Fluorene Thiophene α-Cyanostilbene Hexacatenar-Generating LCs with Hexagonal Columnar Phases and Gels with Helical Morphologies as Well as a Light-Emitting LC Display
Abstract
:1. Introduction
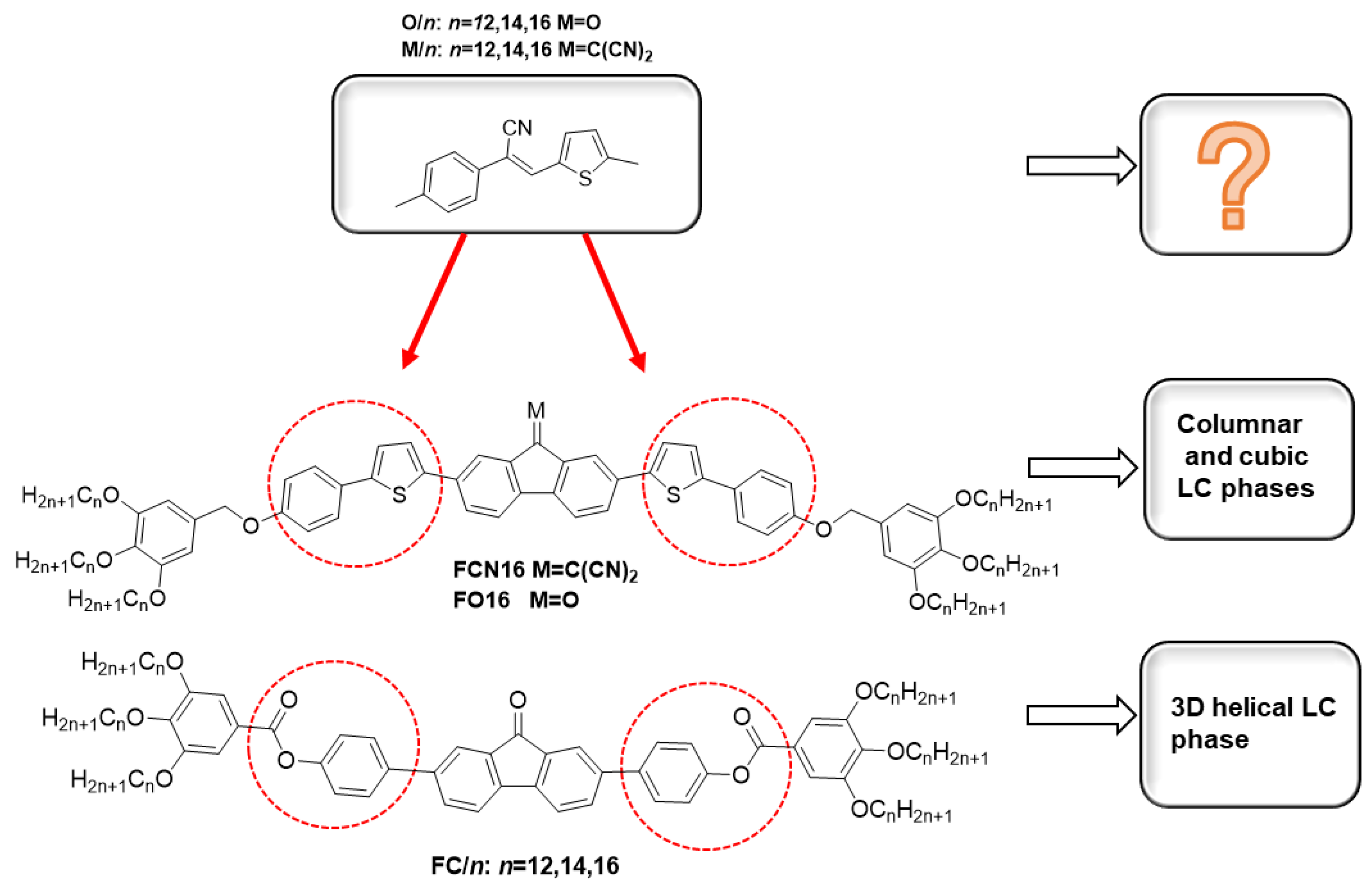
2. Results and Discussion
2.1. Synthesis
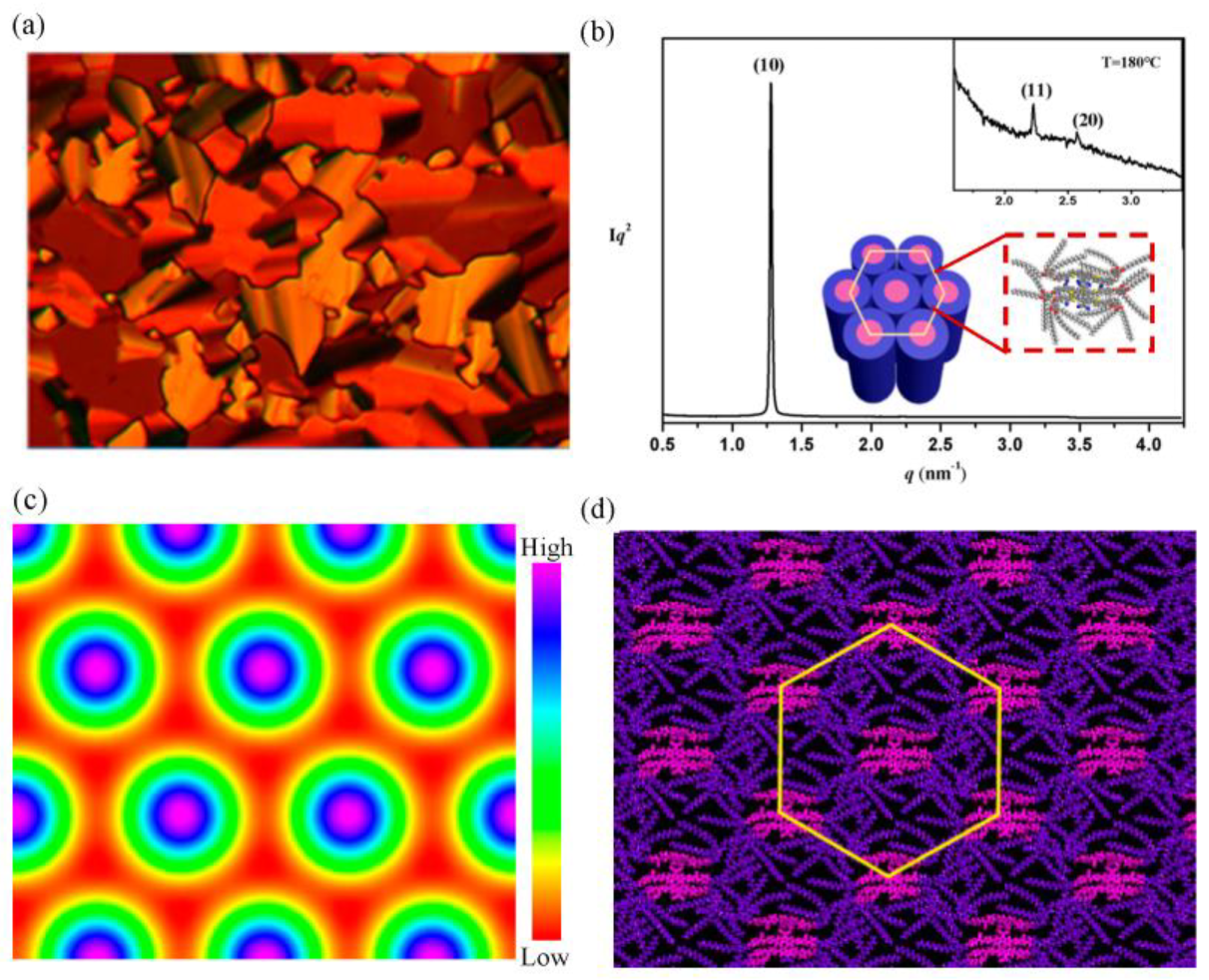
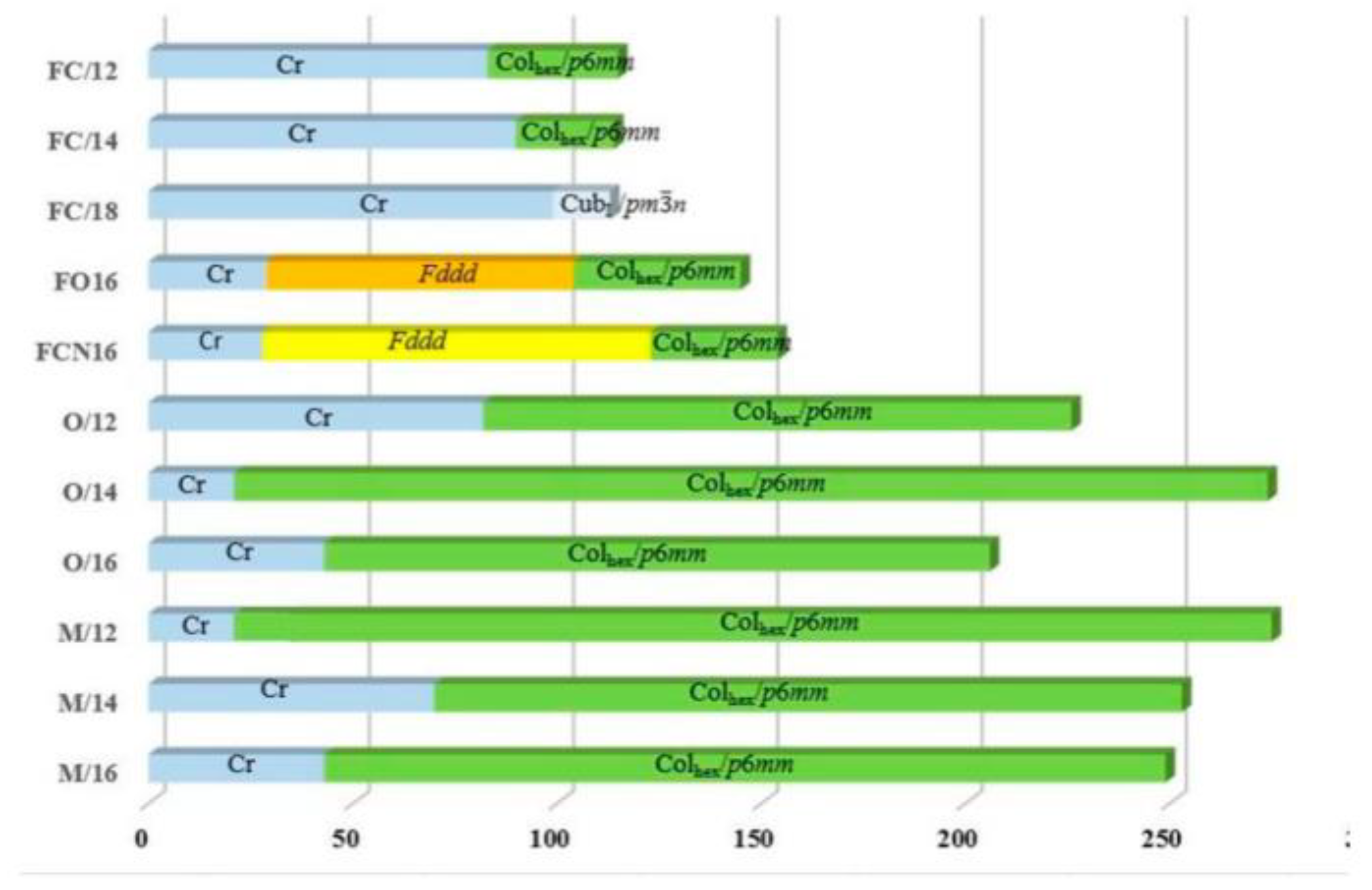
2.2. Mesomorphic Properties
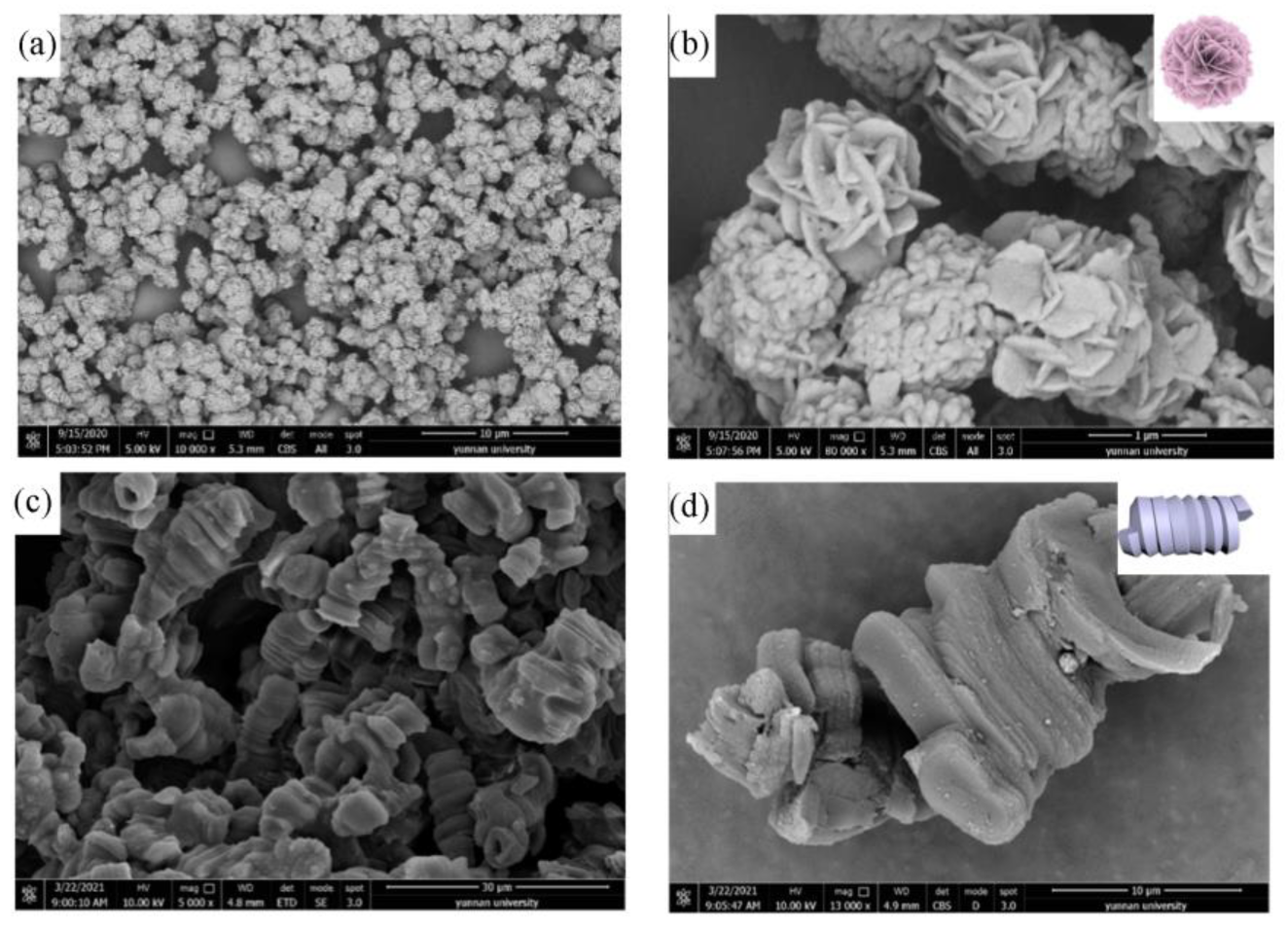
2.3. Gelation Behavior
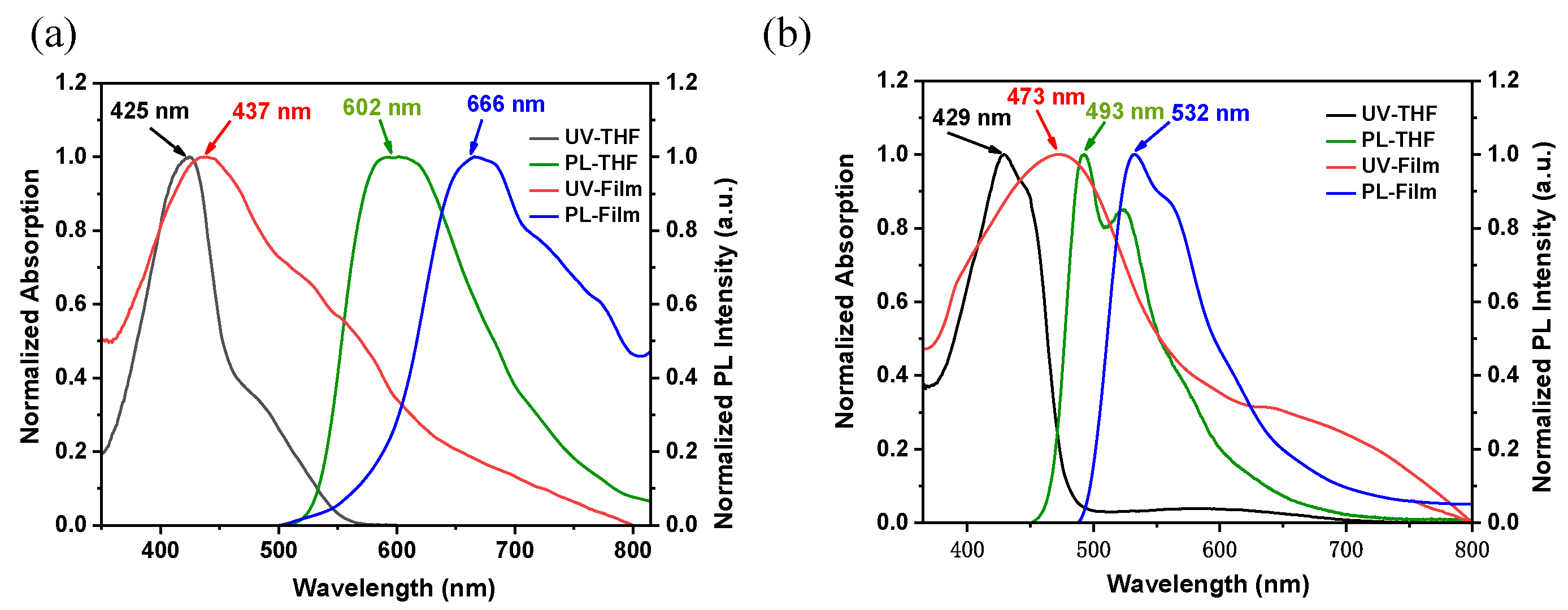
2.4. Photophysical Properties
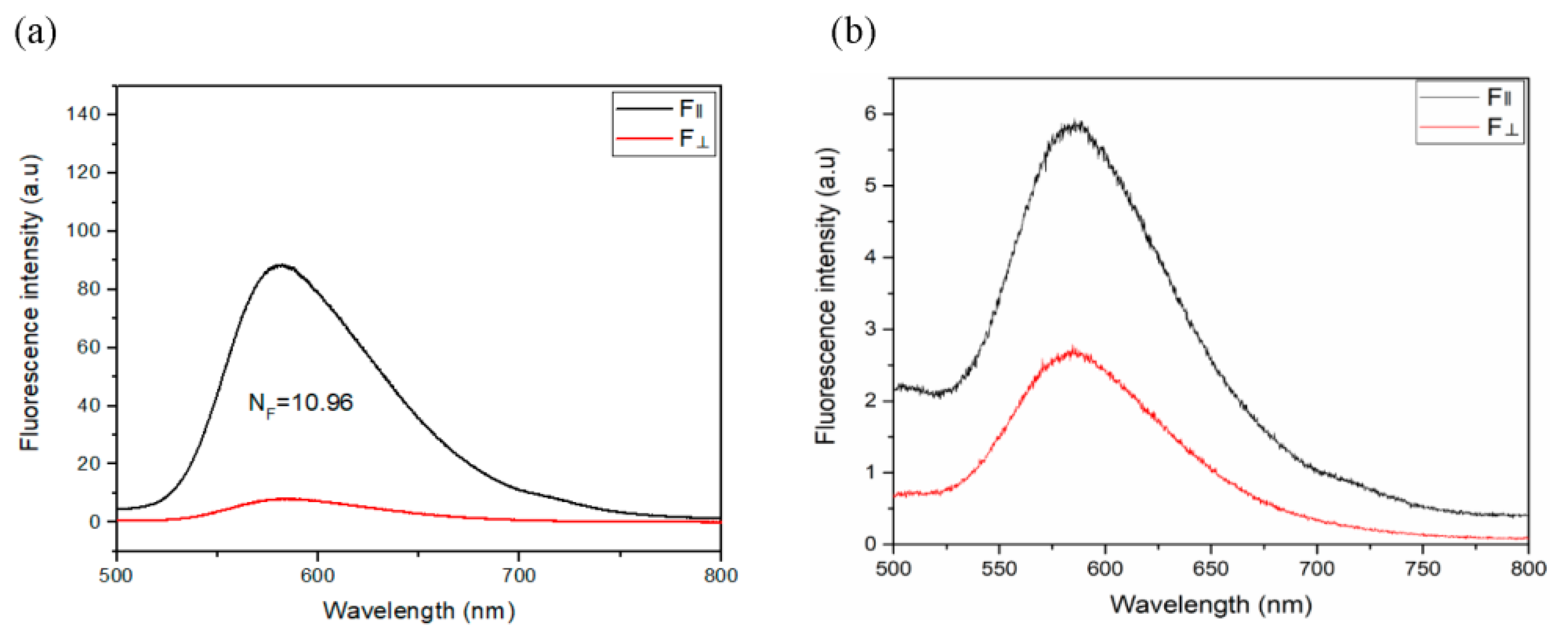
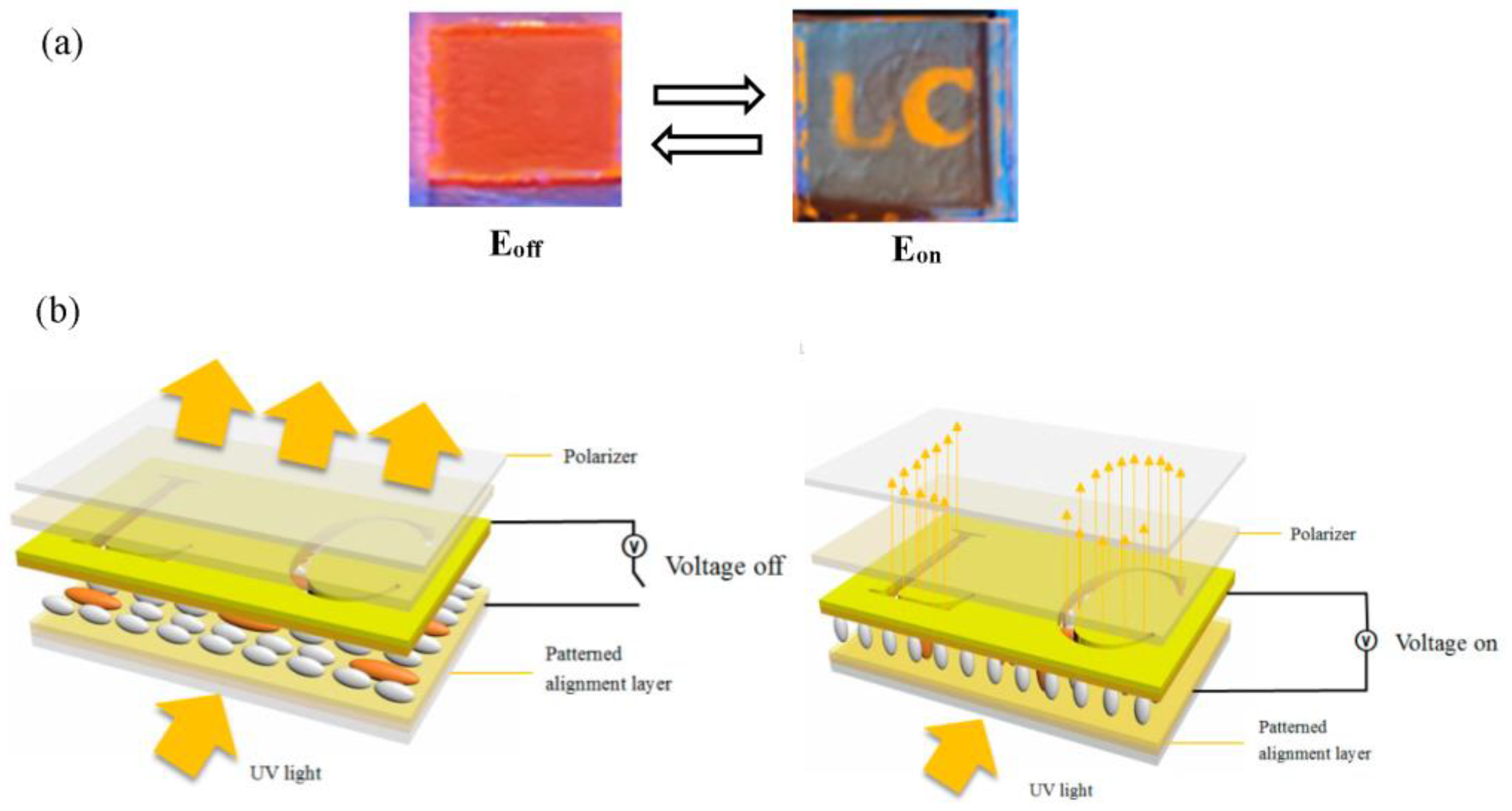
2.5. Polarized Emission Spectra, the Dichroic ratio and LE-LCD Device
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodby, J.W.; Tschierske, C.; Raynes, P.; Gleeson, H.; Kato, T.; Collings, P.J. Laterally Alkyl- and Aryl-Substituted, Swallow-Tailed, and Polycatenar Mesogens: Structural Features and Functionalities. In Handbook of Liquid Crystals; Demus, D., Goodby, J., Gray, G.W., Spiess, H.W., Vill, V., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2014; pp. 1–86. [Google Scholar]
- Nguyen, H.T.; Destrade, C.; Malthete, J. Phasmids and polycatenar mesogens. Adv. Mater. 1997, 9, 375–388. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Gao, S.Y.; Bermeshev, M.V.; Chen, Z.J.; Ren, X.K. Siloxane tethered perylene diimide: From monotropic phase structures to tunable photoconductivity. J. Mater. Chem. C 2021, 9, 9236–9241. [Google Scholar] [CrossRef]
- Eccher, J.; Zajaczkowski, W.; Faria, G.C.; Bock, H.; Seggern, H.V.; Pisula, W.; Bechtold, I.H. Thermal Evaporation versus Spin-Coating: Electrical Performance in Columnar Liquid Crystal OLEDs. ACS Appl. Mater. Interfaces 2015, 7, 16374–16381. [Google Scholar] [CrossRef] [PubMed]
- Hoag, B.P.; Gin, D.L. Fluorescent phasmidic liquid crystals. Adv. Mater. 1998, 10, 1546–1551. [Google Scholar] [CrossRef]
- Levitsky, I.A.; Kishikawa, K.; Eichhorn, S.H.; Swager, T.M. Exciton Coupling and Dipolar Correlations in a Columnar Liquid Crystal: Photophysics of a Bent-Rod Hexacatenar Mesogen. J. Am. Chem. Soc. 2000, 122, 2474–2479. [Google Scholar] [CrossRef]
- Peeters, E.; Van Hal, P.A.; Meskers, S.C.J.; Janssen, R.A.J.; Meijer, E.W. Photoinduced Electron Transfer in a Mesogenic Donor-Acceptor-Donor System. Chem. Eur. 2002, 8, 4470–4474. [Google Scholar] [CrossRef]
- Lincker, F.; Bourgun, P.; Masson, P.; Didier, P.; Guidoni, L.; Bigot, J.Y.; Nicoud, J.F.; Donnio, B.; Guillon, D. Synthesis, Photonic Characteristics, and Mesomorphism of an Oligo Biphenylene Vinylene π-Electron System. Org. Lett. 2005, 7, 1505–1508. [Google Scholar] [CrossRef]
