Lysine Deprivation Suppresses Adipogenesis in 3T3-L1 Cells: A Transcriptome Analysis
Abstract
:1. Introduction
2. Results
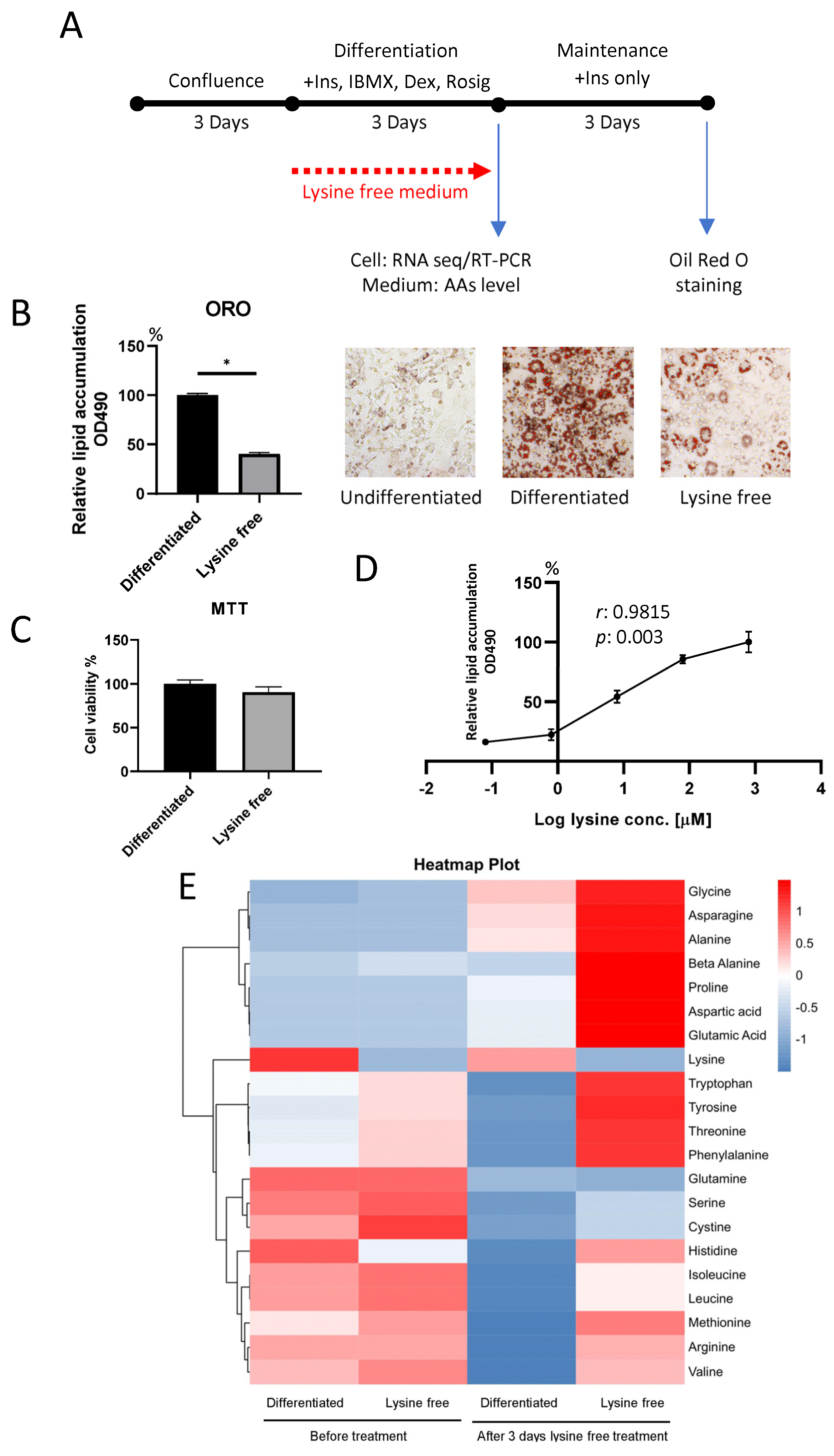
2.1. Lysine Deprivation Suppressed Adipogenesis
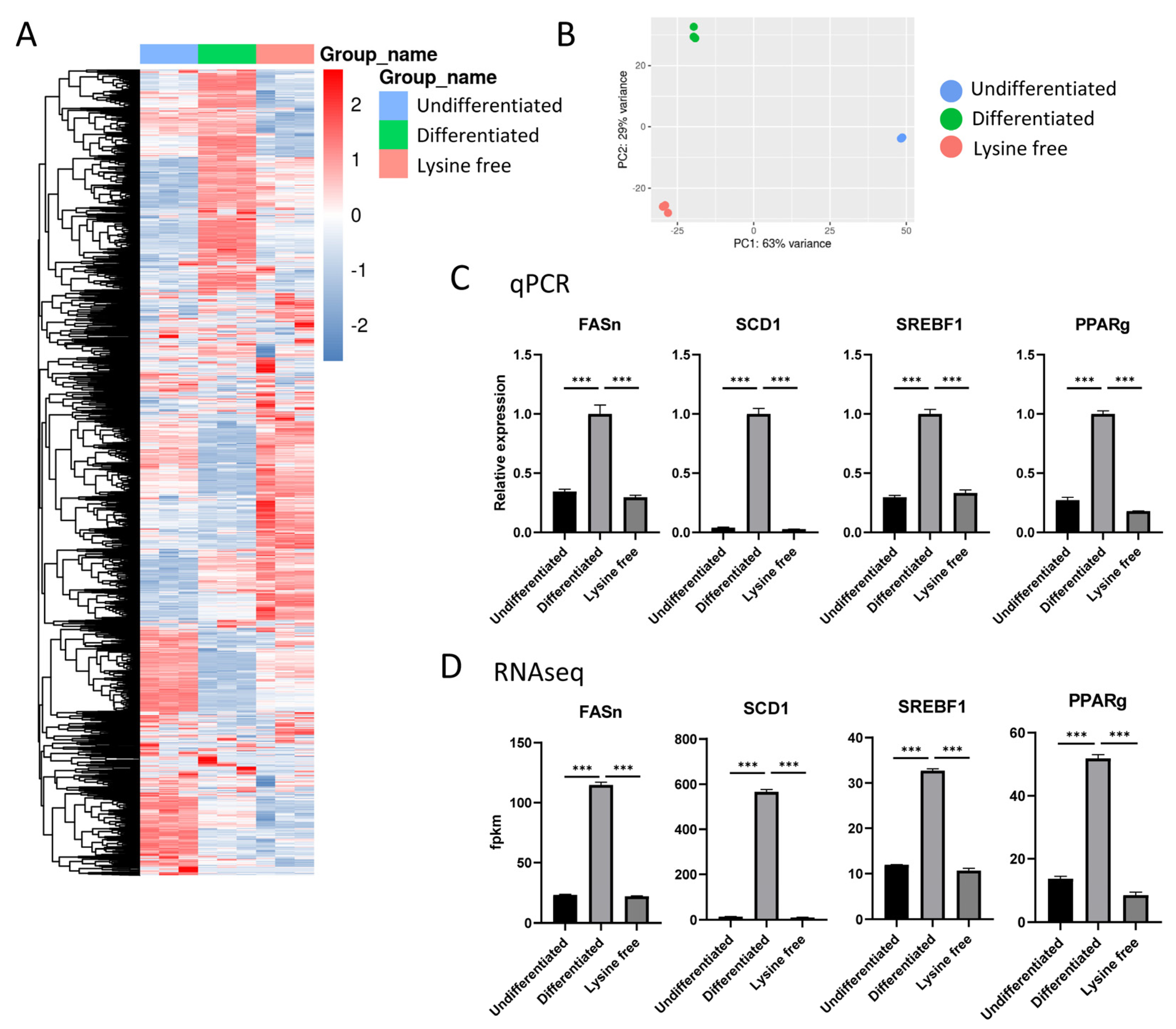
2.2. Transcriptome Profile of the 3T3-L1 Cells
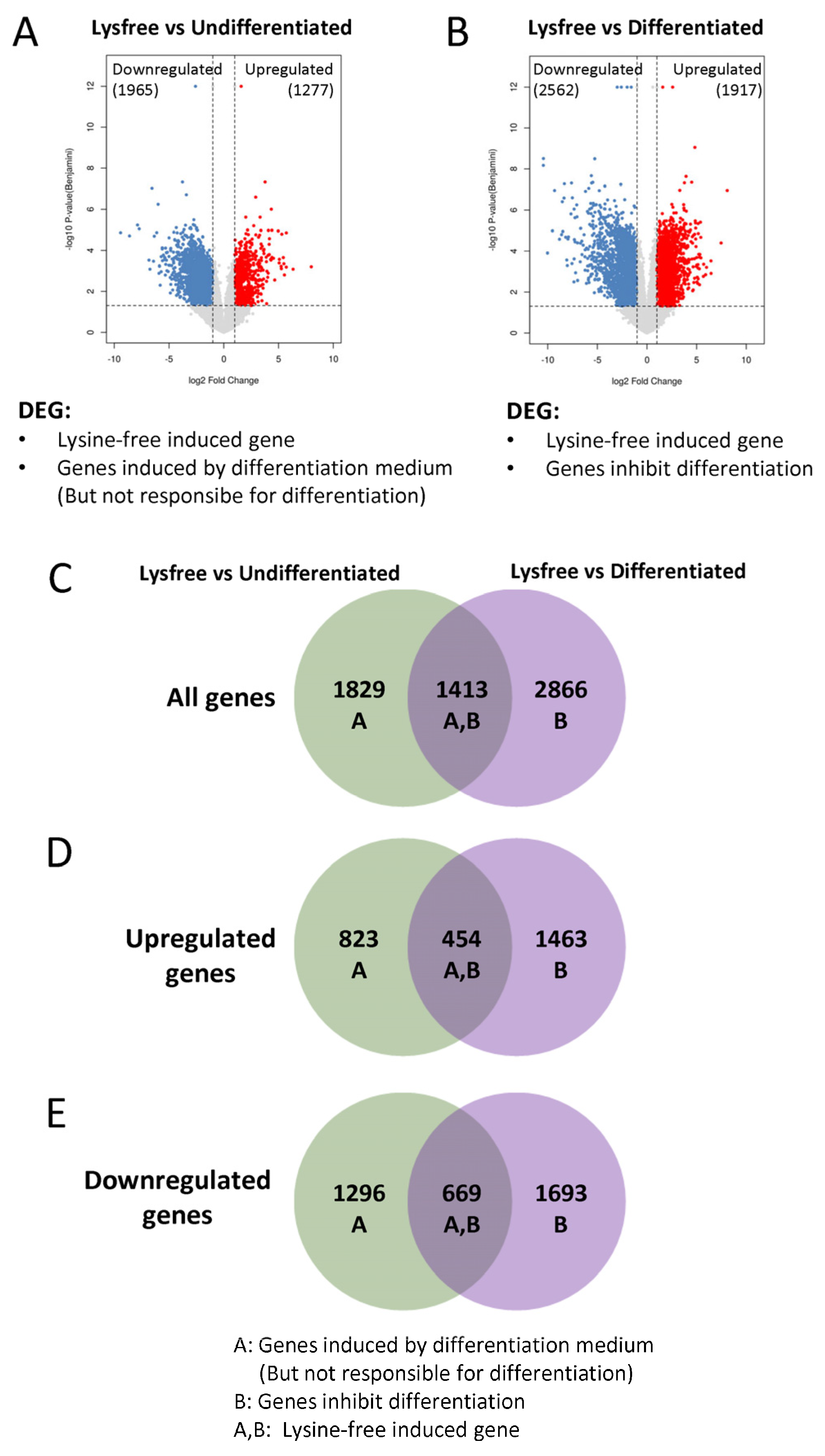
2.3. Comparison between Differentiated and Undifferentiated 3T3-L1 Cells
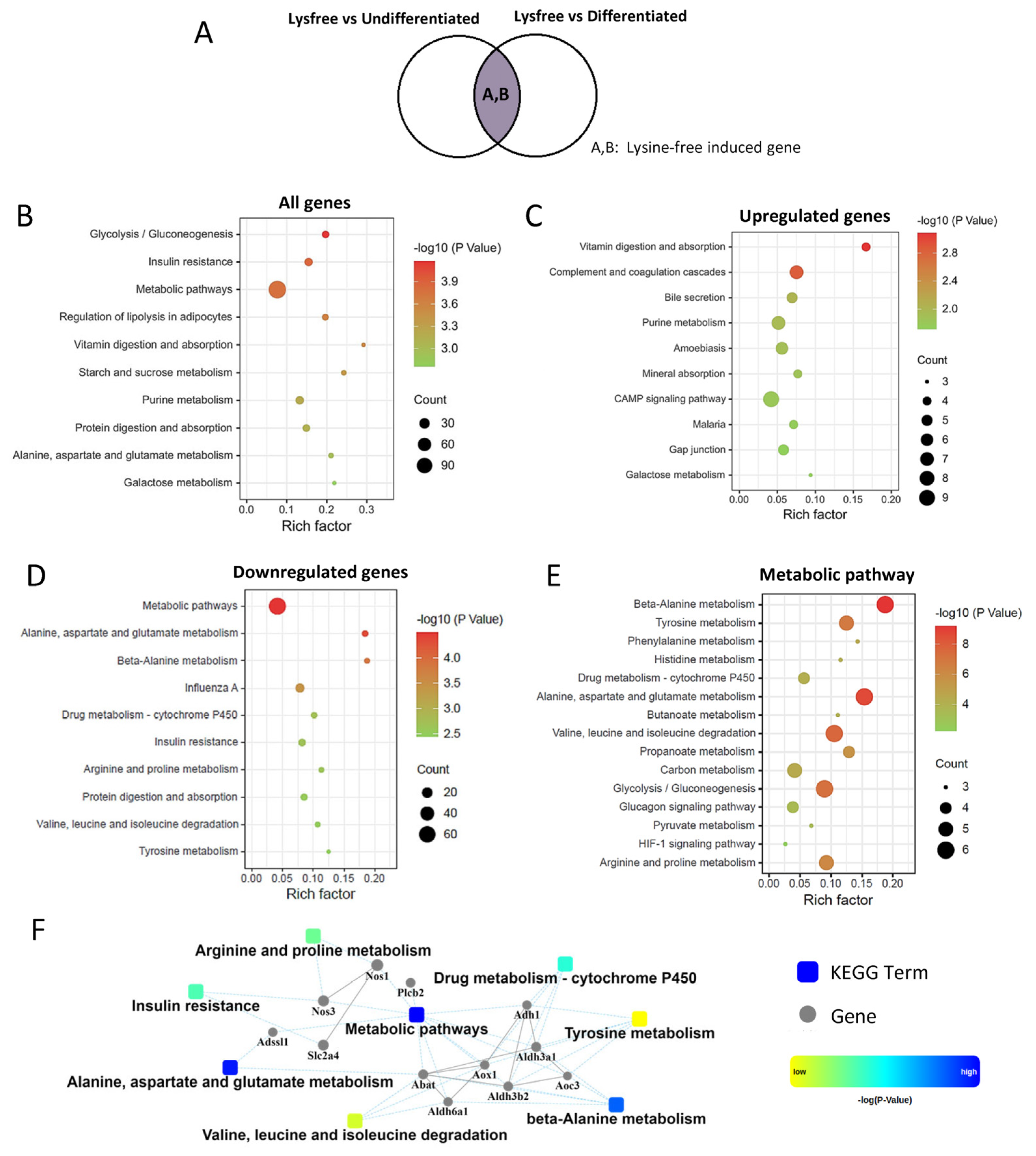
2.4. Identification of Lysine-Depletion-Induced Pathways
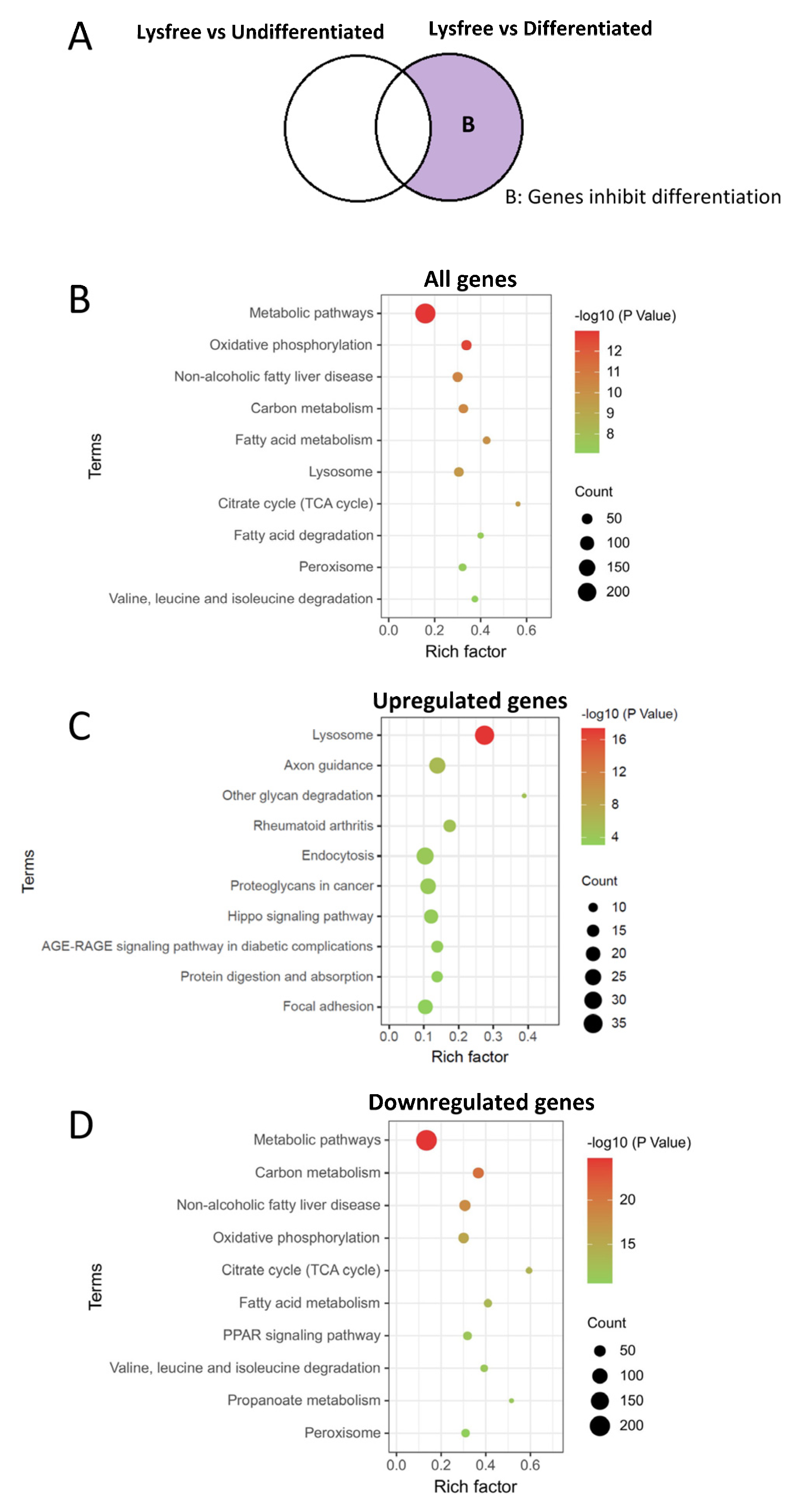
2.5. Genes Critical for Inhibition of 3T3-L1 Cell Differentiation and Adipogenesis
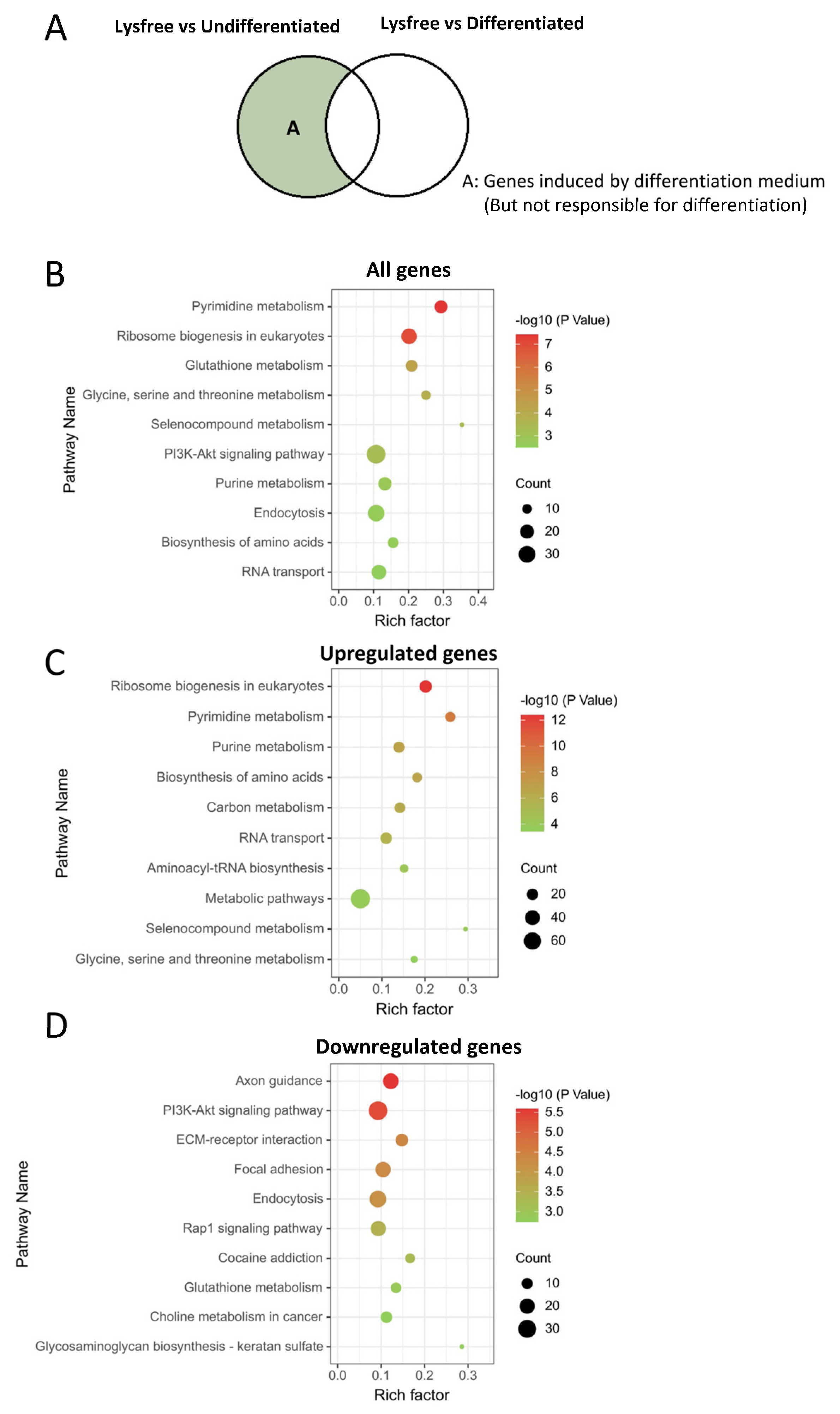
2.6. Pathways That Could Be Altered by Differentiation Medium but Not Critical for the Adipogenesis Process
2.7. Suppression of Adipogenesis Is Not Limited to Lysine Depletion but Also Applied to Methionine and Cystine Deprivation Partially via IL6 Overexpression
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. RNA Extraction, cDNA Synthesis, and RNA Sequencing
4.3. Differentially Expressed GENES (DEG) Analysis
4.4. Functional Annotation Analysis
4.5. Integration of Protein–Protein Interaction (PPI) Network and Module Analysis
4.6. Real-Time PCR Analysis
4.7. Oil-Red O (ORO) Staining
4.8. Medium Amino Acid Analysis
4.9. Western Blotting
4.10. Agilent Seahorse XF ATP Real-Time Rate Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight Fact Sheets; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Blackburn, G.L. Medicalizing Obesity: Individual, Economic, and Medical Consequences. AMA J. Ethics 2011, 13, 890–895. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, F. Impacts of essential amino acids on energy balance. Mol. Metab. 2022, 57, 101393. [Google Scholar] [CrossRef]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Adam, T.; Westerterp-Plantenga, M. Dietary Protein and Energy Balance in Relation to Obesity and Co-morbidities. Front. Endocrinol. 2018, 9, 443. [Google Scholar] [CrossRef]
