1. Introduction
Targeted therapeutic peptides (TTPs) are a series of functional peptides that contain 2–50 amino acids. TTPs have a molecular weight of approximately 0.5–5 kDa [
1]. Apart from artificial synthesis, these peptides also implicitly exist in the proteins of animals, plants, bacteria, and fungi, and can be released during enzymatic digestion, food digestion, or microbial action [
2]. TTPs have been proven to exhibit some biological activities, including antibacterial, anti-inflammatory, growth-promoting, pro-differentiation, anti-hypertensive, and anti-cancer activities [
3,
4]. Compared to traditional small-molecule drugs (SMD), TTPs have a larger binding interface with their targets exhibiting higher specificity and robustness, thus avoiding side effects resulting from off-target effects. In addition, TTPs exhibit low immunogenicity due to their uncomplicated spatial conformation. Therefore, TTPs have been used in many medical fields, and more than 100 TTPs have been approved by the FDA to treat diseases such as tissue trauma, heart failure, hypertension, diabetes, and cancer [
5,
6].
Three conventional methods have been frequently used for screening TTPs in natural proteins: the bioactivity-guided method, the phage display method, and in silico screening method. For the bioactivity-guided screening method, the first step is to divide the enzymatically hydrolyzed crude peptides into products with different molecular weights using membrane filtration technology. The biological activity of each hydrolytic product is analyzed to select the most active component. TTPs in the most active component are isolated and enriched according to the different properties of the peptides, such as molecular weight, polarity, and charge, which are then examined using bioactivity assays. Nong et al. identified a dipeptidyl peptidase-IV inhibitory peptide from the egg yolk hydrolysate of soft-shell turtles [
7]. In the phage display method, a DNA library is separately integrated into the genome of different phages to harbor peptides in their tails allowing positive phages to bind to a fixed target. After extended breeding by infecting bacteria, the positive phages are collected to extract the genome for sequencing required for evaluating TTPs. The EBD fibronectin peptide for targeting prostate cancer was discovered in this way [
8]. In the in silico screening method, the amino acid sequence of the proteins was virtually digested in silico to generate a peptide library. Peptide candidates with highly similar spatial structures and physicochemical properties to those in the database were theoretically simulated and experimentally synthesized to determine their biological activity. Tejano et al. used the BIOPEP-UWM database to screen peptides in the Chlorella sorokiniana protein [
9]. All conventional techniques mentioned above have played an important role in the discovery of TTPs. However, these methods have drawbacks in performing numerous experiments and complex screening processes, in addition to long screening times, which in turn restricts the innovation and clinical translation of peptide drugs due to inefficient screening processes.
Except for the antimicrobial peptides that directly act on the cell membrane of microbes, most TTPs bind to receptors or ion channels through intermolecular forces, such as van der Waals forces, hydrogen bonds, and salt bridges. Extracellular signals originate from the TTP-target complex, enabling cells to respond to external stimuli. For example, glucagon-like peptide 1 (GLP1), a target therapeutic peptide in the treatment of diabetes, binds to the GLP1 receptor on the surface of islet cells and then transmits signals to the nucleus via the insulin signaling pathway to activate insulin secretion and inhibit glucagon release [
10]. This process balances metabolism in the body but consumes the receptors and signal transduction molecules of the islet cells [
11,
12,
13]. To maintain proper cellular function, cells transcribe genes associated with these molecules and translate them to replenish the missing signaling protein. Therefore, when the transcription of cells treated with a peptide library of natural proteins is clear, the TTP-triggered signaling pathways and their receptors are easily determined. A cell receptor is the target of a ligand and the interaction between them is specific and stable. Once the active center of a receptor binds to a suitable ligand, it forms a stable complex [
14]. Hence, as soon as this receptor-ligand complex is captured, the TTPs can be screened.
Co-immunoprecipitation (CoIP) is an effective tool for studying protein–protein interactions based on the specificity between antibodies and antigens [
15]. CoIP comprises the following three steps as follows: (1) the antibody is cross-linked with the magnetic beads to form a complex that can be fixed with magnets; (2) those fixed complexes can capture another complex formed by antigens and unknown proteins to generate a final complex; (3) the final complex is washed and detected by SDS-PAGE, Western blot (WB), or other analytical techniques. The results obtained by CoIP have high accuracy because the interacting proteins are natural proteins that naturally combine with each other by non-covalent bonding. If a different detection method such as liquid chromatography–mass spectrometry (LC-MS), gas chromatography –mass spectrometry (GC-MS), or other modern sequencing techniques is used for CoIP, studying the interaction between receptors and ligands, including proteins, lipids, nucleic acids, and small-molecule drugs, becomes more feasible.
