Preclinical Characterization of the 177Lu-Labeled Prostate Stem Cell Antigen (PSCA)-Specific Monoclonal Antibody 7F5
Abstract
1. Introduction
2. Results
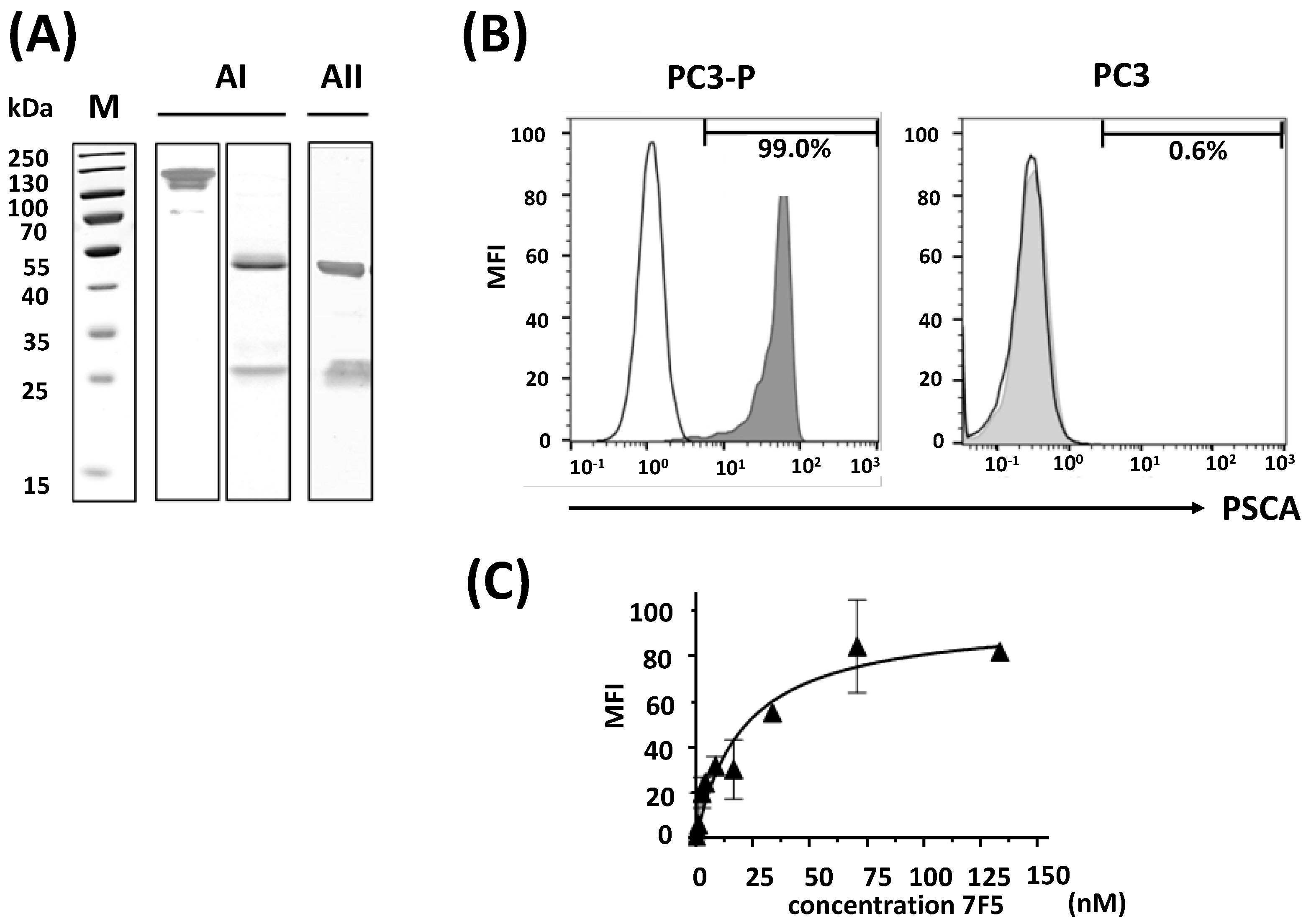
2.1. Purification of the Anti-PSCA mAb 7F5 and Evaluation of Its Specific Binding Capability
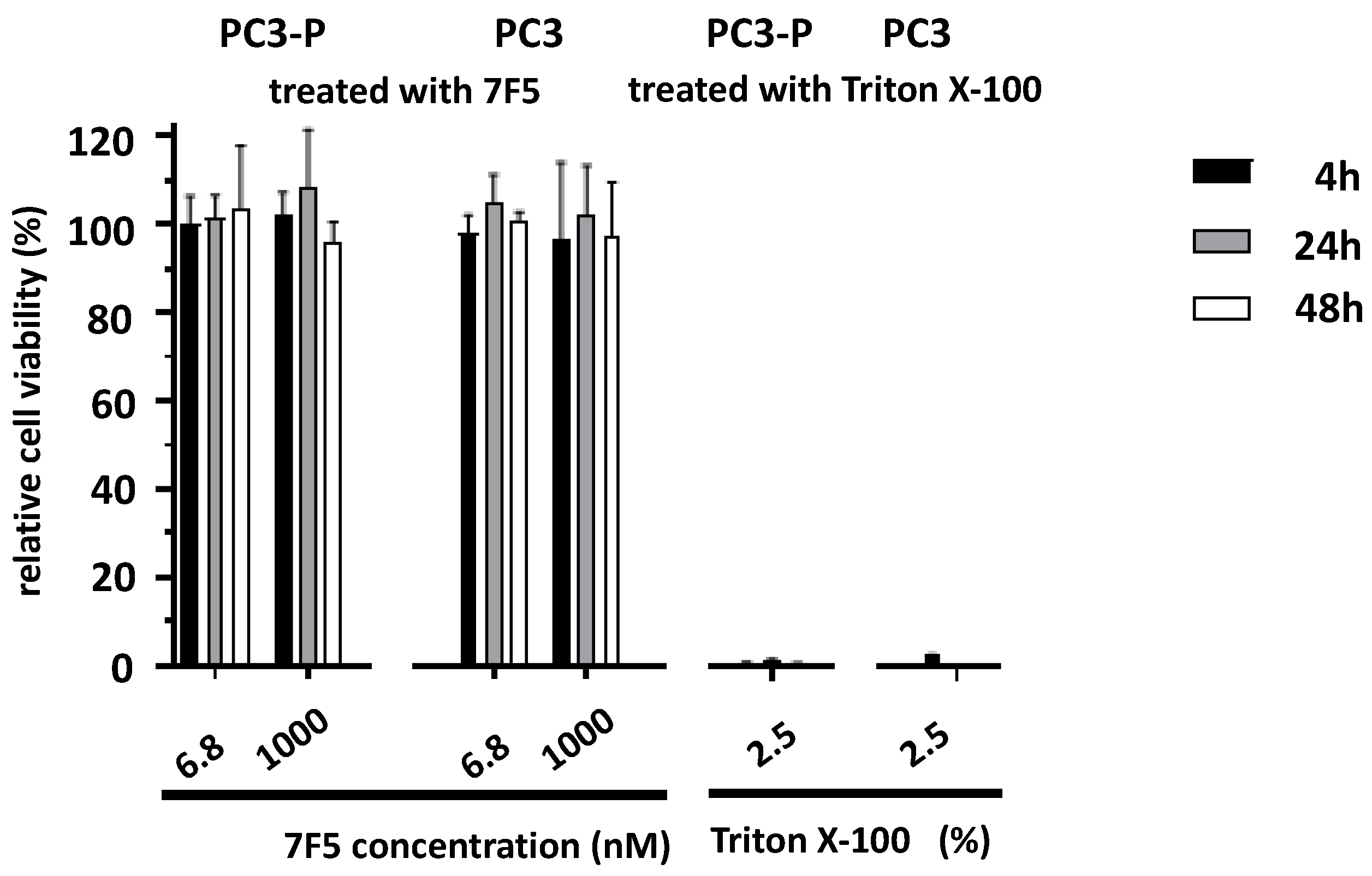
2.2. Effect of the Anti-PSCA mAb 7F5 on the Viability of PSCA-Positive Cells
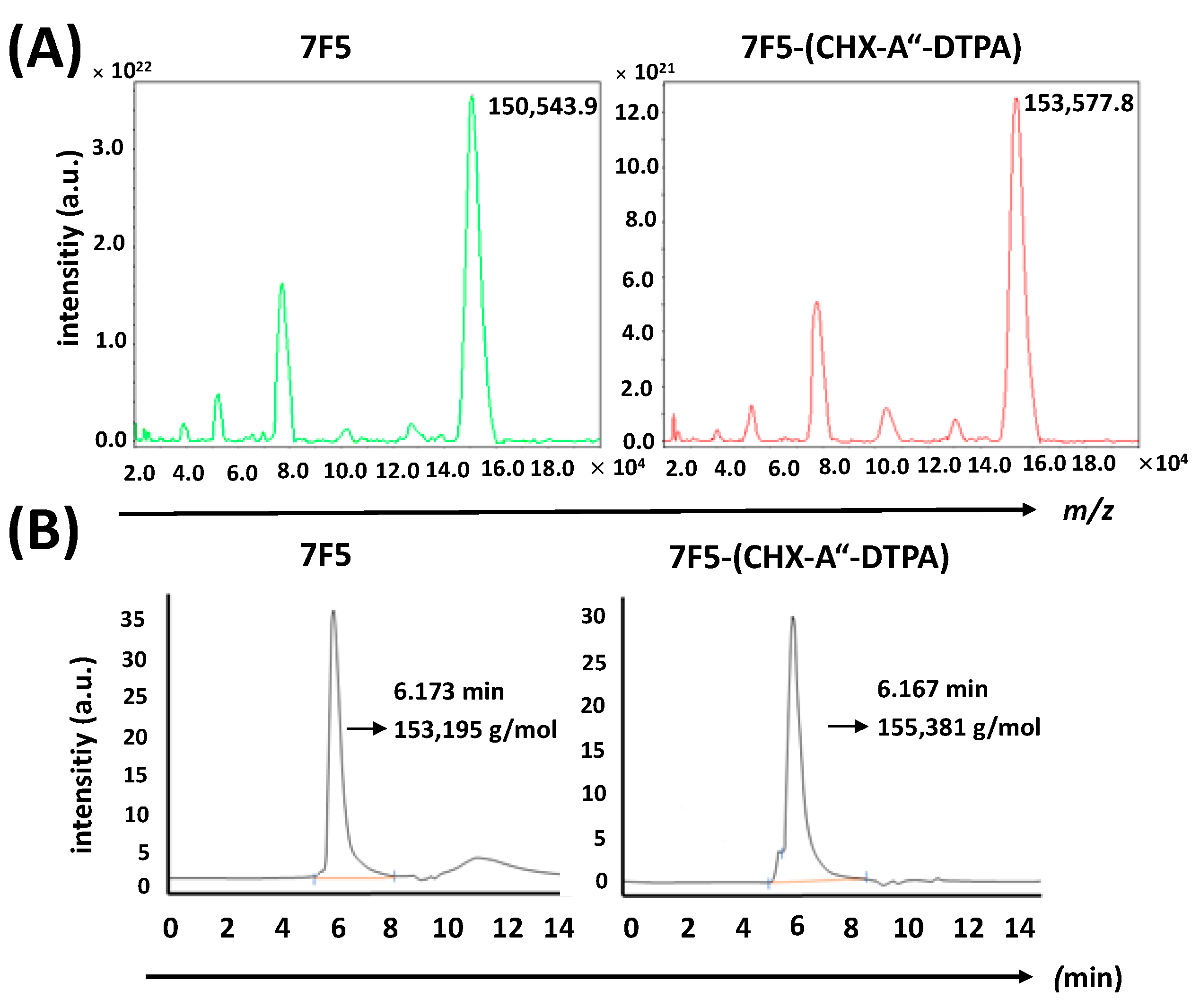
2.3. Conjugation of CHX-A″-DTPA to the Anti-PSCA mAb 7F5
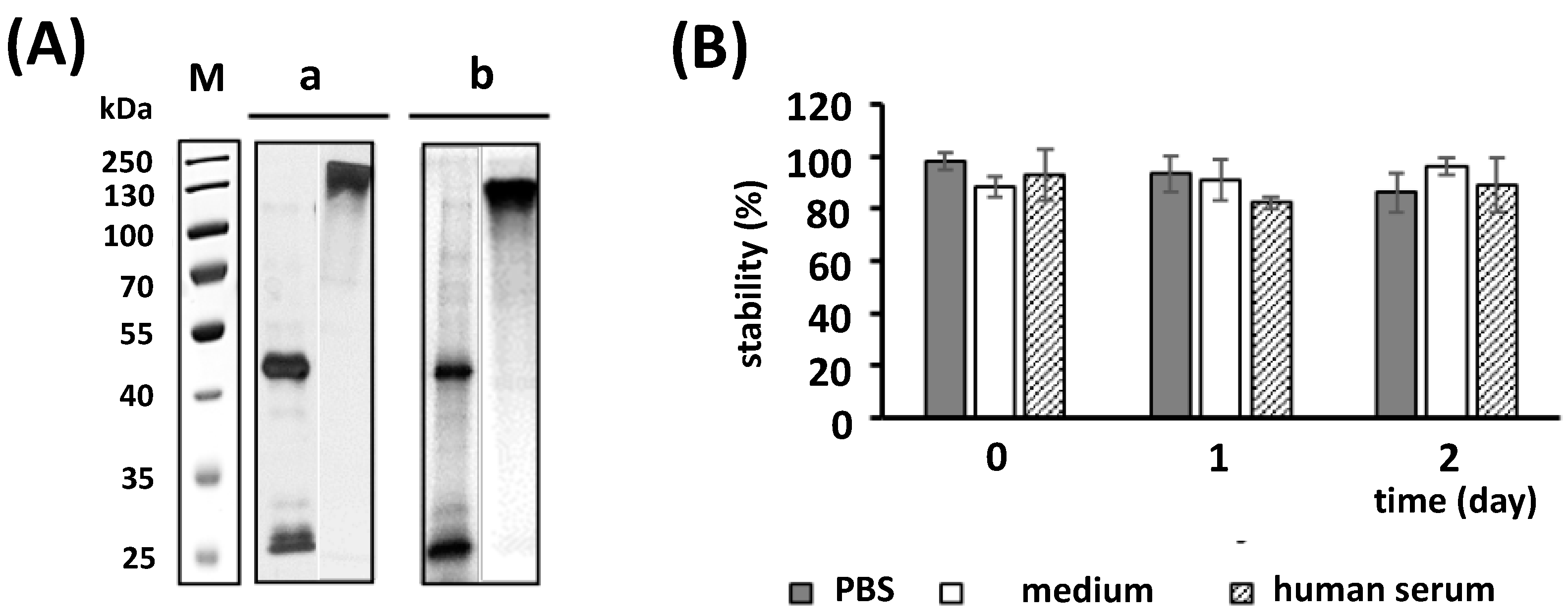
2.4. Radiolabeling and Stability of the Radiolabeled Ab Conjugate
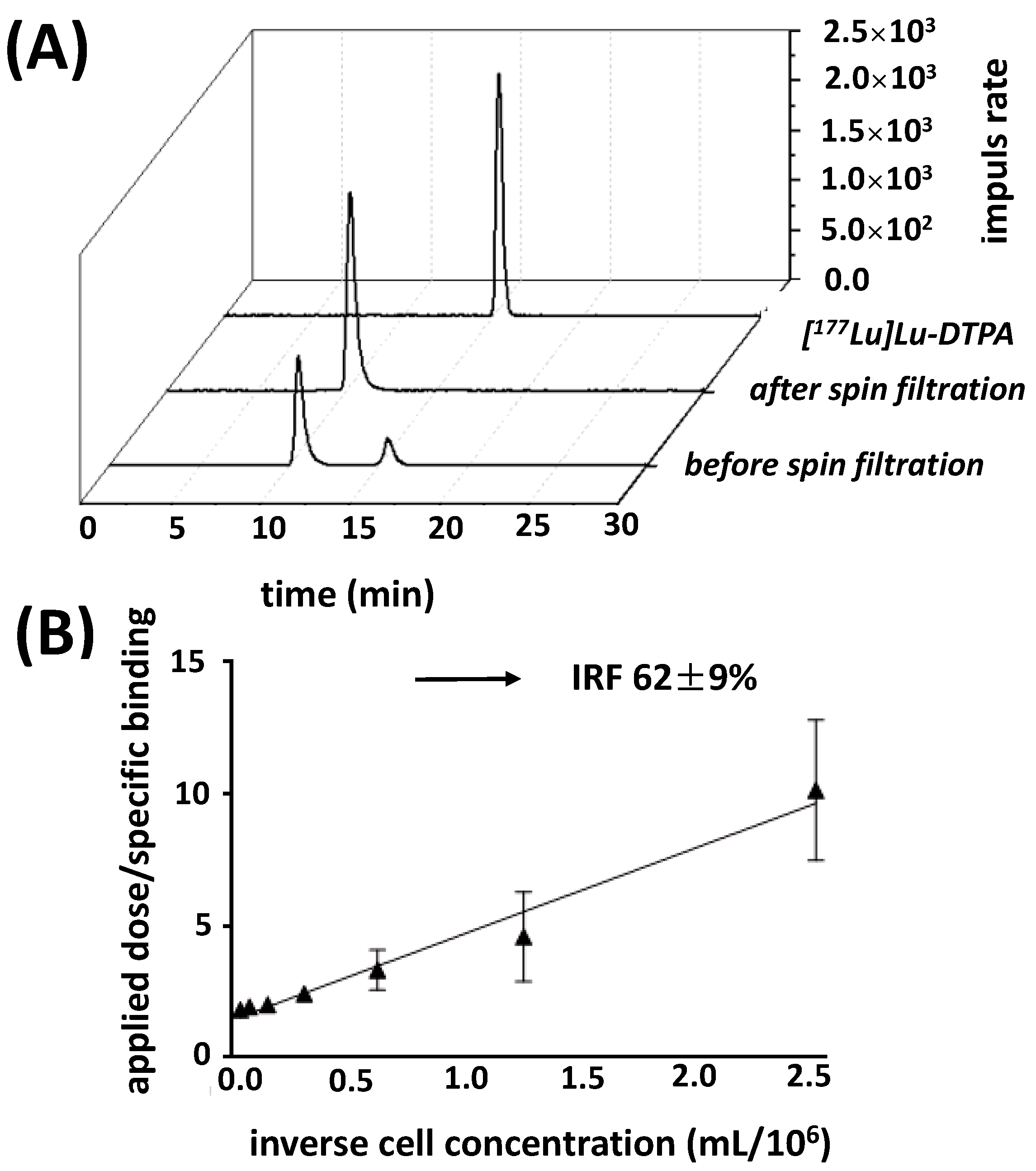
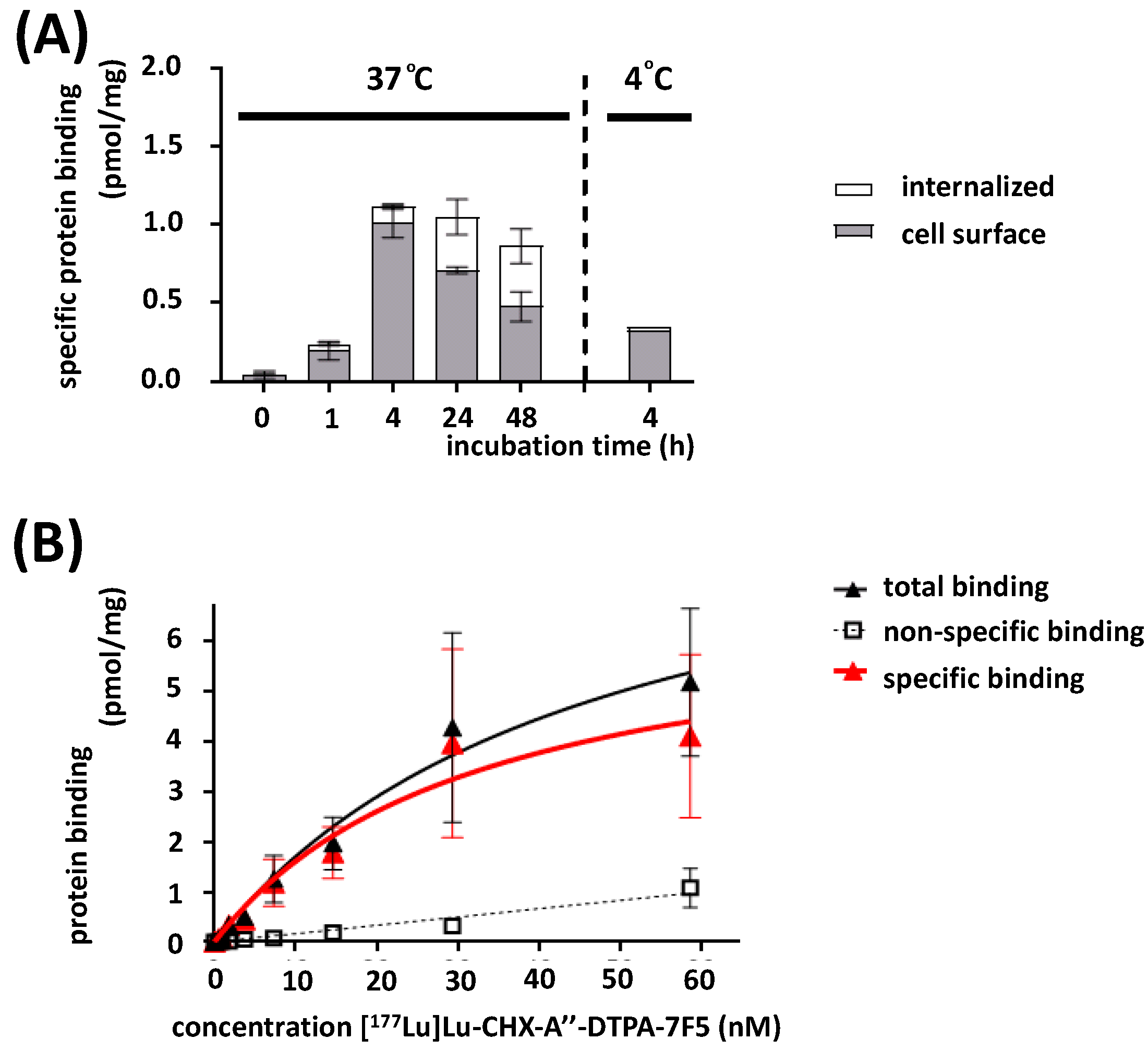
2.5. In Vitro Binding Characteristics
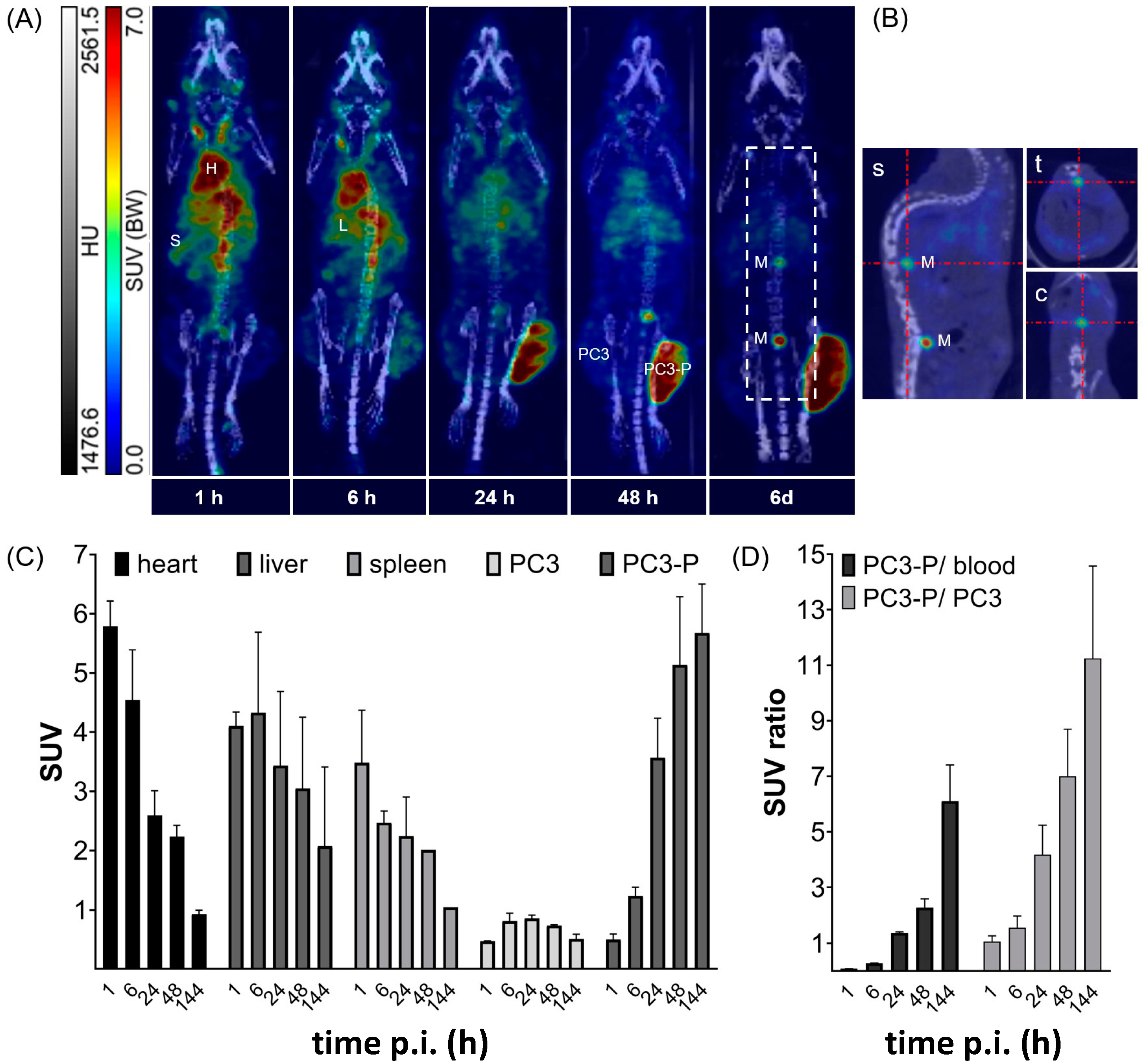
2.6. In Vivo Targeting
2.6.1. Biodistribution Studies
2.6.2. Small Animal Imaging
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.1.1. PCa Cell Lines
4.1.2. Hybridoma Cell Line
4.2. Purification of the Anti-PSCA mAb 7F5 from Hybridoma Cell Culture Supernatant
4.3. Ab Purity and Initial Characterization
4.4. Binding Analysis by Flow Cytometry
4.5. Cell Proliferation Assay
4.6. Ab Conjugation with p-SCN-CHX-A″-DTPA
4.7. Mass Spectroscopy of the Conjugate
4.8. 177Lu-Labeling of [177Lu]Lu-CHX-A″-DTPA-7F5
4.9. Stability Studies
4.10. Radioligand Binding Studies
4.10.1. Immunoreactivity
4.10.2. Internalization
4.10.3. Saturation Binding Studies
4.11. Animal Studies
4.11.1. Biodistribution
4.11.2. Small Animal SPECT/CT-Imaging
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, A.N.; Usman, A.; Morgans, A.; VanderWeele, D.J.; Sosman, J.; Wu, J.D. Past, current, and future of immunotherapies for prostate cancer. Front. Oncol. 2019, 9, 884. [Google Scholar] [CrossRef] [PubMed]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Bouchelouche, K.; Tagawa, S.T.; Goldsmith, S.J.; Turkbey, B.; Capala, J.; Choyke, P. PET/CT imaging and radioimmunotherapy of prostate cancer. Semin. Nucl. Med. 2011, 41, 29–44. [Google Scholar] [CrossRef]
- Liu, Y. The context of prostate cancer genomics in personalized medicine. Oncol. Lett. 2017, 13, 3347–3353. [Google Scholar] [CrossRef]
- Hricak, H.; Choyke, P.L.; Eberhardt, S.C.; Leibel, S.A.; Scardino, P.T. Imaging prostate cancer: A multidisciplinary perspective. Radiology 2007, 243, 28–53. [Google Scholar] [CrossRef]
- Wibmer, A.G.; Burger, I.A.; Sala, E.; Hricak, H.; Weber, W.A.; Vargas, H.A. Molecular imaging of prostate cancer. Radiographics 2016, 36, 142–159. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kiljunen, T.; Joensuu, T.; Kairemo, K.; Uprimny, C.; Virgolini, I. 177Lu-PSMA-617 radioligand therapy for a patient with lymph node metastatic prostate cancer. Oncotarget 2017, 8, 66112–66116. [Google Scholar] [CrossRef]
