Inhibitory Effect of Zinc on Colorectal Cancer by Granzyme B Transcriptional Regulation in Cytotoxic T Cells
Abstract
:1. Introduction
2. Results
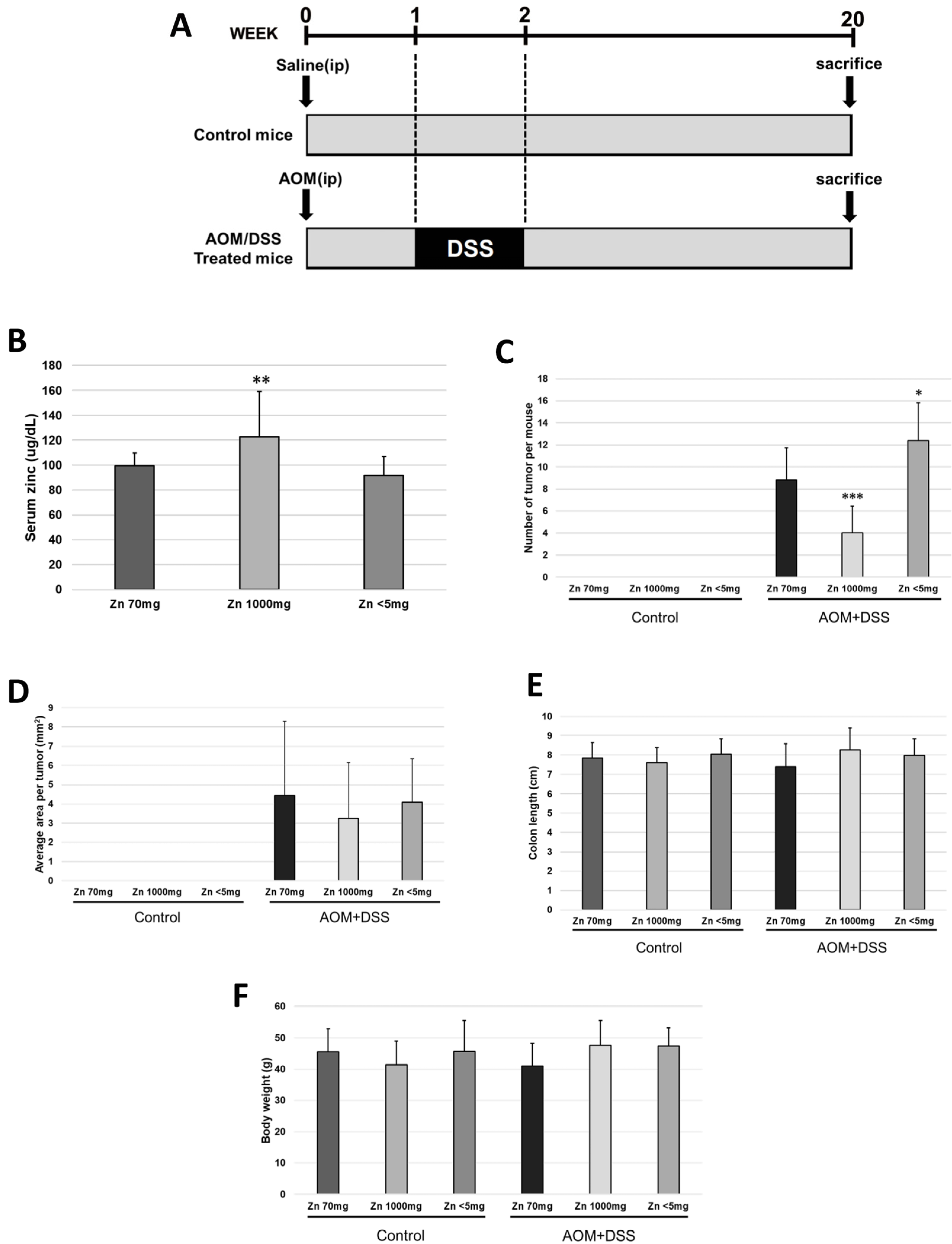
2.1. Increased Zinc Intake Caused a Decrease in the Frequency of Colorectal Malignancies
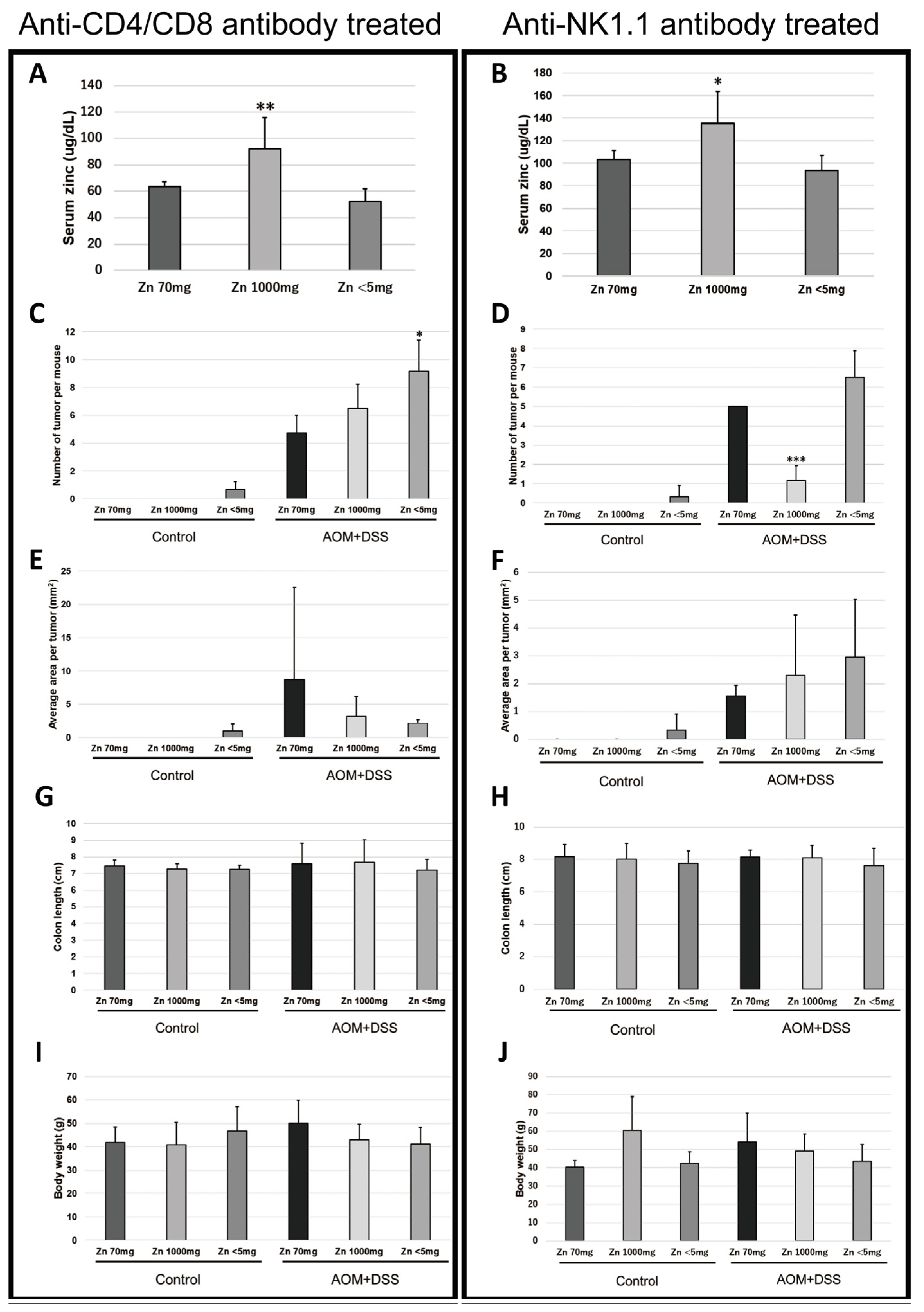
2.2. Removal of CD4- and CD8-Positive Cells Abolished the Difference in Tumor Frequency in the High-Zinc-Intake Group
2.3. Removal of NK1.1-Positive Cells Did Not Change the Difference in Tumor Frequency
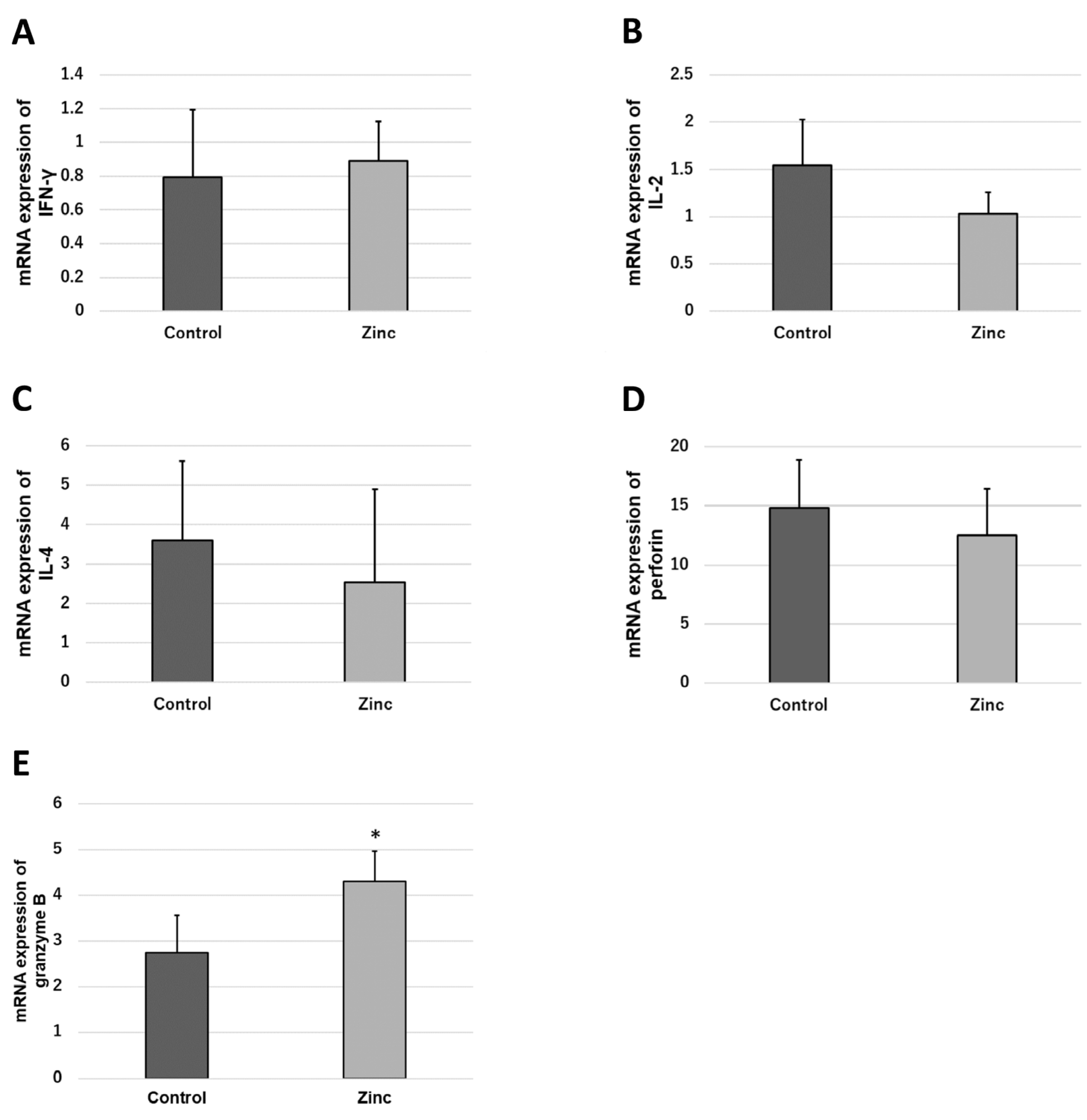
2.4. Effects of Zinc on Differentiation and Proliferation of Immunocompetent Cells
2.5. Effects of Zinc on the Functional Expression of Immunocompetent Cells
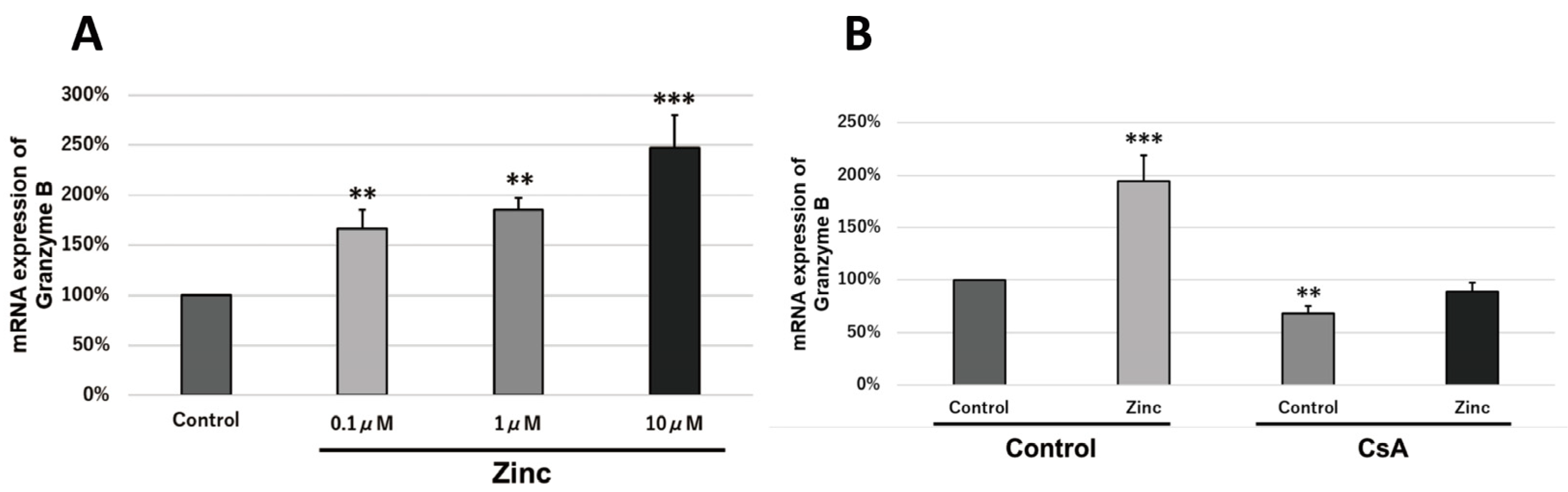
2.6. Granzyme B Is Transcriptionally Activated via the Calcineurin Pathway
2.7. Zinc Increases the Transcriptional Activity of Granzyme B via Intracellular Signaling Molecules
3. Discussion
4. Material and Methods
4.1. Animals and Chemicals
4.2. Quantification of Serum Zinc Levels and Serum Copper Levels
4.3. Induction of Colorectal Cancer
4.4. Mice and Cell Culture
4.5. Measurement of Cytokines
4.6. Real-Time PCR
4.7. Flow Cytometry
4.8. Mineralization of Murine Fecal Samples
4.9. Quantification of Trace Metals in Murine Fecal Samples
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in Wound Healing Modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.W.; Daskalou, D.; Macario, S.; Voruz, F.; Landis, B.N. How to Manage Taste Disorders. Curr. Otorhinolaryngol. Rep. 2022, 10, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Lessons Learned from Experimental Human Model of Zinc Deficiency. J. Immunol. Res. 2020, 2020, 9207279. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; Nagai, J.; Uesawa, Y. Evaluation of antibiotic-induced taste and smell disorders using the FDA adverse event reporting system database. Sci. Rep. 2021, 11, 9625. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, H.; Matsuura, K.; Ishikawa, H.; Hirata, Y.; Kato, N.; Niwa, A.; Narita, Y.; Tomimoto, H. Correlation between Serum Zinc Levels and Levodopa in Parkinson’s Disease. Nutrients 2021, 13, 4114. [Google Scholar] [CrossRef]
- Lazzerini, M.; Wanzira, H. Oral zinc for treating diarrhoea in children. Cochrane Database Syst. Rev. 2016, 12, CD005436. [Google Scholar] [CrossRef]
- Yasui, Y.; Yasui, H.; Suzuki, K.; Saitou, T.; Yamamoto, Y.; Ishizaka, T.; Nishida, K.; Yoshihara, S.; Gohma, I.; Ogawa, Y. Analysis of the predictive factors for a critical illness of COVID-19 during treatment-relationship between serum zinc level and critical illness of COVID-19. Int. J. Infect. Dis. 2020, 100, 230–236. [Google Scholar] [CrossRef]
- Ekemen Keles, Y.; Yilmaz Ciftdogan, D.; Colak, A.; Kara Aksay, A.; Ustundag, G.; Sahin, A.; Yilmaz, N. Serum zinc levels in pediatric patients with COVID-19. Eur. J. Pediatr. 2022, 181, 1575–1584. [Google Scholar] [CrossRef]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and Atopic Dermatitis. J. Nippon Med. Sch. 2021, 88, 171–177. [Google Scholar] [CrossRef]
- Nishida, K.; Uchida, R. Role of Zinc Signaling in the Regulation of Mast Cell-, Basophil-, and T Cell-Mediated Allergic Responses. J. Immunol. Res. 2018, 2018, 5749120. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Hasegawa, A.; Nakae, S.; Oboki, K.; Saito, H.; Yamasaki, S.; Hirano, T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J. Exp. Med. 2009, 206, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Gyorkey, F.; Min, K.W.; Huff, J.A.; Gyorkey, P. Zinc and magnesium in human prostate gland: Normal, hyperplastic, and neoplastic. Cancer Res. 1967, 27, 1348–1353. [Google Scholar]
- Ozeki, I.; Nakajima, T.; Suii, H.; Tatsumi, R.; Yamaguchi, M.; Arakawa, T.; Kuwata, Y. Predictors of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting antiviral treatment: Relationship with serum zinc. J. Clin. Biochem. Nutr. 2020, 66, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.M.; Kasperzyk, J.L.; Andren, O.; Giovannucci, E.L.; Wolk, A.; Hakansson, N.; Andersson, S.O.; Johansson, J.E.; Fall, K.; Mucci, L.A. Dietary zinc and prostate cancer survival in a Swedish cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef]
- Wang, G.; Hiramoto, K.; Ma, N.; Yoshikawa, N.; Ohnishi, S.; Murata, M.; Kawanishi, S. Glycyrrhizin Attenuates Carcinogenesis by Inhibiting the Inflammatory Response in a Murine Model of Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 2609. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.S.; Hahm, K.B.; Surh, Y.J. Effects of Genetic and Pharmacologic Inhibition of COX-2 on Colitis-associated Carcinogenesis in Mice. J. Cancer Prev. 2020, 25, 27–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Pu, W.; Bousquenaud, M.; Cattin, S.; Zaric, J.; Sun, L.K.; Ruegg, C. Emodin Inhibits Inflammation, Carcinogenesis, and Cancer Progression in the AOM/DSS Model of Colitis-Associated Intestinal Tumorigenesis. Front. Oncol. 2020, 10, 564674. [Google Scholar] [CrossRef]