- Sultana, N.H.; Kelly, S.M.; Mansoor, B.; O’Neill, M. Polycatenar oligophenylene liquid crystals. Liq. Cryst. 2007, 34, 1307–1316. [Google Scholar] [CrossRef]
- Matraszek, J.; Mieczkowski, J.; Pociecha, D.; Gorecka, E.; Donnioand, B.; Guillon, D. Molecular Factors Responsible for the Formation of the Axially Polar Columnar Mesophase ColhPA. Chem. Eur. J. 2007, 13, 3377–3385. [Google Scholar] [CrossRef]
- Li, X.; Liu, A.; Xun, S.; Qiao, W.; Wan, X.; Wang, Z.Y. Synthesis and characterization of near-infrared absorbing and fluorescent liquid-crystal chromophore. Org. Lett. 2008, 10, 3785–3787. [Google Scholar] [CrossRef]
- Lifka, T.; Zerban, G.; Seus, P.; Oehlhof, A.; Meier, H. Alkoxy substituted (e,e)-3,6-bis(styryl)pyridazine-a photosensitive mesogen for liquid crystals. Tetrahedron 2008, 64, 6551–6560. [Google Scholar] [CrossRef]
- Cardinaels, T.; Ramaekers, J.; Nockemann, P.; Driesen, K.; Van Hecke, K.; Van Meervelt, L.; Wang, G.; De Feyter, S.; Fernandezglesias, E.; Guillon, D.; et al. Rigid tetracatenar liquid crystals derived from 1, 10-phenanthroline. Soft Matter 2008, 4, 2172–2185. [Google Scholar] [CrossRef]
- Yasuda, T.; Ooi, H.; Morita, J.; Akama, Y.; Minoura, K.; Funahashi, M.; Shimomura, T.; Kato, T. π-conjugated oligothiophene-based polycatenar liquid crystals: Self-organization and photoconductive, luminescent, and redox properties. Adv. Funct. Mater. 2009, 19, 411–419. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, Y.; Wang, L.; Li, Q.; Zheng, K.; Zhan, X.; Liu, Y.; Wan, R.L.J. Synthesis, self-assembly and solution-processed field-effect transistors of a liquid crystalline bis(dithienothiophene) derivative. J. Phys. Chem. C 2009, 113, 16232–16237. [Google Scholar] [CrossRef]
- Sonar, P.; Singh, S.P.; Sudhakar, S.; Dodabalapur, A.; Sellinger, A. High-mobility organic thin film transistors based on benzothiadiazole-sandwiched dihexylquaterthiophenes. Chem. Mater. 2008, 20, 3184–3190. [Google Scholar] [CrossRef]
- Park, Y.S.; Kim, D.; Hoosung, L.; Moon, B. Donor-acceptor-donor-type liquid crystal with a pyridazine core. Org. Lett. 2006, 8, 699–4702. [Google Scholar] [CrossRef]
- Zhang, B.; Xiao, Y.L.; Fang, H.P.; Gao, H.F.; Wang, F.K.; Cheng, X.H. Mesogenic D-A fluorophores based on cyanovinyl and benzothiadiazole. New J. Chem. 2018, 42, 16709–16716. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, M.J.; Song, H.M.; Song, B.J.; Seo, K.D.; Pastore, M.; Anselmi, C.; Fantacci, S.; Angelis, F.D.; Nazeeruddin, M.K.; et al. Organic dyes incorporating low-band-gap chromophores based on π-extended benzothiadiazole for dye-sensitized solar cells. Dyes Pigm. 2011, 91, 192–198. [Google Scholar] [CrossRef]
