclimation: Does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J. 2008, 275, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Muller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Cantrell, M.; Peers, G.A. Mutant of Chlamydomonas without LHCSR maintains high rates of photosynthesis, but has reduced cell division rates in sinusoidal light conditions. PLoS ONE 2017, 12, e179395. [Google Scholar] [CrossRef]
- Barera, S.; Dall’Osto, L.; Bassi, R. Effect of lhcsr gene dosage on oxidative stress and light use efficiency by Chlamydomonas reinhardtii cultures. J. Biotechnol. 2021, 328, 12–22. [Google Scholar] [CrossRef]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- De, S.A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar]
- Leonelli, L.; Brooks, M.D.; Niyogi, K.K. Engineering the lutein epoxide cycle into Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E7002–E7008. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Shikanai, T. PGR5-dependent cyclic electron flow protects photosystem I under fluctuating light at donor and acceptor sides. Plant Physiol. 2019, 179, 588–600. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Shikanai, T. Regulatory network of proton motive force: Contribution of cyclic electron transport around photosystem I. Photosynth. Res. 2016, 129, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; McAusland, L.; Lawson, T.; Raines, C.A. Overexpression of the Rieske FeS protein increases electron transport rates and biomass yield. Plant Physiol. 2017, 175, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ermakova, M.; Lopez-Calcagno, P.E.; Raines, C.A.; Furbank, R.T.; Susanne, V.C. Overexpression of the Rieske FeS protein of the cytochrome b6f complex increases C4 photosynthesis in Setaria viridis. Commun. Bio. 2019, 2, 314. [Google Scholar] [CrossRef]
- Reizer, J.; Reizer, A.; Saier, M.H. Is the ribulose monophosphate pathway widely distributed in bacteria? Microbiology (Reading) 1997, 143 Pt 8, 2519–2520. [Google Scholar] [CrossRef]
- Strauss, G.; Fuchs, G. Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle. Eur. J. Biochem. 1993, 215, 633–643. [Google Scholar] [CrossRef]
- Peterhansel, C.; Offermann, S. Re-engineering of carbon fixation in plants—challenges for plant biotechnology to improve yields in a high-CO2 world. Curr. Opin. Biotechnol. 2012, 23, 204–208. [Google Scholar] [CrossRef]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef]
- Iqbal, W.A.; Miller, I.G.; Moore, R.L.; Hope, I.J.; Cowan-Turner, D.; Kapralov, M.V. Rubisco substitutions predicted to enhance crop performance through carbon uptake modelling. J. Exper. Bot. 2021, 72, 6066–6075. [Google Scholar] [CrossRef]
- Ishikawa, C.; Hatanaka, T.; Misoo, S.; Miyake, C.; Fukayama, H. Functional incorporation of sorghum small subunit increases the catalytic turnover rate of Rubisco in transgenic rice. Plant Physiol. 2011, 156, 1603–1611. [Google Scholar] [CrossRef]
- Matsumura, H.; Shiomi, K.; Yamamoto, A.; Taketani, Y.; Kobayashi, N.; Yoshizawa, T.; Tanaka, S.I.; Yoshikawa, H.; Endo, M.; Fukayama, H. Hybrid Rubisco with complete replacement of rice Rubisco small subunits by sorghum counterparts confers C4- plant-like high catalytic activity. Mol. Plant 2020, 13, 1570–1581. [Google Scholar] [CrossRef]
- Lin, M.T.; Occhialini, A.; Andralojc, P.J.; Devonshire, J.; Hines, K.M.; Parry, M.; Hanson, M.R. β-Carboxysomal proteins assemble into highly organized structures in Nicotiana chloroplasts. Plant J. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Tanaka, M.; Keira, M.; Yoon, D.K.; Mae, T.; Ishida, H.; Makino, A.; Ishiyama, K. Photosynthetic enhancement, lifespan extension, and leaf area enlargement in flag leaves increased the yield of transgenic rice plants overproducing Rubisco under sufficient N fertilization. Rice 2022, 15, 10. [Google Scholar] [CrossRef]