- Muzzioli, M.; Stecconi, R.; Donnini, A.; Re, F.; Provinciali, M. Zinc improves the development of human CD34+ cell progenitors towards Natural Killer cells and induces the expression of GATA-3 transcription factor. Int. J. Biochem. Cell Biol. 2007, 39, 955–965. [Google Scholar] [CrossRef]
- Kim, P.W.; Sun, Z.Y.; Blacklow, S.C.; Wagner, G.; Eck, M.J. A zinc clasp structure tethers Lck to T cell coreceptors CD4 and CD8. Science 2003, 301, 1725–1728. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Chen, X.; Wang, Z.; Liang, X.; Zhang, T.; Liu, M.; Zhou, N.; Lv, J.; Tang, K.; et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol. 2020, 5, eaax7969. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Xia, S.; Kong, Q.; Li, S.; Liu, X.; Junqueira, C.; Meza-Sosa, K.F.; Mok, T.M.Y.; Ansara, J.; et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 2020, 579, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef] [PubMed]
- Volkel, H.; Scholz, M.; Link, J.; Selzle, M.; Werner, P.; Tunnemann, R.; Jung, G.; Ludolph, A.C.; Reuter, A. Superoxide dismutase mutations of familial amyotrophic lateral sclerosis and the oxidative inactivation of calcineurin. FEBS Lett. 2001, 503, 201–205. [Google Scholar] [CrossRef]
- Cousins, R.J. Gastrointestinal factors influencing zinc absorption and homeostasis. Int. J. Vitam. Nutr. Res. 2010, 80, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Saito, M.; Horiguchi, D.; Kina, K. A highly sensitive colorimetric determination of serum zinc using water-soluble pyridylazo dye. Clin. Chim. Acta 1982, 120, 127–135. [Google Scholar] [PubMed]
- Abe, A.; Yamashita, S.; Noma, A. Sensitive, direct colorimetric assay for copper in serum. Clin. Chem. 1989, 35, 552–554. [Google Scholar] [CrossRef]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003, 94, 965–973. [Google Scholar] [CrossRef]