- Seo, K.D.; Choi, I.T.; Park, Y.G.; Kang, S.; Lee, J.Y.; Kim, H.K. Novel D-A-π-A coumarin dyes containing low band-gap chromophores for dye-sensitised solar cells. Dyes Pigm. 2012, 94, 469–474. [Google Scholar] [CrossRef]
- Thomas, K.R.J.; Singh, P.; Baheti, A.; Hsu, Y.C.; Ho, K.C.; Lin, J.T. Electro-optical Properties of New Anthracene Based Organic Dyes for Dye-sensitized Solar Cells. Dyes Pigm. 2011, 91, 33–43. [Google Scholar] [CrossRef]
- Guo, L.X.; Xing, Y.B.; Wang, M.; Sun, Y.; Zhang, X.Q.; Lin, B.Q.; Yang, H. Luminescent liquid crystals bearing aggregation-induced emission active tetraphenylthiophene fluorophore. J. Mater. Chem. C 2019, 7, 4828–4837. [Google Scholar] [CrossRef]
- Yu, W.L.; Cao, Y.; Pei, J.; Huang, W.; Heeger, A.J. Blue polymer light-emitting diodes from poly (9,9-dihexylfluorene-alt-co-2, 5-didecyloxypara-phenylene). Appl. Phys. Lett. 1998, 75, 3270–3272. [Google Scholar] [CrossRef]
- Yu, W.L.; Pei, J.; Cao, Y.; Huang, W.; Heeger, A.J. New efficient blue light emitting polymer for light emitting diodes. Chem. Commun. 1999, 18, 1837–1838. [Google Scholar] [CrossRef]
- Perepichka, I.F.; Perepichka, D.F. Functional Oligothiophene-based Materials: Nanoarchitectues and Applications. In Handbook of Thiophene-Based Materials: Applications in Organic Electronics and Photonics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–155. [Google Scholar]
- Funahashi, M.; Yasuda, T.; Kato, T. Liquid Crystal Semiconductors: Oligothiophene and Related Materials. In Handbook of Liquid Crystals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–34. [Google Scholar]
- Calcaterra, A.; D’Acquarica, I. The market of chiral drugs: Chiral switches versus de novo enantiomerically pure compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Kan, X.; Shang, J.Y.; Qiao, H.; Dong, Y.B. Catalytic Asymmetric Synthesis of Chiral Covalent Organic Frameworks from Prochiral Monomers for Heterogeneous Asymmetric Catalysis. J. Am. Chem. Soc. 2020, 142, 16915–16920. [Google Scholar] [CrossRef]
- Roberts, J.C.; Hudson, Z.M.; Lemieux, R.P. The influence of alkoxy chain length on the ferroelectric properties of chiral fluorenol liquid crystals. J. Mater. Chem. 2008, 18, 3361–3365. [Google Scholar] [CrossRef]