- Rahbar, K.; Bode, A.; Weckesser, M.; Avramovic, N.; Claesener, M.; Stegger, L.; Bögemann, M. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin. Nucl. Med. 2016, 41, 522–528. [Google Scholar] [CrossRef]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–1670. [Google Scholar] [CrossRef]
- Jang, A.; Kendi, A.T.; Sartor, O. Status of PSMA-targeted radioligand therapy in prostate cancer: Current data and future trials. Ther. Adv. Med. Oncol. 2023, 15, 17588359231157632. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Malcher, A.; Eder, M.; Eisenhut, M.; Linhart, H.G.; Hadaschik, B.A.; Holland-Letz, T.; Giesel, F.L.; Kratochwil, C.; Haufe, S.; et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: Biodistribution in humans and first evaluation of tumour lesions. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Eder, M.; Neels, O.; Müller, M.; Bauder-Wüst, U.; Remde, Y.; Schäfer, M.; Hennrich, U.; Eisenhut, M.; Afshar-Oromieh, A.; Haberkorn, U.; et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: A new PET tracer for imaging of prostate cancer. Pharmaceuticals 2014, 7, 779–796. [Google Scholar] [CrossRef] [PubMed]
- Eiber, M.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Ruffani, A.; Haller, B.; Graner, F.-P.; Kübler, H.; Haberkorn, U.; Eisenhut, M.; et al. Evaluation of hybrid ⁶⁸Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J. Nucl. Med. 2015, 56, 668–674. [Google Scholar] [CrossRef]
- Hoffmann, M.A.; Buchholz, H.G.; Wieler, H.J.; Hofner, T.; Muller-Hubenthal, J.; Trampert, L.; Schreckenberger, M. The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Oncotarget 2019, 10, 6124–6137. [Google Scholar] [CrossRef]
- Giesel, F.L.; Will, L.; Lawal, I.; Lengana, T.; Kratochwil, C.; Vorster, M.; Neels, O.; Reyneke, F.; Haberkon, U.; Kopka, K.; et al. Intraindividual comparison of (18)F-PSMA-1007 and (18)F-DCFPyL PET/CT in the prospective evaluation of patients with newly diagnosed prostate carcinoma: A pilot study. J. Nucl. Med. 2018, 59, 1076–1080. [Google Scholar] [CrossRef]
- Arndt, C.; Feldmann, A.; Koristka, S.; Schafer, M.; Bergmann, R.; Mitwasi, N.; Berndt, N.; Bachmann, D.; Kegler, A.; Schmitz, M.; et al. A theranostic PSMA ligand for PET imaging and retargeting of T cells expressing the universal chimeric antigen receptor UniCAR. Oncoimmunology 2019, 8, 1659095. [Google Scholar] [CrossRef]