Sericin, one of the major components of the silkworm cocoon, is a group of natural macromolecular glycoproteins with a molecular weight of approximately 20–400 kDa. It is synthesized and secreted by the middle silk glands of silkworms and covers the surface of the silk fibroin [
16]. Previous studies have demonstrated that sericin and its hydrolysate have many biological activities, such as cell proliferation and synthesis of extracellular matrix proteins [
17,
18], which are beneficial for curing illnesses such as wound repair and colitis treatment [
19,
20]. In this study, a novel strategy for screening TTPs in the raw material of sericin was developed based on transcriptomics and co-immunoprecipitation techniques. The proposed strategy is based on the construction of peptide libraries, screening of TTP-triggered signaling pathways, CoiP, and identification and functional analysis of target peptides. The results showed that the two most active and selective peptides were successfully screened and could significantly promote the transcription and synthesis of extracellular matrix proteins. Our proposed strategy can also be utilized for screening other drug candidates, including proteins, lipids, nucleic acids, and small-molecule drugs.
3. Discussion
TTPs are used in the treatment of many diseases worldwide, including hypertension, diabetes, and oncology, because of their specificity, high safety, and low price. The preliminary pharmaceutical market analysis stated that the sales of TTP drugs exceeded
$50 billion in 2019, accounting for approximately 5% of the global pharmaceutical market and that percentage increases annually [
22]. Therefore, the development of TTP drugs is a hot research topic that has attracted the attention of many researchers.
Sericin, as a natural product, is often processed as a scaffold, drug carrier, biological tracer, or directly used as a wound dressing because of its good moisturizing property, low immunogenicity, good biocompatibility, and biodegradability [
23]. However, the high-molecular-weight sericin proteins obtained using the traditional methods maintain their advanced structure, and the content of TTPs is not effectively released, resulting in fewer specific pharmaceutical properties [
24]. Therefore, we created trypsinogen-based self-degummed silk in the early stage, which allows us to easily obtain sericin peptides [
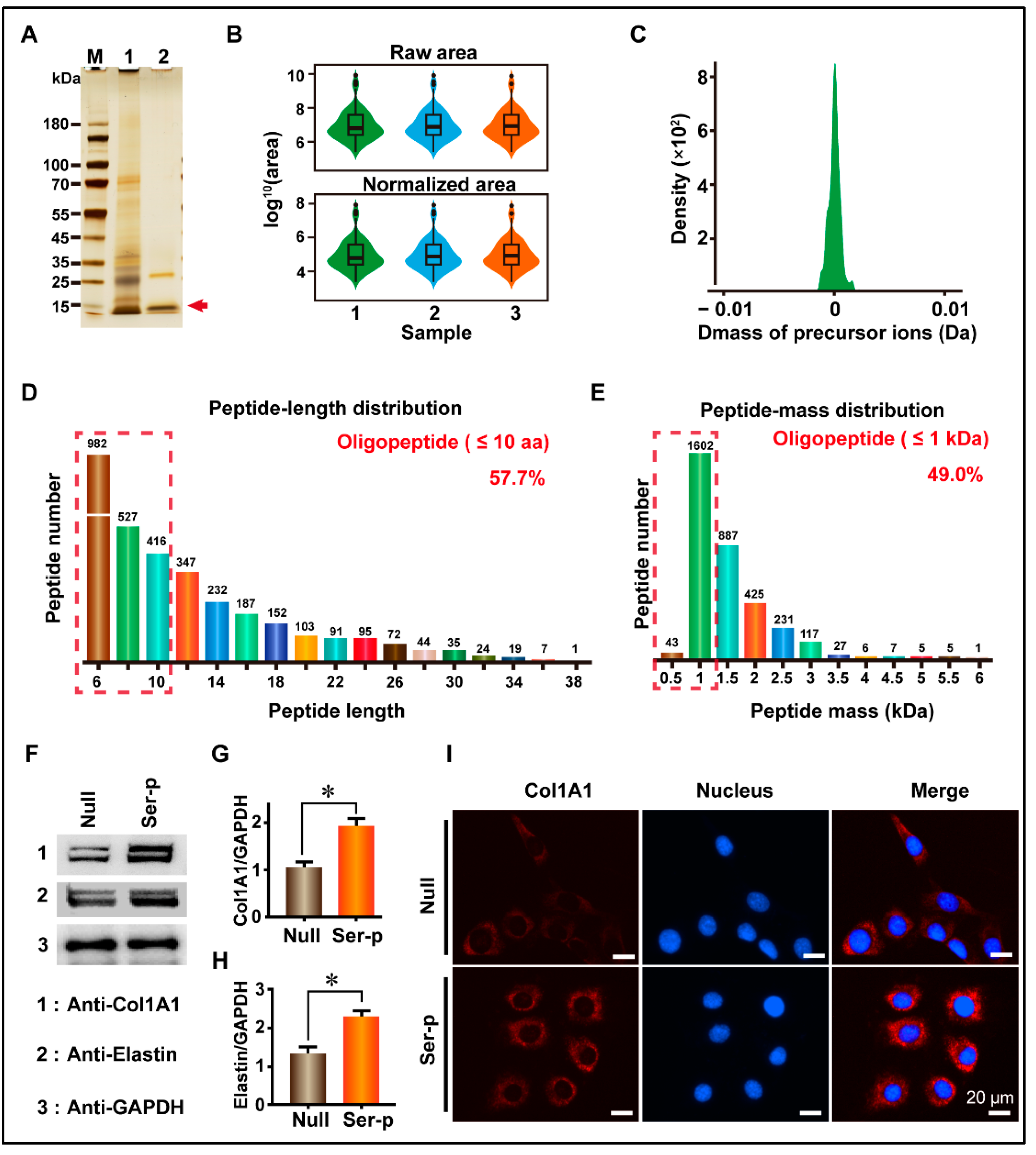
17]. However, we found that the self-degummed sericin can be completely separated from the silk fibroin, but it cannot be fully degraded into low-molecular-weight peptides, which would affect the filtration of the sericin solution. Hence, in this study, we first collected the self-degummed sericin peptides with lower molecular weights and retained their cell proliferation-promoting properties. Subsequently, the remaining sericin peptides with large molecular weights were further digested by chymotrypsin. The construction of a sericin peptide library using this stepwise enzymatic method can not only reduce the loss of sericin protein and greatly improve its utilization rate but also make the sericin peptide library exhibit the characteristics of low molecular weight peptides. In the enzymatically generated sericin peptide library, the content of oligopeptides with amino acids <10 is approximately 57.7%, and the content of oligopeptides with molecular weight <1 kDa is approximately 49.0%, which is within the range of skin absorption. In addition, it can promote cell proliferation and extracellular matrix protein synthesis. This enzymatic sericin peptide library can be potentially used to produce anti-aging agents or adjuvants for the skin [