- Ferraz-Bannitz, R.; Beraldo, R.A.; Peluso, A.A.; Dall, M.; Babaei, P.; Foglietti, R.C.; Martins, L.M.; Gomes, P.M.; Marchini, J.S.; Suen, V.M.M.; et al. Dietary Protein Restriction Improves Metabolic Dysfunction in Patients with Metabolic Syndrome in a Randomized, Controlled Trial. Nutrients 2022, 14, 2670. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.-W.; Wood, C.; Bertinato, J. Dietary supplementation with l-lysine affects body weight and blood hematological and biochemical parameters in rats. Mol. Biol. Rep. 2019, 46, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Shipelin, V.A.; Trusov, N.V.; Apryatin, S.A.; Shumakova, A.A.; Balakina, A.S.; Riger, N.A.; Gmoshinski, I.V.; Nikityuk, D.B. Effects of Tyrosine and Tryptophan in Rats with Diet-Induced Obesity. Int. J. Mol. Sci. 2021, 22, 2429. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, X.; Hu, L.; Chen, J.; Zhu, J.; Shan, A. Leucine and isoleucine have similar effects on reducing lipid accumulation, improving insulin sensitivity and increasing the browning of WAT in high-fat diet-induced obese mice. Food Funct. 2020, 11, 2279–2290. [Google Scholar] [CrossRef]
- Kasaoka, S.; Tsuboyama-Kasaoka, N.; Kawahara, Y.; Inoue, S.; Tsuji, M.; Ezaki, O.; Kato, H.; Tsuchiya, T.; Okuda, H.; Nakajima, S. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition 2004, 20, 991–996. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, X.; Sun, Y.; Hu, L.; Zhu, J.; Shao, C.; Meng, Q.; Shan, A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020, 68, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Du, Y.; Lv, Z.; Chen, S.; Zhu, J.; Sheng, H.; Guo, F. Effects of essential amino acids on lipid metabolism in mice and humans. J. Mol. Endocrinol. 2016, 57, 223–231. [Google Scholar] [CrossRef]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine Deprivation Increases Hepatic Insulin Sensitivity via GCN2/mTOR/S6K1 and AMPK Pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef]