- Arndt, C.; Bachmann, M.; Bergmann, R.; Berndt, N.; Feldmann, A.; Koristka, S. Theranostic CAR T cell targeting: A brief review. J. Label. Compd. Radiopharm. 2019, 62, 533–540. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Koristka, S.; Berndt, N.; Bergmann, R.; Bachmann, M.P. Conventional CARs versus modular CARs. Cancer Immunol. Immunother. 2019, 68, 1713–1719. [Google Scholar] [CrossRef]
- Bachmann, M. The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 2019, 211, 13–22. [Google Scholar] [CrossRef]
- Roll, W.; Bode, A.; Weckesser, M.; Bögemann, M.; Rahbar, K. Excellent response to 177Lu-PSMA-617 radioligand therapy in a patient with advanced metastatic castration resistant prostate cancer evaluated by 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2017, 42, 152–153. [Google Scholar] [CrossRef] [PubMed]
- Rischpler, C.; Beck, T.I.; Okamoto, S.; Schlitter, A.M.; Knorr, K.; Schwaiger, M.; Gschwend, J.; Maurer, T.; Meyer, P.T.; Eiber, M. (68)Ga-PSMA-HBED-CC uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J. Nucl. Med. 2018, 59, 1406–1411. [Google Scholar] [CrossRef] [PubMed]
- Rowe, S.P.; Deville, C.; Paller, C.; Cho, S.Y.; Fishman, E.K.; Pomper, M.G.; Ross, A.E.; Gorin, M.A. Uptake of (18)F-DCFPyL in Paget’s disease of bone, an important potential pitfall in clinical interpretation of PSMA PET Studies. Tomography 2015, 1, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Law, W.P.; Fiumara, F.; Fong, W.; Miles, K.A. Gallium-68 PSMA uptake in adrenal adenoma. J. Med. Imaging Radiat. Oncol. 2016, 60, 514–517. [Google Scholar] [CrossRef]
- Kanthan, G.L.; Drummond, J.; Schembri, G.P.; Izard, M.A.; Hsiao, E. Follicular thyroid adenoma showing avid uptake on 68Ga PSMA-HBED-CC PET/CT. Clin. Nucl. Med. 2016, 41, 331–332. [Google Scholar] [CrossRef]
- Mannweiler, S.; Amersdorfer, P.; Trajanoski, S.; Terrett, J.A.; King, D.; Mehes, G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol. Oncol. Res. 2009, 15, 167–172. [Google Scholar] [CrossRef]
- Shetty, D.; Patel, D.; Le, K.; Bui, C.; Mansberg, R. Pitfalls in gallium-68 PSMA PET/CT interpretation—A pictorial review. Tomography 2018, 4, 182–193. [Google Scholar] [CrossRef]
- Taieb, D.; Foletti, J.M.; Bardies, M.; Rocchi, P.; Hicks, R.J.; Haberkorn, U. PSMA-targeted radionuclide therapy and salivary gland toxicity: Why does it matter? J. Nucl. Med. 2018, 59, 747–748. [Google Scholar] [CrossRef]
- Ono, H.; Sakamoto, H.; Yoshida, T.; Saeki, N. Prostate stem cell antigen is expressed in normal and malignant human brain tissues. Oncol. Lett. 2018, 15, 3081–3084. [Google Scholar] [CrossRef]