- Lin, M.T.; Orr, D.J.; Worrall, D.; Parry, M.; Carmo-Silva, E.; Hanson, M.R. A procedure to introduce point mutations into the Rubisco large subunit gene in wild-type plants. Plant J. 2021, 106, 876–887. [Google Scholar] [CrossRef]
- Lorimer, G.H.; Miziorko, H.M. Carbamate formation on the epsilon-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and Mg2+. Biochemistry 1980, 19, 5321–5328. [Google Scholar] [CrossRef]
- Portis, A.J. Rubisco activase—Rubisco’s catalytic chaperone. Photosynth. Res. 2003, 75, 11–27. [Google Scholar] [CrossRef]
- Parry, M.A.; Keys, A.J.; Madgwick, P.J.; Carmo-Silva, A.E.; Andralojc, P.J. Rubisco regulation: A role for inhibitors. J. Exp. Bot. 2008, 59, 1569–1580. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Relationship between the heat tolerance of photosynthesis and the thermal stability of rubisco activase in plants from contrasting thermal environments. Plant Physiol. 2004, 134, 1460–1470. [Google Scholar] [CrossRef]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef]
- Kumar, A.; Li, C.; Portis, A.J. Arabidopsis thaliana expressing a thermostable chimeric Rubisco activase exhibits enhanced growth and higher rates of photosynthesis at moderately high temperatures. Photosynth. Res. 2009, 100, 143–153. [Google Scholar] [CrossRef]
- Yamori, W.; Masumoto, C.; Fukayama, H.; Makino, A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 2012, 71, 871–880. [Google Scholar] [CrossRef]
- Suganami, M.; Suzuki, Y.; Tazoe, Y.; Yamori, W.; Makino, A. Co-overproducing Rubisco and Rubisco activase enhances photosynthesis in the optimal temperature range in rice. Plant Physiol. 2021, 185, 108–119. [Google Scholar] [CrossRef]
- Zhu, X.G.; Eric, D.S.; Long, S.P. Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: A numerical simulation using an evolutionary algorithm. Plant Physiol. 2007, 145, 513–526. [Google Scholar] [CrossRef]
- Rosenthal, D.M.; Locke, A.M.; Khozaei, M.; Raines, C.A.; Long, S.P.; Ort, D.R. Over-expressing the C(3) photosynthesis cycle enzyme sedoheptulose-1–7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (FACE). BMC Plant Biol. 2011, 11, 123. [Google Scholar] [CrossRef]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef]
- Mangan, N.M.; Flamholz, A.; Hood, R.D.; Milo, R.; Savage, D.F. pH determines the energetic efficiency of the cyanobacterial CO2 concentrating mechanism. Proc. Natl. Acad. Sci. USA 2016, 113, E5354–E5362. [Google Scholar] [CrossRef]
- Jenkins, C.L.; Furbank, R.T.; Hatch, M.D. Mechanism of C(4) photosynthesis: A model describing the inorganic carbon pool in bundle sheath cells. Plant Physiol. 1989, 91, 1372–1381. [Google Scholar] [CrossRef]
- Wang, P.; Khoshravesh, R.; Karki, S.; Tapia, R.; Balahadia, C.P.; Bandyopadhyay, A.; Quick, W.P.; Furbank, R.; Sage, T.L.; Langdale, J.A. Re-creation of a key step in the evolutionary switch from C(3) to C(4) leaf anatomy. Curr. Biol. 2017, 27, 3278–3287. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Li, J.; Wei, S.; Yan, Y.; Yang, J.; Zhao, M.; Langdale, J.A.; Zhou, W. Maize GOLDEN2-LIKE genes enhance biomass and grain yields in rice by improving photosynthesis and reducing photoinhibition. Commun. Biol. 2020, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Borden, J.S.; Savage, D.F. New discoveries expand possibilities for carboxysome engineering. Curr. Biol. 2021, 61, 58–66. [Google Scholar] [CrossRef]