- Ambele, M.A.; Dhanraj, P.; Giles, R.; Pepper, M.S. Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int. J. Mol. Sci. 2020, 21, 4283. [Google Scholar] [CrossRef]
- Siersbæk, R.D.; Nielsen, R.; Mandrup, S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol. Metab. 2012, 23, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Mori, M.A.; Lee, K.Y.; Smyth, G.; Liew, C.W.; Macotela, Y.; Rourk, M.; Bluher, M.; Russell, S.J.; Kahn, C.R. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat. Commun. 2012, 3, 902. [Google Scholar] [CrossRef]
- Zhu, D.; Shi, S.; Wang, H.; Liao, K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.K.; Madsen, L.; Pedersen, L.M.; Hallenborg, P.; Hagland, H.; Viste, K.; Døskeland, S.O.; Kristiansen, K. Cyclic AMP (cAMP)-Mediated Stimulation of Adipocyte Differentiation Requires the Synergistic Action of Epac- and cAMP-Dependent Protein Kinase-Dependent Processes. Mol. Cell. Biol. 2008, 28, 3804–3816. [Google Scholar] [CrossRef]
- El-Safadi, M.; Shinwari, T.; Al-Malki, S.; Manikandan, M.; Mahmood, A.; Aldahmash, A.; Alfayez, M.; Kassem, M.; Alajez, N.M. Convergence of TGFβ and BMP signaling in regulating human bone marrow stromal cell differentiation. Sci. Rep. 2019, 9, 4977. [Google Scholar] [CrossRef]
- Abou-Ezzi, G.; Supakorndej, T.; Zhang, J.; Anthony, B.; Krambs, J.; Celik, H.; Karpova, D.; Craft, C.S.; Link, D.C. TGF-β Signaling Plays an Essential Role in the Lineage Specification of Mesenchymal Stem/Progenitor Cells in Fetal Bone Marrow. Stem Cell Rep. 2019, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Bowers, R.R.; Lane, M.D. Wnt signaling and adipocyte lineage commitment. Cell Cycle 2008, 7, 1191–1196. [Google Scholar] [CrossRef]