- Sahoo, D.; Imam, M.R.; Peterca, M.; Partridge, B.E.; Wilson, D.A.; Zeng, X.B.; Ungar, G.; Heiney, P.A.; Percec, V. Hierarchical Self-Organization of Chiral Columns from Chiral Supramolecular Spheres. J. Am. Chem. Soc. 2018, 140, 13478–13487. [Google Scholar] [CrossRef]
- Wang, Y.X.; Li, X.J.; Li, F.; Sun, W.Y.; Zhu, C.J.; Cheng, Y.X. Strong circularly polarized luminescence induced from chiral supramolecular assembly of helical nanorods. Chem. Commun. 2017, 53, 7505–7508. [Google Scholar] [CrossRef]
- Mokashi-Punekar, S.; Zhou, Y.; Brooks, S.C.; Rosi, N.L. Construction of Chiral, Helical Nanoparticle Superstructures: Progress and Prospects. Adv. Mater. 2019, 32, 1905975. [Google Scholar] [CrossRef]
- Felix, H.; Quentin, E.; Guillaume, C.; Yan, S.; Carole, D.; Olivier, G.; Frederic, G.; Boris, L.G.L.; Thierry, G.; Vincent, D.; et al. Chiral supramolecular nanotubes of single-chain magnets. Angew. Chem. Int. Edit. 2019, 4, 780–784. [Google Scholar]
- Timoneda, M.A.M.; Calama, M.C.; Reinhoudt, D.N. Supramolecular chirality of self-assembled systems in solution. Chem. Soc. Rev. 2004, 33, 363–372. [Google Scholar] [CrossRef]
- Link, D.R.; Natale, G.; Shao, R.; Maclennan, J.E.; Clark, N.A.; Korblova, E.; Walba, D.M. Spontaneous Formation of Macroscopic Chiral Domains in a Fluid Smectic Phase of Achiral Molecules. Science 1997, 278, 1924–1927. [Google Scholar] [CrossRef]
- Tschierske, C.; Ungar, G. Mirror Symmetry Breaking by Chirality Synchronisation in Liquids and Liquid Crystals of Achiral Molecules. Chem. Phys. Chem. 2016, 17, 9–16. [Google Scholar] [CrossRef]
- Dressel, C.; Reppe, T.; Poppe, S.; Prehm, M.; Lu, H.J.; Zeng, X.B.; Ungar, G.; Tschierske, C. Supramolecular Networks: Helical Networks of π-Conjugated Rods-A Robust Design Concept for Bicontinuous Cubic Liquid Crystalline Phases with Achiral Ia d and Chiral I23 Lattice. Adv. Funct. Mater. 2020, 30, 2004353. [Google Scholar] [CrossRef]
- Prins, L.J.; Huskens, J.; Jong, F.D.; Timmerman, P.; Reinhoudt, D.N. Complete asymmetric induction of supramolecular chirality in a hydrogen-bonded assembly. Nature 1999, 398, 498–502. [Google Scholar] [CrossRef]
- Duan, P.; Cao, H.; Zhang, L.; Liu, M.H. Gelation induced supramolecular chirality: Chirality transfer, amplification and application. Soft Matter 2014, 10, 5428–5448. [Google Scholar] [CrossRef]
- Ouyang, G.H.; Liu, M.H. Self-assembly of chiral supra-amphiphiles. Mate. Chem. Front. 2020, 4, 155–167. [Google Scholar] [CrossRef]
- Lincker, F.; Attias, A.J.; Mathevet, F.; Heinrich, B.; Donnio, B.; Fave, J.L.; Rannou, P.; Demadrille, R. Influence of polymorphism on charge transport properties in isomers of fluorenone-based liquid crystalline semiconductors. Chem. Commun. 2012, 48, 3209–3211. [Google Scholar] [CrossRef]