- Link, T.; Kuithan, F.; Ehninger, A.; Kuhlmann, J.D.; Kramer, M.; Werner, A.; Gatzweiler, A.; Richter, B.; Ehninger, G.; Baretton, G.; et al. Exploratory investigation of PSCA-protein expression in primary breast cancer patients reveals a link to HER2/neu overexpression. Oncotarget 2017, 8, 54592–54603. [Google Scholar] [CrossRef]
- Cunha, A.C.; Weigle, B.; Kiessling, A.; Bachmann, M.; Rieber, E.P. Tissue-specificity of prostate specific antigens: Comparative analysis of transcript levels in prostate and non-prostate tissues. Cancer Lett. 2006, 236, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Thomas, G.; Yamashiro, J.; Shintaku, I.P.; Dorey, F.; Raitano, A.; Witte, O.N.; Said, J.W.; Loda, M.; Reiter, R.E. Prostate stem cell antigen (PSCA) expression increases with high gleason score, advanced stage and bone metastasis in prostate cancer. Oncogene 2000, 19, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, A.; Wehner, R.; Füssel, S.; Bachmann, M.; Wirth, M.P.; Schmitz, M. Tumor-associated antigens for specific immunotherapy of prostate cancer. Cancers 2012, 4, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Han, K.R.; Seligson, D.B.; Liu, X.; Horvath, S.; Shintaku, P.I.; Thomas, G.V.; Said, J.W.; Reiter, R.E. Prostate stem cell antigen expression is associated with gleason score, seminal vesicle invasion and capsular invasion in prostate cancer. J. Urol. 2004, 171, 1117–1121. [Google Scholar] [CrossRef]
- Lam, J.S.; Yamashiro, J.; Shintaku, I.P.; Vessella, R.L.; Jenkins, R.B.; Horvath, S.; Said, J.W.; Reiter, R.E. Prostate stem cell antigen is overexpressed in prostate cancer metastases. Clin. Cancer Res. 2005, 11, 2591–2596. [Google Scholar] [CrossRef]
- Koristka, S.; Cartellieri, M.; Arndt, C.; Bippes, C.C.; Feldmann, A.; Michalk, I.; Wiefel, K.; Stamova, S.; Schmitz, M.; Ehninger, G.; et al. Retargeting of regulatory T cells to surface-inducible autoantigen La/SS-B. J. Autoimmun. 2013, 42, 105–116. [Google Scholar] [CrossRef]
- Cartellieri, M.; Koristka, S.; Arndt, C.; Feldmann, A.; Stamova, S.; von Bonin, M.; Topfer, K.; Kruger, T.; Geib, M.; Michalk, I.; et al. A novel ex vivo isolation and expansion procedure for chimeric antigen receptor engrafted human T cells. PLoS ONE 2014, 9, e93745. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Bergmann, R.; Loff, S.; Cartellieri, M.; Bachmann, D.; Aliperta, R.; Hetzenecker, M.; Ludwig, F.; Albert, S.; et al. Retargeting of T lymphocytes to PSCA- or PSMA positive prostate cancer cells using the novel modular chimeric antigen receptor platform technology “UniCAR”. Oncotarget 2017, 8, 31368–31385. [Google Scholar] [CrossRef]