- Fajas, L.; Egler, V.; Reiter, R.; Hansen, J.; Kristiansen, K.; Debril, M.-B.; Miard, S.; Auwerx, J. The Retinoblastoma-Histone Deacetylase 3 Complex Inhibits PPARγ and Adipocyte Differentiation. Dev. Cell 2002, 3, 903–910. [Google Scholar] [CrossRef]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; Phillips, S.A.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Zaganjor, E.; Yoon, H.; Spinelli, J.B.; Nunn, E.R.; Laurent, G.; Keskinidis, P.; Sivaloganathan, S.; Joshi, S.; Notarangelo, G.; Mulei, S.; et al. SIRT4 is an early regulator of branched-chain amino acid catabolism that promotes adipogenesis. Cell Rep. 2021, 36, 109345. [Google Scholar] [CrossRef]
- Tsurugizawa, T.; Uematsu, A.; Uneyama, H.; Torii, K. Reversible brain response to an intragastric load of l-lysine under l-lysine depletion in conscious rats. Br. J. Nutr. 2013, 109, 1323–1329. [Google Scholar] [CrossRef]
- Cruz, J.F.D.; Pacunla, K.W.M.; Hwang, S.G. Low lysine stimulates adipogenesis through ZFP423 upregulation in bovine stromal vascular cells. J. Anim. Sci. Technol. 2022, 64, 1173–1183. [Google Scholar] [CrossRef]
- Beloor, J.; Kang, H.K.; Yun, C.-H.; Kim, S.H.; Moon, Y.S. Low Lysine Treatment Increases Adipogenic Potential of Bovine Intramuscular Preadipocytes. Asian-Australas. J. Anim. Sci. 2009, 22, 721–726. [Google Scholar] [CrossRef]
- Kyoya, T.; Ishida, A.; Nakashima, K.; Nakajima, I.; Toyoda, A.; Nakamura, Y.; Katsumata, M. The effects of concentrations of lysine in media on differentiation of 3T3-L1 preadipocytes. Anim. Sci. J. 2011, 82, 565–570. [Google Scholar] [CrossRef]
- Sun, W.; Yu, Z.; Yang, S.; Jiang, C.; Kou, Y.; Xiao, L.; Tang, S.; Zhu, T. A Transcriptomic Analysis Reveals Novel Patterns of Gene Expression During 3T3-L1 Adipocyte Differentiation. Front. Mol. Biosci. 2020, 7, 564339. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine Deprivation Decreases Fat Mass by Stimulation of Lipolysis in White Adipose Tissue and Upregulation of Uncoupling Protein 1 (UCP1) in Brown Adipose Tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Meng, Q.; Zhang, Q.; Guo, F. Isoleucine or valine deprivation stimulates fat loss via increasing energy expenditure and regulating lipid metabolism in WAT. Amino Acids 2012, 43, 725–734. [Google Scholar] [CrossRef]