- Lin, M.T.; Occhialini, A.; Andralojc, P.J.; Parry, M.A.; Hanson, M.R. A faster Rubisco with potential to increase photosynthesis in crops. Nature 2014, 513, 547–550. [Google Scholar] [CrossRef]
- Long, B.M.; Hee, W.Y.; Sharwood, R.E.; Rae, B.D.; Kaines, S.; Lim, Y.L.; Nguyen, N.D.; Massey, B.; Bala, S.; Susanne, V.C.; et al. Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nat. Commun. 2018, 9, 3570. [Google Scholar] [CrossRef] [PubMed]
- Orr, D.J.; Worrall, D.; Lin, M.T.; Carmo-Silva, E.; Hanson, M.R.; Parry, M. Hybrid cyanobacterial-tobacco Rubisco supports autotrophic growth and procarboxysomal aggregation. Plant Physiol. 2020, 182, 807–818. [Google Scholar] [CrossRef]
- Chen, T.; Riaz, S.; Davey, P.; Zhao, Z.; Sun, Y.; Dykes, G.F.; Zhou, F.; Hartwell, J.; Lawson, T.; Nixon, P.J.; et al. Producing fast and active Rubisco in tobacco to enhance photosynthesis. Plant Cell 2023, 35, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Bloom, A. Photorespiration: The futile cycle? Plants 2021, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Kebeish, R.; Niessen, M.; Thiruveedhi, K.; Bari, R.; Hirsch, H.J.; Rosenkranz, R.; St, B.N.; Schönfeld, N.B.; Kreuzaler, F.; Peterh, N.C. Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat. Biotechnol. 2007, 25, 593. [Google Scholar] [CrossRef]
- Trudeau, D.L.; Edlich-Muth, C.; Zarzycki, J.; Scheffen, M.; Goldsmith, M.; Khersonsky, O.; Avizemer, Z.; Fleishman, S.J.; Cotton, C.; Erb, T.J.; et al. Design and in vitro realization of carbon-conserving photorespiration. Proc. Natl. Acad. Sci. USA 2018, 115, E11455–E11464. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Liu, H.W.; Ort, D.R. Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 2019, 363, 45. [Google Scholar] [CrossRef]
- Shen, B.R.; Wang, L.M.; Lin, X.L.; Yao, Z.; Xu, H.W.; Zhu, C.H.; Teng, H.Y.; Cui, L.L.; Liu, E.E.; Zhang, J.J.; et al. Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Mol. Plant 2019, 12, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Zsogon, A.; Cermak, T.; Voytas, D.; Peres, L.E. Genome editing as a tool to achieve the crop ideotype and de novo domestication of wild relatives: Case study in tomato. Plant Sci. 2017, 256, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Palmgren, M.G.; Edenbrandt, A.K.; Vedel, S.E.; Andersen, M.M.; Landes, X.; Osterberg, J.T.; Falhof, J.; Olsen, L.I.; Christensen, S.B.; Sandoe, P.; et al. Are we ready for back-to-nature crop breeding? Trends Plant Sci. 2015, 20, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Shelef, O.; Weisberg, P.J.; Provenza, F.D. The value of native plants and local production in an era of global agriculture. Front. Plant Sci. 2017, 8, 2069. [Google Scholar] [CrossRef] [PubMed]
- Wolter, F.; Schindele, P.; Puchta, H. Plant breeding at the speed of light: The power of CRISPR/Cas to generate directed genetic diversity at multiple sites. BMC Plant Biol. 2019, 19, 176. [Google Scholar] [CrossRef]
- Zsogon, A.; Cermak, T.; Naves, E.R.; Notini, M.M.; Edel, K.H.; Weinl, S.; Freschi, L.; Voytas, D.F.; Kudla, J.; Peres, L. De novo domestication of wild tomato using genome editing. Nat. Biotechnol. 2018, 36, 1211–1216. [Google Scholar] [CrossRef]
- Wu, J.; Lawit, S.J.; Weers, B.; Sun, J.; Mongar, N.; Van, H.J.; Melo, R.; Meng, X.; Rupe, M.; Clapp, J.; et al. Overexpression of zmm28 increases maize grain yield in the field. Proc. Natl. Acad. Sci. USA 2019, 116, 23850–23858. [Google Scholar] [CrossRef]
- Barampuram, S.; Zhang, Z.J. Recent advances in plant transformation. Methods Mol. Biol. 2011, 701, 1–35. [Google Scholar]