- Feldmann, A.; Arndt, C.; Topfer, K.; Stamova, S.; Krone, F.; Cartellieri, M.; Koristka, S.; Michalk, I.; Lindemann, D.; Schmitz, M.; et al. Novel humanized and highly efficient bispecific antibodies mediate killing of prostate stem cell antigen-expressing tumor cells by CD8+ and CD4+ T cells. J. Immunol. 2012, 189, 3249–3259. [Google Scholar] [CrossRef]
- Morgenroth, A.; Cartellieri, M.; Schmitz, M.; Gunes, S.; Weigle, B.; Bachmann, M.; Abken, H.; Rieber, E.P.; Temme, A. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate 2007, 67, 1121–1131. [Google Scholar] [CrossRef]
- Feldmann, A.; Stamova, S.; Bippes, C.C.; Bartsch, H.; Wehner, R.; Schmitz, M.; Temme, A.; Cartellieri, M.; Bachmann, M. Retargeting of T cells to prostate stem cell antigen expressing tumor cells: Comparison of different antibody formats. Prostate 2011, 71, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Arndt, C.; Feldmann, A.; Koristka, S.; Cartellieri, M.; Dimmel, M.; Ehninger, A.; Ehninger, G.; Bachmann, M. Simultaneous targeting of prostate stem cell antigen and prostate-specific membrane antigen improves the killing of prostate cancer cells using a novel modular T cell-retargeting system. Prostate 2014, 74, 1335–1346. [Google Scholar] [CrossRef]
- Koristka, S.; Kegler, A.; Bergmann, R.; Arndt, C.; Feldmann, A.; Albert, S.; Cartellieri, M.; Ehninger, A.; Ehninger, G.; Middeke, J.M.; et al. Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J. Autoimmun. 2018, 90, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, A.; Hoffmann, A.; Bergmann, R.; Koristka, S.; Berndt, N.; Arndt, C.; Rodrigues Loureiro, L.; Kittel-Boselli, E.; Mitwasi, N.; Kegler, A.; et al. Versatile chimeric antigen receptor platform for controllable and combinatorial T cell therapy. OncoImmunology 2020, 9, 1785608. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Arndt, C.; Koristka, S.; Berndt, N.; Bergmann, R.; Feldmann, A.; Schmitz, M.; Pietzsch, J.; Steinbach, J.; Bachmann, M. From mono- to bivalent: Improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro und in vivo. Oncotarget 2018, 9, 25597–25616. [Google Scholar] [CrossRef] [PubMed]
- Lindmo, T.; Boven, E.; Cuttitta, F.; Fedorko, J.; Bunn, P.A. Determination of the Immunoreactive Fraction of Radiolabeled Monoclonal-Antibodies by Linear Extrapolation to Binding at Infinite Antigen Excess. J. Immunol. Methods 1984, 72, 77–89. [Google Scholar] [CrossRef]
- Ren, J.; Wang, F.; Wei, G.; Yang, Y.; Liu, Y.; Wei, M.; Huan, Y.; Larson, A.C.; Zhang, Z. MRl of prostate cancer antigen expression for diagnosis and immunotherapy. PLoS ONE 2012, 7, e38350. [Google Scholar] [CrossRef]