- Moro, J.; Roisné-Hamelin, G.; Chaumontet, C.; Even, P.C.; Blais, A.; Cansell, C.; Piedcoq, J.; Gaudichon, C.; Tomé, D.; Azzout-Marniche, D. Lysine or Threonine Deficiency Decreases Body Weight Gain in Growing Rats despite an Increase in Food Intake without Increasing Energy Expenditure in Response to FGF21. Nutrients 2022, 15, 197. [Google Scholar] [CrossRef]
- Palma-Granados, P.; Seiquer, I.; Benítez, R.; Óvilo, C.; Nieto, R. Effects of lysine deficiency on carcass composition and activity and gene expression of lipogenic enzymes in muscles and backfat adipose tissue of fatty and lean piglets. Animal 2019, 13, 2406–2418. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Chen, X.; Gao, C.; Jiao, L.; Wang, J.; Xu, G.; Fu, H.; Guo, X.; Zhao, Y. IL-6 induces lipolysis and mitochondrial dysfunction, but does not affect insulin-mediated glucose transport in 3T3-L1 adipocytes. J. Bioenerg. Biomembr. 2011, 43, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Rotter, V.; Nagaev, I.; Smith, U. Interleukin-6 (IL-6) Induces Insulin Resistance in 3T3-L1 Adipocytes and Is, Like IL-8 and Tumor Necrosis Factor-α, Overexpressed in Human Fat Cells from Insulin-resistant Subjects. J. Biol. Chem. 2003, 278, 45777–45784. [Google Scholar] [CrossRef]
- Kraakman, M.J.; Allen, T.L.; Whitham, M.; Iliades, P.; Kammoun, H.L.; Estévez, E.; Lancaster, G.I.; Febbraio, M.A. Targeting gp130 to prevent inflammation and promote insulin action. Diabetes Obes. Metab. 2013, 15 (Suppl. 3), 170–175. [Google Scholar] [CrossRef] [PubMed]
- Almuraikhy, S.; Kafienah, W.; Bashah, M.; Diboun, I.; Jaganjac, M.; Al-Khelaifi, F.; Abdesselem, H.; Mazloum, N.A.; Alsayrafi, M.; Mohamed-Ali, V.; et al. Interleukin-6 induces impairment in human subcutaneous adipogenesis in obesity-associated insulin resistance. Diabetologia 2016, 59, 2406–2416. [Google Scholar] [CrossRef]