- Oliveira, S.F.; Lichtenstein, G.; Alseekh, S.; Rosado-Souza, L.; Conte, M.; Suguiyama, V.F.; Lira, B.S.; Fanourakis, D.; Usadel, B.; Bhering, L.L.; et al. The genetic architecture of photosynthesis and plant growth-related traits in tomato. Plant Cell Environ. 2018, 41, 327–341. [Google Scholar]
- Flexas, J.; Clemente-Moreno, M.J.; Bota, J.; Brodribb, T.J.; Gago, J.; Mizokami, Y.; Nadal, M.; Perera-Castro, A.V.; Roig-Oliver, M.; Sugiura, D.; et al. Cell wall thickness and composition are involved in photosynthetic limitation. J. Exp. Bot. 2021, 72, 3971–3986. [Google Scholar] [CrossRef]
- Xiong, D.; Douthe, C.; Flexas, J. Differential coordination of stomatal conductance, mesophyll conductance, and leaf hydraulic conductance in response to changing light across species. Plant Cell Environ. 2018, 41, 436–450. [Google Scholar] [CrossRef]
- Harrison, E.L.; Arce, C.L.; Gray, J.E.; Hepworth, C. The influence of stomatal morphology and distribution on photosynthetic gas exchange. Plant J. 2020, 101, 768–779. [Google Scholar] [CrossRef]
- Gago, J.; Daloso, D.M.; Carriqui, M.; Nadal, M.; Morales, M.; Araujo, W.L.; Nunes-Nesi, A.; Flexas, J. Mesophyll conductance: The leaf corridors for photosynthesis. Biochem. Soc. Trans. 2020, 48, 429–439. [Google Scholar] [CrossRef]
- Chen, T.W.; Stutzel, H.; Kahlen, K. High light aggravates functional limitations of cucumber canopy photosynthesis under salinity. Ann. Bot. 2018, 121, 797–807. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Tang, Y.; Zhu, X.G. Stomata conductance as a goalkeeper for increased photosynthetic efficiency. Curr. Opin. Biol. 2022, 70, 102310. [Google Scholar] [CrossRef]
- Sakoda, K.; Yamori, W.; Groszmann, M.; Evans, J.R. Stomatal, mesophyll conductance, and biochemical limitations to photosynthesis during induction. Plant Physiol. 2021, 185, 146–160. [Google Scholar] [CrossRef]
- Masle, J.; Gilmore, S.R.; Farquhar, G.D. The ERECTA gene regulates plant transpiration efficiency in Arabidopsis. Nature 2005, 436, 866–870. [Google Scholar] [CrossRef]
- Takai, T.; Adachi, S.; Taguchi-Shiobara, F.; Sanoh-Arai, Y.; Iwasawa, N.; Yoshinaga, S.; Hirose, S.; Taniguchi, Y.; Yamanouchi, U.; Wu, J.; et al. A natural variant of NAL1, selected in high-yield rice breeding programs, pleiotropically increases photosynthesis rate. Sci. Rep. 2013, 3, 2149. [Google Scholar] [CrossRef]
- Lehmeier, C.; Pajor, R.; Lundgren, M.R.; Mathers, A.; Sloan, J.; Bauch, M.; Mitchell, A.; Bellasio, C.; Green, A.; Bouyer, D.; et al. Cell density and airspace patterning in the leaf can be manipulated to increase leaf photosynthetic capacity. Plant J. 2017, 92, 981–994. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Erashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research Progress in Improving Photosynthetic Efficiency. Int. J. Mol. Sci. 2023, 24, 9286. https://doi.org/10.3390/ijms24119286
Li R, He Y, Chen J, Zheng S, Zhuang C. Research Progress in Improving Photosynthetic Efficiency. International Journal of Molecular Sciences. 2023; 24(11):9286. https://doi.org/10.3390/ijms24119286
Chicago/Turabian StyleLi, Ruiqi, Ying He, Junyu Chen, Shaoyan Zheng, and Chuxiong Zhuang. 2023. "Research Progress in Improving Photosynthetic Efficiency" International Journal of Molecular Sciences 24, no. 11: 9286. https://doi.org/10.3390/ijms24119286
APA StyleLi, R., He, Y., Chen, J., Zheng, S., & Zhuang, C. (2023). Research Progress in Improving Photosynthetic Efficiency. International Journal of Molecular Sciences, 24(11), 9286. https://doi.org/10.3390/ijms24119286