- Gu, Z.; Yamashiro, J.; Kono, E.; Reiter, R.E. Anti-prostate stem cell antigen monoclonal antibody 1G8 induces cell death in vitro and inhibits tumor growth in vivo via a Fc-independent mechanism. Cancer Res. 2005, 65, 9495–9500. [Google Scholar] [CrossRef]
- Saffran, D.C.; Raitano, A.; Hubert, R.S.; Witte, O.N.; Reiter, R.E.; Jakobovits, A. Anti-PSCA mAbs inhibit tumor growth and metastasis formation and prolong the survival of mice bearing human prostate cancer xenografts. Proc. Natl. Acad. Sci. USA 2001, 98, 2658–2663. [Google Scholar] [CrossRef]
- Pillai, A.M.; Knapp, F.F., Jr. Evolving important role of lutetium-177 for therapeutic nuclear medicine. Curr. Radiopharm. 2015, 8, 78–85. [Google Scholar] [CrossRef]
- Lassmann, M.; Eberlein, U. Radiation dosimetry aspects of 117Lu. Curr. Radiopharm. 2015, 8, 139–144. [Google Scholar] [CrossRef]
- O’Donoghue, J.A.; Bardies, M.; Wheldon, T.E. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J. Nucl. Med. 1995, 36, 1902–1909. [Google Scholar]
- Smith-Jones, P.M. Radioimmunotherapy of prostate cancer. Q. J. Nucl. Med. Mol. Imaging 2004, 48, 297–304. [Google Scholar] [PubMed]
- Milenic, D.E.; Brady, E.D.; Brechbiel, M.W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D.; Brechbiel, M.W. Part II: The science of targeted radionuclide therapy, Chapter 6: Chelation Chemistry. In Targeted Radionuclide Therapy; Speer, T.W., Ed.; Lippincott Williams & Wilkins, a Wolters Kluwer Business: Philadelphia, PA, USA, 2011; Volume 1. [Google Scholar]
- Vallabhajosula, S. Part III: Special Topics in Radionuclide Therapy, Capter 19: The Chemistry of Therapeutic Radiopharmaceuticals. In Nuclear Medicine Therpay: Principles and Clinical Applications; Aktolun, C., Goldsmith, S.J., Eds.; Springer: New York, NY, USA, 2013; Volume 1. [Google Scholar]
- Kosmas, C.; Snook, D.; Gooden, C.S.; Courtenay-Luck, N.S.; McCall, M.J.; Meares, C.F.; Epenetos, A.A. Development of humoral immune responses against a macrocyclic chelating agent (DOTA) in cancer patients receiving radioimmunoconjugates for imaging and therapy. Cancer Res. 1992, 52, 904–911. [Google Scholar] [PubMed]
- Chakravarty, R.; Chakraborty, S.; Sarma, H.D.; Nair, K.V.; Rajeswari, A.; Dash, A. (90) Y/(177) Lu-labelled Cetuximab immunoconjugates: Radiochemistry optimization to clinical dose formulation. J. Label. Compd. Radiopharm. 2016, 59, 354–363. [Google Scholar] [CrossRef]
- Breeman, W.A.; van der Wansem, K.; Bernard, B.F.; van Gameren, A.; Erion, J.L.; Visser, T.J.; Krenning, E.P.; de Jong, M. The addition of DTPA to [177Lu-DOTA0,Tyr3]octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 312–315. [Google Scholar]