- Huang, D.; Maulu, S.; Ren, M.; Liang, H.; Ge, X.; Ji, K.; Yu, H. Dietary Lysine Levels Improved Antioxidant Capacity and Immunity via the TOR and p38 MAPK Signaling Pathways in Grass Carp, Ctenopharyngodon idellus Fry. Front. Immunol. 2021, 12, 635015. [Google Scholar] [CrossRef] [PubMed]
- Belalcázar, A.D.; Ball, J.G.; Frost, L.M.; Valentovic, M.A.; Wilkinson, J. Transsulfuration Is a Significant Source of Sulfur for Glutathione Production in Human Mammary Epithelial Cells. ISRN Biochem. 2013, 2013, 637897. [Google Scholar] [CrossRef]
- Wallis, K.F.; Morehead, L.C.; Bird, J.T.; Byrum, S.D.; Miousse, I.R. Differences in cell death in methionine versus cysteine depletion. Environ. Mol. Mutagen. 2021, 62, 216–226. [Google Scholar] [CrossRef]
- Pan, Y.; Qiao, Q.Y.; Pan, L.H.; Zhou, D.C.; Hu, C.; Gu, H.F.; Fu, S.K.; Liu, X.L.; Jin, H.M. Losartan Reduces Insulin Resistance by Inhibiting Oxidative Stress and Enhancing Insulin Signaling Transduction. Exp. Clin. Endocrinol. Diabetes 2015, 123, 170–177. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Cranberries (Oxycoccus quadripetalus) inhibit pro-inflammatory cytokine and chemokine expression in 3T3-L1 adipocytes. Food Chem. 2016, 196, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Cavener, D.R. The GCN2 eIF2α Kinase Regulates Fatty-Acid Homeostasis in the Liver during Deprivation of an Essential Amino Acid. Cell Metab. 2007, 5, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Qiu, H.; Garcia-Barrio, M.; Anderson, J.; Hinnebusch, A.G. Uncharged tRNA Activates GCN2 by Displacing the Protein Kinase Moiety from a Bipartite tRNA-Binding Domain. Mol. Cell 2000, 6, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Vattem, K.M.; Wek, R.C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 2004, 101, 11269–11274. [Google Scholar] [CrossRef]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2alpha/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef]