- Mehta, S.Q.; Behl, S.; Day, P.L.; Delgado, A.M.; Larson, N.B.; Stromback, L.R.; Huebner, A.R.; DeGrado, T.R.; Davis, J.M.; Jannetto, P.J.; et al. Evaluation of Zn, Cu, and Se Levels in the North American Autism Spectrum Disorder Population. Front. Mol. Neurosci. 2021, 14, 665686. [Google Scholar] [CrossRef]
- Xu, J.; Church, S.J.; Patassini, S.; Begley, P.; Waldvogel, H.J.; Curtis, M.A.; Faull, R.L.M.; Unwin, R.D.; Cooper, G.J.S. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017, 9, 1106–1119. [Google Scholar] [CrossRef]


| AOM/DSS Treatment | Diet | |
|---|---|---|
| Group 1 | Untreated | 70 mg/kg zinc |
| Group 2 | Untreated | 1000 mg/kg zinc |
| Group 3 | Untreated | <5 mg/kg zinc |
| Group 4 | Treated | 70 mg/kg zinc |
| Group 5 | Treated | 1000 mg/kg zinc |
| Group 6 | Treated | <5 mg/kg zinc |
| Target | Primer | Sequence |
|---|---|---|
| IL-2 | Forward Primer | 5′-CCTGAGCAGGATGGAGAATTACA-3′ |
| Reverse Primer | 5′-TCCAGAGACATGCCGCAGAG-3′ | |
| IL-4 | Forward Primer | 5′-TCTCGAATGTACCAGGAGCCATATC-3′ |
| Reverse Primer | 5′-AGCACCTTGGAAGCCCTACAGA-3′ | |
| IFN-γ | Forward Primer | 5′-CGGCACAGTCATTGAAAGCCTA-3′ |
| Reverse Primer | 5′-GTTGCTGATGGCCTGATTGTC-3′ | |
| Perforin | Forward Primer | 5′-GCAATTTCCGGGCAGAACA-3′ |
| Reverse Primer | 5′-CTGAACTCCTGGCCACCAAAG-3′ | |
| Granzyme B | Forward Primer | 5′-GACTTTGTGCTGACTGCTGCTC-3′ |
| Reverse Primer | 5′-GGGATGACTTGCTGGGTCTTC-3′ | |
| G3PDH | Forward Primer | 5′-AGCTGAACGGGAAGCTCACT-3′ |
| Reverse Primer | 5′-TGAAGTCGCAGGAGACAACC-3′ |
| Monitored Isotopes | Concentration Range in Standards (ppb) | Concentration Range in Analytical Samples (ppb) | Amount in QC Sample (ng) * | Mean Recovery (%) ** |