- Lincker, F.; Heinrich, B.; Bettignies, R.D.; Rannou, P.; Pécaut, J.; Grevin, B.; Pron, A.; Donnio, B.; Demadrille, R. Fluorenone core donor-acceptor-donor π-conjugated molecules end-capped with dendritic oligo(thiophene)s: Synthesis, liquid crystalline behaviour, and photovoltaic applications. J. Mater. Chem. 2011, 21, 5238–5247. [Google Scholar] [CrossRef]
- Do, T.T.; Rundel, K.; Gu, Q.Y.; Gann, E.; Manzhos, S.; Feron, K.; Bella, J.; McNeillb, C.R.; Sonar, P. 9-Fluorenone and 9,10-anthraquinone potential fused aromatic building blocks to synthesize electron acceptors for organic solar cell. New J. Chem. 2017, 41, 2899–2909. [Google Scholar] [CrossRef]
- Wang, C.S.; Batsanov, A.S.; Bryce, M.R.; Sage, I. Nanoscale Aryleneethynylene Molecular Wires with Reversible Fluorenone Electrochemistry for Self-Assembly onto Metal Surfaces. Org. Lett. 2004, 6, 2181–2184. [Google Scholar] [CrossRef]
- McCubbin, J.A.; Tong, X.; Zhao, Y.; Snieckus, V.; Lemieux, R.P. Directed Metalation-Cross Coupling Route to Ferroelectric Liquid Crystals with a Chiral Fluorenol Core: The Effect of Intermolecular Hydrogen Bonding on Polar Order. Chem. Mater. 2005, 17, 2574–2581. [Google Scholar] [CrossRef]
- Percec, V.; Glodde, M.; Bera, T.K.; Miura, Y.; Shiyanovskaya, I.; Singer, K.D.; Balagurusamy, V.S.K.; Heiney, P.A.; Schnell, I.; Rapp, A.; et al. Self-organization of supramolecular helical dendrimers into complex electronic materials. Nature 2002, 419, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Xiao, Y.L.; Guo, C.X.; Chang, Q.; Gao, H.F.; Cheng, X.H. Control the self-assembly of fluorenone-based polycatenars by tuning chain length. Tetrahedron 2019, 75, 409–415. [Google Scholar] [CrossRef]
- Li, Y.X.; Gao, H.F.; Zhang, R.B.; Gabana, K.; Chang, Q.; Gehring, G.; Cheng, X.H.; Zeng, X.B.; Ungar, G. A case of antiferrochirality Liquid crystal phase of counter-rotating staircases. Nat. Commun. 2022, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, Y.T.; Tao, Y.; Wang, Y.; Miao, X.R.; Cheng, X.H.; Deng, W.L. Concentration-Dependent Conformational Isomerization of Fluorenone-Based Polycatenar in 2D Polymorphic Self-Assembly. J. Phys. Chem. C 2020, 124, 25396–25402. [Google Scholar] [CrossRef]