25,
26].
The use of traditional methods to screen TTPs from enzymatic peptide libraries in natural resources is a complex, time-consuming, and inefficient process, which hinders the development of new drugs. Comparing the three conventional screening approaches, we found that previous researchers have made little or no use of the working principle of TTP: ligand > receptor > intracellular signal > cellular transcription and translation > protein [
27]. Under normal circumstances, cells can compensate for missing proteins through gene transcription and translation. Therefore, if we comprehensively investigate the changes in cell signaling pathways, we can easily determine the receptor and signaling pathways activated by TTPs. Transcriptomics or translatomics is an effective method to accurately reflect changes in intracellular mRNA or protein levels [
28,
29]. However, the former is more commonly utilized by researchers because of its well-developed technology, short test cycle, and low price. Therefore, we innovatively combined transcriptomics with CoIP technology to establish a simple and effective screening method that allows us to easily screen TS263/1000 from an enzymatic sericin peptide library that can promote the synthesis of extracellular matrix proteins. This can effectively avoid (1) the problems of performing too many validation experiments in the bioactivity-guided method, (2) the complexity of constructing the library in the phage display method, and (3) excessive false positives in the virtual screening method in silico. In addition, the receptors used in this method are directly derived from cells, which saves time and the cost required for recombinant expression, maintains their natural activity, and greatly improves the efficiency of screening pharmaceutical peptides.
The specificity of peptide drugs is also one of the important factors in determining their use for disease treatment. Off-targeting drugs can cause some unpredicted side effects [
30]. The TGF-β signaling pathway can regulate many cellular processes, including growth, differentiation, and extracellular matrix synthesis [
31]. The TGF-β signaling pathway can be divided into two types: classical and non-classical pathways [
32]. The non-classical TGF-β signaling pathways always cooperate with three para-signaling pathways to promote cell proliferation, including the JNK, ERK, and P38 signaling pathways [
33]. Therefore, in our study, we validated the specificity of TS263. The key factors of the four signaling pathways mentioned above were considered to investigate the levels of transcription, translation, and phosphorylation of Smad2/3, JNK, ERK1/2, and P38. TS263 has been suggested to only promote the transcription and phosphorylation of Smad2/3 through the TGFβR2 receptor, but it cannot affect the transcription and phosphorylation of JNK, ERK, and P38. We also examined cell viability and proliferation in the Null and TS263 groups and found no significant differences between them. Therefore, we believe that TTPs screening using our approach can specifically regulate cellular processes.