- Ballabio, A.; Bonifacino, J.S. Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 101–118. [Google Scholar] [CrossRef]
- Orentreich, N.; Matias, J.R.; DeFelice, A.; Zimmerman, J.A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.G.; Morrison, C.D.; Gettys, T.W. Remodeling of Lipid Metabolism by Dietary Restriction of Essential Amino Acids. Diabetes 2013, 62, 2635–2644. [Google Scholar] [CrossRef]
- Malloy, V.L.; Krajcik, R.A.; Bailey, S.J.; Hristopoulos, G.; Plummer, J.D.; Orentreich, N. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 2006, 5, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Stone, K.P.; Forney, L.A.; Wanders, D.; Gettys, T.W. Nutritional Regulation of Hepatic FGF21 by Dietary Restriction of Methionine. Front. Endocrinol. 2021, 12, 773975. [Google Scholar] [CrossRef]
- Han, Q.; Tan, Y.; Hoffman, R.M. Oral dosing of Recombinant Methioninase Is Associated with a 70% Drop in PSA in a Patient with Bone-metastatic Prostate Cancer and 50% Reduction in Circulating Methionine in a High-stage Ovarian Cancer Patient. Anticancer Res. 2020, 40, 2813–2819. [Google Scholar] [CrossRef]
- Tashiro, Y.; Han, Q.; Tan, Y.; Sugisawa, N.; Yamamoto, J.; Nishino, H.; Inubushi, S.; Higuchi, T.; Aoki, T.; Murakami, M.; et al. Oral Recombinant Methioninase Prevents Obesity in Mice on a High-fat Diet. Vivo 2020, 34, 489–494. [Google Scholar] [CrossRef]
- Zhang, Y.; Higgins, C.B.; Van Tine, B.A.; Bomalaski, J.S.; DeBosch, B.J. Pegylated arginine deiminase drives arginine turnover and systemic autophagy to dictate energy metabolism. Cell Rep. Med. 2022, 3, 100498. [Google Scholar] [CrossRef] [PubMed]
- Lukasheva, E.V.; Babayeva, G.; Karshieva, S.S.; Zhdanov, D.D.; Pokrovsky, V.S. L-Lysine α-Oxidase: Enzyme with Anticancer Properties. Pharmaceuticals 2021, 14, 1070. [Google Scholar] [CrossRef]