- Kim, H.J.; Nandajan, P.C.; Gierschner, J.; Park, S.Y. Light-Harvesting Fluorescent Supramolecular Block Copolymers Based on Cyanostilbene Derivatives and Cucurbit [8] urils in Aqueous Solution. Adv. Funct. Mater. 2017, 28, 1705141. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar. Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Martinez-Abadı′a, M.; Gimenez, R.; Ros, M.B. Self-Assembled α-Cyanostilbenes for Advanced Functional Materials. Adv. Mater. 2017, 20, 1704161. [Google Scholar]
- Fang, W.Y.; Zhao, W.; Pei, P.; Liu, R.; Zhang, Y.Y.; Kong, L.; Yang, J.X. A Λ-shaped cyanostilbene derivative: Multi-stimuli responsive fluorescence sensors, rewritable information storage and colour converter for w-LEDs. J. Mater. Chem. C 2018, 6, 9269–9276. [Google Scholar] [CrossRef]
- Gopinath, A.; Manivannan, N.; Mandal, S.; Mathivanan, N.; Nasar, A.S. Ubstituent enhanced fluorescence properties of star α-cyanostilbenes and their application in bioimaging. J. Mater. Chem. B 2019, 7, 6010–6023. [Google Scholar] [CrossRef]
- Rodriguez, J.G.; Tejedor, J.L.; Parraa, T.L.; Diazb, C. Synthesis of conjugated 2,7-bis(trimethylsilylethynyl)-(phenylethynyl)nfluoren-9-one and 9-(p-methoxyphenyl)-9-methyl derivatives: Optical properties. Tetrahedron 2006, 62, 3355–3361. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, X.; Jiang, S.S.; Liu, L.L.; Weeksb, B.L.; Zhang, Z. Highly efficient synthesis of 9-fluorenones from 9H-fluorenes by air oxidation. Green Chem. 2011, 13, 1891–1896. [Google Scholar] [CrossRef]
- Tan, X.P.; Kong, L.Y.; Dai, H.; Cheng, X.H.; Liu, F.; Tschierske, C. Triblock Polyphiles through Click Chemistry: Self-Assembled Thermotropic Cubic Phases Formed by Micellar and Monolayer Vesicular Aggregates. Chem. Eur. J. 2013, 19, 16303–16313. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Tan, X.P.; Xing, W.; Zhao, K.; Zhang, B.; Cheng, X.H. Coumarin-based emissive hexacatenars: Synthesis, 2D and 3D self-assembly and photodimerization. J. Mater. Chem. C 2018, 6, 10782–10792. [Google Scholar] [CrossRef]
- Marrocchi, A.; Seri, M.; Kim, C.; Facchetti, A.; Taticchi, A.; Marks, T.J. Low-Dimensional Arylacetylenes for Solution-Processable Organic Field-Effect Transistors. Chem. Mater. 2009, 21, 2592–2594. [Google Scholar] [CrossRef]
- Umberger, J.Q.; LaMer, V.K. The kinetics of diffusion controlled molecular and ionic reactions in solution as determined by measurements of the quenching of fluorescence. J. Am. Chem. Soc. 1945, 67, 1099–1109. [Google Scholar] [CrossRef]
- Pron, A.; Gawrys, P.; Zagorska, M. Electroactive materials for organic electronics: Preparation strategies, structural aspects and characterization techniques. Chem. Soc. Rev. 2010, 39, 2577–2632. [Google Scholar] [CrossRef]