- Almqvist, Y.; Steffen, A.C.; Tolmachev, V.; Divgi, C.R.; Sundin, A. In vitro and in vivo characterization of 177Lu-huA33: A radioimmunoconjugate against colorectal cancer. Nucl. Med. Biol. 2006, 33, 991–998. [Google Scholar]
- Fani, M.; Bouziotis, P.; Harris, A.L.; Psimadas, D.; Gourni, E.; Loudos, G.; Varvarigou, A.D.; Maecke, H.R. 177Lu-labeled-VG76e monoclonal antibody in tumor angiogenesis: A comparative study using DOTA and DTPA chelating systems. Radiochim. Acta 2007, 95, 351–357. [Google Scholar] [CrossRef]
- Pandey, U.; Kameswaran, M.; Gamre, N.; Dash, A. Preparation of (177) Lu-labeled Nimotuzumab for radioimmunotherapy of EGFR-positive cancers: Comparison of DOTA and CHX-A’’-DTPA as bifunctional chelators. J. Label. Compd. Radiopharm. 2019, 62, 158–165. [Google Scholar]
- Dho, S.H.; Kim, S.Y.; Chung, C.; Cho, E.H.; Lee, S.Y.; Kim, J.Y.; Kim, L.K.; Min, S.W.; Lee, J.; Jung, S.H.; et al. Development of a radionuclide-labeled monoclonal anti-CD55 antibody with theranostic potential in pleural metastatic lung cancer. Sci. Rep. 2018, 8, 8960. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Tolmachev, V.; Andersson, K.; Gedda, L.; Sandstrom, M.; Carlsson, J. [(177)Lu]pertuzumab: Experimental studies on targeting of HER-2 positive tumour cells. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Hens, M.; Vaidyanathan, G.; Zhao, X.G.; Bigner, D.D.; Zalutsky, M.R. Anti-EGFRvIII monoclonal antibody armed with 177Lu: In vivo comparison of macrocyclic and acyclic ligands. Nucl. Med. Biol. 2010, 37, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Kameswaran, M.; Pandey, U.; Dhakan, C.; Pathak, K.; Gota, V.; Vimalnath, K.V.; Dash, A.; Samuel, G. Synthesis and preclinical evaluation of 177Lu-CHX-A’’-DTPA-rituximab as aradioimmunotherapeutic agent for Non-Hodgkin’s Lymphoma. Cancer Biother. Radiopharm. 2015, 30, 240–246. [Google Scholar] [PubMed]
- Fujimori, K.; Covell, D.G.; Fletcher, J.E.; Weinstein, J.N. A modeling analysis of monoclonal antibody percolation through tumors: A binding-site barrier. J. Nucl. Med. 1990, 31, 1191–1198. [Google Scholar]
- Rudnick, S.I.; Adams, G.P. Affinity and avidity in antibody-based tumor targeting. Cancer Biother. Radiopharm. 2009, 24, 155–161. [Google Scholar] [CrossRef]
- Carpenter, G.; Cohen, S. 125I-Labeled human epidermal growth factor. J. Cell. Biol. 1976, 71, 159–171. [Google Scholar] [CrossRef]
- Riedl, T.; van Boxtel, E.; Bosch, M.; Parren, P.W.; Gerritsen, A.F. High-throughput screening for internalizing antibodies by homogeneous fluorescence imaging of a pH-activated probe. J. Biomol. Screen 2016, 21, 12–23. [Google Scholar] [CrossRef]