4. Methods and Materials
4.1. Cell Culture
Human immortal keratinocytes (HaCaT) and human foreskin fibroblasts (HFF-1) were purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, China) and cultured in a DMEM medium supplemented with 10% fetal bovine serum (Gibco, New York, NY, USA) at 37 °C in an incubator with 5% CO2.
4.2. Preparation of Sericin Peptide Library
Ten grams of self-degummed silk were immersed in 500 mL of 1 mM of Tris-HCl solution for 24 h. The supernatant was then collected by centrifugation at 1.8 × 104 rpm and heated at 121 °C for 30 min to inactivate trypsin activity. Finally, the sericin solution was desalted using a dialysis bag with a molecular retention of 500 kDa and filtered through a 0.22 µm membrane. The remaining sericin on the membrane with a higher molecular weight was collected and reprocessed with chymotrypsin heated, desalted, and filtered. The collected solution was mixed, freeze-dried, and stored at −80 °C.
4.3. Sericin Peptide Library Promotes Transcription and Synthesis of Extracellular Matrix Proteins
When cell fusion reached 70–80% in the tissue culture dish, HaCaT cells or HFF-1 cells were re-suspended with trypsin (Gibco, New York, NY, USA) and planted in a 24-well plate at a density of 1.0 × 104 cells/well. These cell-containing wells were divided into Null and Ser-p groups and replaced with 200 μL of fresh DMEM medium with 10% FBS after 12 h. After treating each well of the Ser-p group with a 0.4 μg sericin peptide library for 48 h, the medium was collected, and the cells were lysed with RIPA (Beyontime, Shanghai, China).
4.4. SDS-PAGE and Western Blotting
Medium or cell lysates containing 20 ug of protein were separated on a polyacrylamide gel with a gradient of 4–20% (Gencript, Nanjing, China) and then transferred to PVDF membranes. After blocking with 5% skim milk solution, washing with 1× TBST, incubation with a primary antibody, washing with 1× TBST, incubation with a secondary antibody, and washing with 1× TBST, the membrane was transferred to a chemiluminescence imaging system (Champchemi 610 plus, Clinx, Shanghai, China) to record the signal.
The primary antibodies used in this study were anti-Col1A1, anti-Elastin, anti-TGFβR2, anti-Smad2/3, anti-phosphorylated-Smad2/3, anti-ERK1/2, anti-phosphorylated-ERK1/2, anti-JNK, anti-phosphorylated-JNK, anti-P38, anti-phosphorylated-P38, and anti-GAPDH. All antibodies were purchased from CST except for anti-elastin (Abcam, Cambridge, UK). The secondary antibody used was goat anti-rabbit IGG (Beyotime, Shanghai, China). The primary and secondary antibodies were diluted with 1× TBST at a ratio of 1:10,000 (v/v).
4.5. Transcriptome Analysis and Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total rRNA was extracted as previously described [
34]. Cells treated with sericin or TTP were collected and washed with 1× PBS. Total RNA was extracted using Total RNA Kit I (Omega, New Orleans, LA, USA) according to the manufacturer’s instructions.
For transcriptome analysis, 1 μg of mRNA was enriched using oligo (dT) to construct the sequencing library, followed by sequencing. Data were recorded and analyzed by Qiantang Biotechnology Co., Ltd. (Suzhou, China).
For reverse transcription polymerase chain reaction (RT-PCR), 1 μg of mRNA was inverted to cDNA using a TransScript
® II All-in-One First-Strand cDNA Synthesis SuperMix Kit (Transgen, Beijing, China), and RT-PCR was performed using an ABI Prism 7500 sequence detection system (ABI, Waltham, MA, USA). Primers used in this study are listed in
Table 6.
4.6. Immunofluorescence Staining
After removal of the culture medium, cells were processed using the following steps: washed with 1× PBS, fixed with 4% paraformaldehyde for 15 min, neutralized with 1× TBST solution containing glycine at a concentration of 1 mg/mL, blocked with 1×TBST solution containing 5% goat serum for 2 h, incubated with a primary antibody for 2 h, washed with 1× TBST, and incubated with a secondary antibody for 2 h. After washing twice with 1× TBST, the cells were placed on an EVOS FL AUTO fluorescence microscope (Thermo Fisher Scientific, Waltham, MA, USA) to record the fluorescence signal.