- Zou, C.; Wang, J.; Wang, M. Patterning of discotic liquid crystals with tunable molecular orientation for electronic applications. Small 2018, 14, 1800557. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Pschirer, N.G. Polyphenylene-based materials for organic photovoltaics. Chem. Rev. 2010, 110, 6817–6855. [Google Scholar] [CrossRef]
- Popov, P.; Honaker, L.W.; Kooijman, E.E. A liquid crystal biosensor for specific detection of antigens. Sens. Biosensing Res. 2016, 8, 31–35. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jakli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, Z.; Mo, G.; Xing, X.; Liu, P. A small-angle x-ray scattering station at beijing synchrotron radiation facility. Instrum Sci. Technol. 2014, 42, 128–141. [Google Scholar] [CrossRef]
- Williams, A.T.R.; Winfield, S.A.; Miller, J.N. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst 1983, 108, 1067–1071. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.Z.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In Vitro and In Vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Hu, R.R.; Lager, E.; Aguilar-Aguilar, A.; Liu, J.Z.; Lam, J.W.Y.; Sung, H.H.Y.; Williams, I.D.; Zhong, Y.C.; Wong, Y.S.; Pena-Cabrera, E.; et al. Twisted Intramolecular Charge Transfer and Aggregation-Induced Emission of BODIPY Derivatives. J. Phys. Chem. C 2009, 113, 15845–15853. [Google Scholar] [CrossRef]
- Gobel, D.; Clamora, N.; Nachtsheim, B.J. Regioselective ortho-functionalization of bromofluorenecarbaldehydes using TMPMgCl·LiCl. Org. Biomol. Chem. 2018, 16, 4071–4075. [Google Scholar] [CrossRef]
- Lee, T.; Landis, C.A.; Dhar, B.M.; Jung, B.J.; Sun, J.; Sarjeant, A.; Lee, H.J.; Katz, H. Synthesis, Structural Characterization, and Unusual Field-Effect Behavior of Organic Transistor Semiconductor Oligomers: Inferiority of Oxadiazole Compared with Other Electron-Withdrawing Subunits. J. Am. Chem. Soc. 2009, 131, 1692–1705. [Google Scholar] [CrossRef]
- Tan, X.P.; Zhang, R.L.; Guo, C.X.; Cheng, X.H.; Gao, H.F.; Liu, F.; Bruckner, J.R.; Giesselmann, F.; Prehmeand, M.; Tschierske, C. Amphotropic azobenzene derivatives with oligooxyethylene and glycerol based polar groups. J. Mater. Chem. C 2015, 3, 11202–11211. [Google Scholar] [CrossRef]
- Huang, D.X.; Prehm, M.; Gao, H.F.; Cheng, X.H.; Liu, Y.S.; Tschierske, C. Synthesis and self-assembly of luminescent hexacatenar molecules incorporating a 4,7-diphenyl-2,1,3-benzothiadiazole core. RSC Adv. 2016, 6, 21387–21395. [Google Scholar] [CrossRef]


| Comp. | n | T/°C [∆H/kJ·mol−1] | a/nm (T/°C) | μ |