- Ross, S.; Spencer, S.D.; Holcomb, I.; Tan, C.; Hongo, J.A.; Devaux, B.; Rangell, L.; Keller, G.A.; Schow, P.; Steeves, R.M.; et al. Prostate stem cell antigen as therapy target: Tissue expression and in vivo efficacy of an immunoconjugate. Caner Res. 2002, 62, 2546–2553. [Google Scholar]
- Altai, M.; Membreno, R.; Cook, B.; Tolmachev, V.; Zeglis, B.M. Pretargeted imaging and therapy. J. Nucl. Med. 2017, 58, 1553–1559. [Google Scholar] [CrossRef]
- Patra, M.; Zarschler, K.; Pietzsch, H.J.; Stephan, H.; Gasser, G. New insights into the pretargeting approach to image and treat tumours. Chem. Soc. Rev. 2016, 45, 6415–6431. [Google Scholar] [CrossRef] [PubMed]
- David, T.; Hlinova, V.; Kubicek, V.; Bergmann, R.; Striese, F.; Berndt, N.; Szollosi, D.; Kovacs, T.; Mathe, D.; Bachmann, M.; et al. Improved conjugation, 64-Cu radiolabeling, in vivo stability, and imaging using nonprotected bifunctional macrocyclic ligands: Bis(phosphinate) cyclam (BPC) chelators. J. Med. Chem. 2018, 61, 8774–8796. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Striese, F.; Neuber, C.; Gräßel, S.; Arndt, C.; Ullrich, M.; Steinbach, J.; Pietzsch, J.; Bergmann, R.; Pietzsch, H.-J.; Sihver, W.; et al. Preclinical Characterization of the 177Lu-Labeled Prostate Stem Cell Antigen (PSCA)-Specific Monoclonal Antibody 7F5. Int. J. Mol. Sci. 2023, 24, 9420. https://doi.org/10.3390/ijms24119420
Striese F, Neuber C, Gräßel S, Arndt C, Ullrich M, Steinbach J, Pietzsch J, Bergmann R, Pietzsch H-J, Sihver W, et al. Preclinical Characterization of the 177Lu-Labeled Prostate Stem Cell Antigen (PSCA)-Specific Monoclonal Antibody 7F5. International Journal of Molecular Sciences. 2023; 24(11):9420. https://doi.org/10.3390/ijms24119420
Chicago/Turabian StyleStriese, Franziska, Christin Neuber, Sandy Gräßel, Claudia Arndt, Martin Ullrich, Jörg Steinbach, Jens Pietzsch, Ralf Bergmann, Hans-Jürgen Pietzsch, Wiebke Sihver, and et al. 2023. "Preclinical Characterization of the 177Lu-Labeled Prostate Stem Cell Antigen (PSCA)-Specific Monoclonal Antibody 7F5" International Journal of Molecular Sciences 24, no. 11: 9420. https://doi.org/10.3390/ijms24119420
APA StyleStriese, F., Neuber, C., Gräßel, S., Arndt, C., Ullrich, M., Steinbach, J., Pietzsch, J., Bergmann, R., Pietzsch, H.-J., Sihver, W., Frenz, M., Feldmann, A., & Bachmann, M. P. (2023). Preclinical Characterization of the 177Lu-Labeled Prostate Stem Cell Antigen (PSCA)-Specific Monoclonal Antibody 7F5. International Journal of Molecular Sciences, 24(11), 9420. https://doi.org/10.3390/ijms24119420