The primary antibodies used in this experiment were anti-Col1A1 and anti-elastin, whereas the secondary antibody was a goat anti-rabbit IGG. The dilution ratio of the antibodies was 1:500.
4.7. Co-Immunoprecipitation (CoIP)
Cell membrane proteins (CMP) were extracted from HaCaT cells using the Minute™ Plasma Membrane Protein Isolation and Cell Fractionation Kit (Invent, Eden Prairie, MN, USA) and stored at −80 °C. Subsequently, 5 ug of anti-TGFβR2 antibody and protein G beads (Dynabeads, Thermo Fisher, Waltham, MA, USA) were co-incubated for 2 h and then cross-linked using bis (sulfosuccinimidyl) suberate for 15 min. After termination with 1 M Tris-HCl, the complex was washed with 1 × TBST and incubated with CMP overnight. The supernatant was removed, and the sample was incubated with 500 μL of sericin at a concentration of 1 mg/mL for 2 h. After washing with 1× PBS, the sample was dissolved in 5× SDS-PAGE loading buffer, with or without R250 dye, and heated in a water bath at 100 °C for 10 min. Finally, the supernatant was collected to perform SDS-PAGE and LC-MS analysis.
4.8. LC-MS Analysis
The sericin peptide library or the sample from CoIP was filtered using an ultrafiltration tube with molecular retention of 10 kDa (GE, Boston, MA, USA). The filtrate was collected, freeze-dried, re-dissolved in 0.1% (w/v) trifluoroacetic acid, and desalted using a desalination column (GE, Boston, MA, USA). Samples were then freeze-dried and redissolved in 0.1% (v/v) formic acid solution. Finally, the data were collected using a Thermo Scientific™ liquid chromatography–mass spectrometer (Thermo Fisher) and processed using Byonic v2.16.11 (Protein Metrics, San Carlos, CA, USA) software.
4.9. Molecular Docking, Molecular Dynamics, and Calculation of Molecular Free Energy
The spatial conformation, static charge, and energy of the TS263/1000 ligand were optimized to simulate the physiological environment using Schrödinger2021-3 software. The TGFβR2 receptor (ID:1KTZ) downloaded from the PDB database was modified using Maestro software (version 12.9) by removing water molecules, adding hydrogen atoms, and optimizing the force field. Subsequently, the centroid of the eutectic ligand in the TGFβR2 crystal structure was selected as the center of the active pocket for docking with the TS-263/1000 ligand using the SP precision algorithm.
In the molecular dynamics study, the ligand and receptor were described by the ff14SB protein force field, and the Na
+/Cl
− system was used to balance the charge after adding the cross-section of the octahedral TIP3P solvent cassette at a distance of 10 Å in the docking system. The energy of the docking system with constant volume was then optimized using the steepest descent method of 2500 steps and conjugate gradient method, the steepest descent method of 2500 steps, and rapidly heated from 0 K to 298.15 K within 200 ps and was maintained at 298.15 K. To ensure uniform distribution of solvent molecules in a solvent box, the docking system was run using the NVT (isothermal and isobaric) ensemble simulation for 500 ps and the NPT (isothermal and isobaric) ensemble simulation for 500 ps. Finally, the composite system was subjected to the NPT (isothermal and isobaric) ensemble simulations under periodic boundary conditions. The parameters used in the process of dynamic calculation were as follows: truncation distance of non-bond force at 10 Å; long-range electrostatic interaction; particle mesh Ewald (PME) method [
35]; limitation of the length of the hydrogen bond (SHAKE method) [
36]; and temperature control (Langevin algorithm) [
37].
To calculate the binding free energy between the ligand and receptor, the MM/GBSA method was used to calculate the MD trajectory for 40–50 ns [
38]. The calculation formula is as follows:
In this formula, ΔE
internal, ΔE
VDW, ΔE
elec, ΔE
GB, and ΔE
SA represent the internal energy, van der Waals interaction, electrostatic interaction, polar solvation-free energy, and non-polar solvation-free energy, respectively. The entropy change was omitted in this study because of its low accuracy and excessive consumption of computing resources [
39].
4.10. Statistical Analysis
Data are presented as the mean ± standard error of the mean (SEM), and Student’s t-test was used for statistical analysis in this study. Statistical significance was set at p < 0.05.