|---|---|---|---|---|
| O/12 | 12 | Cr 82 Colhex/p6mm 226 Iso (Iso 222 Colhex/p6mm 73 Cr)c | 5.37 (200) | 3.44 |
| O/14 | 14 | Cr 21 [28.84] Colhex/p6mm 238 [2.07] Iso (Iso 233 [2.08] Colhex/p6mm 11 [28.17] Cr) | 5.65 (180) | 3.44 |
| O/16 | 16 | Cr 43 [65.61] Colhex/p6mm 206 [1.09] Iso (Iso 206 [1.03] Colhex/p6mm 28 [53.00] Cr) | 6 (180) | 3.16 |
| M/12 | 12 | Cr20c Colhex/p6mmb 275 [0.89] Iso (Iso 259 [0.60] Colhex/p6mm Cr20)c | - | - |
| M/14 | 14 | Cr 70c Colhex/p6mm 253 [2.62] Iso (Iso 230 [1.39] Colhex/p6mm 68c Cr) | 5.65 (180) | 3.36 |
| M/16 | 16 | Cr 43 [22.65] Colhex/p6mm b 249 [2.21] Iso (Iso 243 [0.96] Colhex/p6mm17 [12.78] Cr) | - | - |
| Solvent | O/16 | M/14 |
|---|---|---|
| Ethyl acetate | P | P |
| Acetone | P | P |
| n-hexane | P | P |
| Toluene | S | S |
| Acetonitrile | P | P |
| t-butanol | S | S |
| Dichloromethane | S | S |
| Petroleum ether | S | S |
| 1,4-Dioxane | G | G |
| Ethanol | P | P |
| Compd. | Abs λmax(nm) | PL a λmax (nm) | Stokes Shift b(nm) | (V) | (V) | EHOMO (eV) | ELUMO (eV) | (eV) | (eV) | (eV) |
|---|---|---|---|---|---|---|---|---|---|---|
| O/12 | 425 c (437) d | 602 c (666) d | 177 c (229)d | −0.70 | 1.90 | −5.32 | −2.73 | 2.75 | 2.59 | 2.60 |
| M/12 | 429 c (473) d | 493 c (532) d | 64 c (59) d | −0.70 | 1.45 | −5.39 | −3.41 | 2.20 | 1.98 | 2.20 |
| Solvents | λabs(nm) | λem(nm) a | Stokes Shift (nm) b | ΦFLc |
|---|---|---|---|---|
| Hexane | 420 | 655 | 235 | 0.061 |
| Toluene | 424 | 582 | 158 | 0.579 |
| DCM | 425 | 637 | 212 | 0.114 |
| THF | 425 | 602 | 177 | 0.484 |
| 1,4-Dioxane | 421 | 592 | 171 | 0.40 |
| solid state | - | - | - | 0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Cheng, X. Fluorene Thiophene α-Cyanostilbene Hexacatenar-Generating LCs with Hexagonal Columnar Phases and Gels with Helical Morphologies as Well as a Light-Emitting LC Display. Int. J. Mol. Sci. 2023, 24, 9337. https://doi.org/10.3390/ijms24119337
Zhao H, Cheng X. Fluorene Thiophene α-Cyanostilbene Hexacatenar-Generating LCs with Hexagonal Columnar Phases and Gels with Helical Morphologies as Well as a Light-Emitting LC Display. International Journal of Molecular Sciences. 2023; 24(11):9337. https://doi.org/10.3390/ijms24119337
Chicago/Turabian StyleZhao, Hongmei, and Xiaohong Cheng. 2023. "Fluorene Thiophene α-Cyanostilbene Hexacatenar-Generating LCs with Hexagonal Columnar Phases and Gels with Helical Morphologies as Well as a Light-Emitting LC Display" International Journal of Molecular Sciences 24, no. 11: 9337. https://doi.org/10.3390/ijms24119337