- Kusakabe, H.; Kodama, K.; Kuninaka, A.; Yoshino, H.; Soda, K. Effect ofl-Lysine α-Oxidase on Growth of Mouse Leukemic Cells. Biosci. Biotechnol. Biochem. 1980, 44, 387–392. [Google Scholar] [CrossRef]
- Smriga, M.; Kameishi, M.; Uneyama, H.; Torii, K. Dietary L-Lysine Deficiency Increases Stress-Induced Anxiety and Fecal Excretion in Rats. J. Nutr. 2002, 132, 3744–3746. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Sato, Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020, 29, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward | Reverse |
|---|---|---|
| FASn | CCCAGCGGTAGAGAATAGCA | GGGTCCACTAAACTGAGCCT |
| SCD1 | TCCAACTCATGTGCCTCTGT | AACAACCAACCCTCGCATTC |
| SREBF1 | TTGTTCCTTTGCCTTCCAGC | GATGCCGACCAGATTCCCTA |
| PPARg | TGACAGACCTCAGGCAGATC | AGAAGGAACACGTTGTCAGC |
| IL6 | TCCTTCCTACCCCAATTTCCA | GTCCACAAACTGATATGCTTAGG |
| Tbp | TGCTGTTGGTGATTGTTGGT | ACTGGGAAGGCGGAATGTAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.M.-Y.; Lin, Z.-Q.; Zheng, L.-X.; Tu, Y.-F.; So, Y.-H.; Zheng, X.-H.; Feng, T.-J.; Wang, X.-Y.; Wong, W.-T.; Leung, Y.-C. Lysine Deprivation Suppresses Adipogenesis in 3T3-L1 Cells: A Transcriptome Analysis. Int. J. Mol. Sci. 2023, 24, 9402. https://doi.org/10.3390/ijms24119402
Lee LM-Y, Lin Z-Q, Zheng L-X, Tu Y-F, So Y-H, Zheng X-H, Feng T-J, Wang X-Y, Wong W-T, Leung Y-C. Lysine Deprivation Suppresses Adipogenesis in 3T3-L1 Cells: A Transcriptome Analysis. International Journal of Molecular Sciences. 2023; 24(11):9402. https://doi.org/10.3390/ijms24119402
Chicago/Turabian StyleLee, Leo Man-Yuen, Zhi-Qiang Lin, Lu-Xi Zheng, Yi-Fan Tu, Yik-Hing So, Xiu-Hua Zheng, Tie-Jun Feng, Xi-Yue Wang, Wai-Ting Wong, and Yun-Chung Leung. 2023. "Lysine Deprivation Suppresses Adipogenesis in 3T3-L1 Cells: A Transcriptome Analysis" International Journal of Molecular Sciences 24, no. 11: 9402. https://doi.org/10.3390/ijms24119402