|---|---|---|---|---|
| 53Cr | 0.750–250 | 3.60–21.69 | 225 | 77.1 |
| 55Mn | 1.243–124.25 | 1.74–7.06 | 1118.25 | 101.9 |
| 57Fe | 3.72–1240 | 125.2–1158 | 1116 | 97.2 |
| 59Co | 0.025–25.0 | 0.039–0.261 | 22.5 | 107.8 |
| 63Cu | 0.750–250 | 27.90–177.4 | 225 | 92.7 |
| 66Zn | 3.713–1237.5 | 4.52–761.0 | 1113.75 | 125.6 |
| 78Se | 0.249–74.7 | (0.236) ***, 0.256–3.40 | 224.1 | 100.6 |
| 95Mo | 0.074–24.78 | 0.131–1.41 | 222.975 | 95.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, N.; Fujisawa, Y.; Xiang, H.; Kitamura, H.; Nishida, K. Inhibitory Effect of Zinc on Colorectal Cancer by Granzyme B Transcriptional Regulation in Cytotoxic T Cells. Int. J. Mol. Sci. 2023, 24, 9457. https://doi.org/10.3390/ijms24119457
Nakagawa N, Fujisawa Y, Xiang H, Kitamura H, Nishida K. Inhibitory Effect of Zinc on Colorectal Cancer by Granzyme B Transcriptional Regulation in Cytotoxic T Cells. International Journal of Molecular Sciences. 2023; 24(11):9457. https://doi.org/10.3390/ijms24119457
Chicago/Turabian StyleNakagawa, Naoya, Yutaka Fujisawa, Huihui Xiang, Hidemitsu Kitamura, and Keigo Nishida. 2023. "Inhibitory Effect of Zinc on Colorectal Cancer by Granzyme B Transcriptional Regulation in Cytotoxic T Cells" International Journal of Molecular Sciences 24, no. 11: 9457. https://doi.org/10.3390/ijms24119457
APA StyleNakagawa, N., Fujisawa, Y., Xiang, H., Kitamura, H., & Nishida, K. (2023). Inhibitory Effect of Zinc on Colorectal Cancer by Granzyme B Transcriptional Regulation in Cytotoxic T Cells. International Journal of Molecular Sciences, 24(11), 9457. https://doi.org/10.3390/ijms24119457





