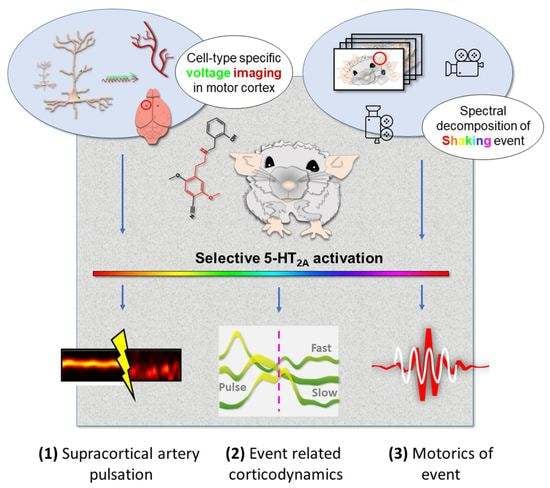
Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging
Abstract
1. Introduction
2. Results
2.1. 25CN-NBOH Altered Blood-Volume Related Signal Oscillations (BvSOs) in the Motor Cortex
2.2. 25CN-NBOH Did Not Substantially Alter Gross Motor Output
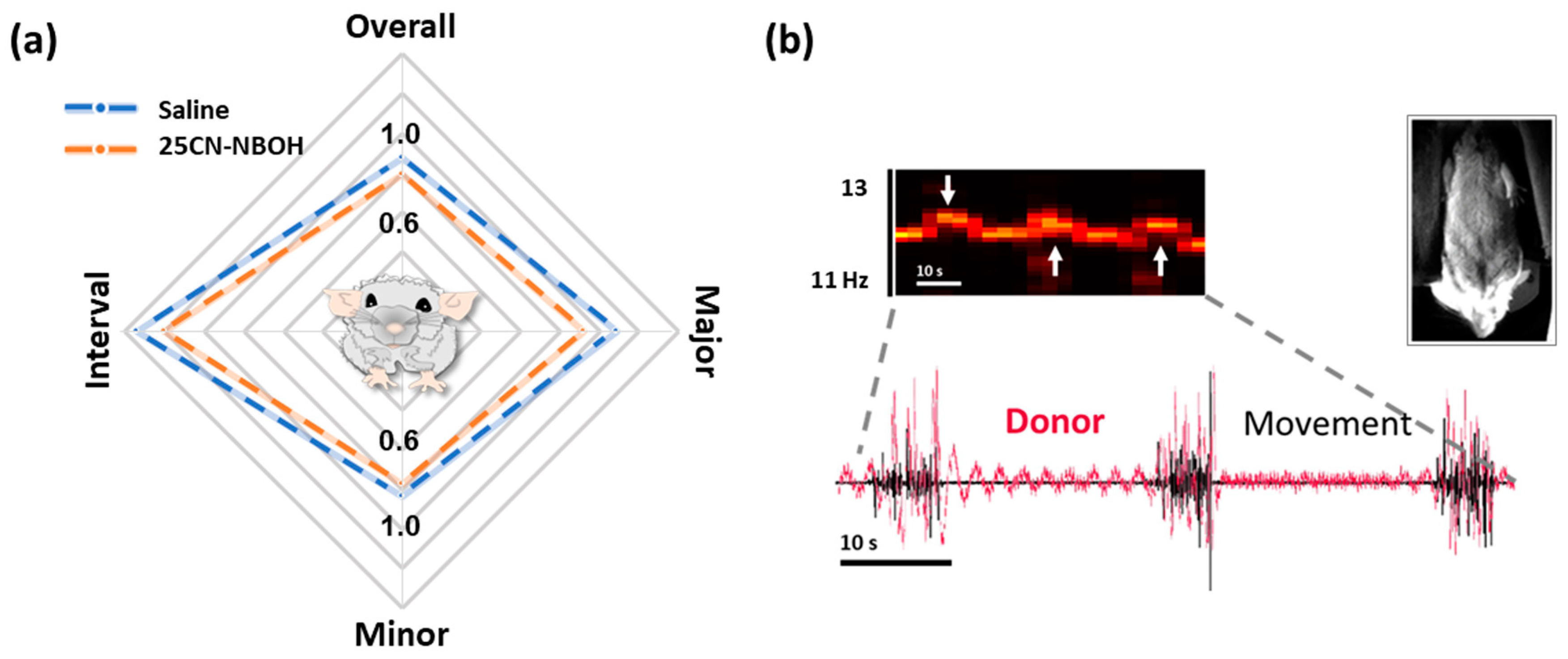
2.3. 25CN-NBOH Induced Shaking Behavior
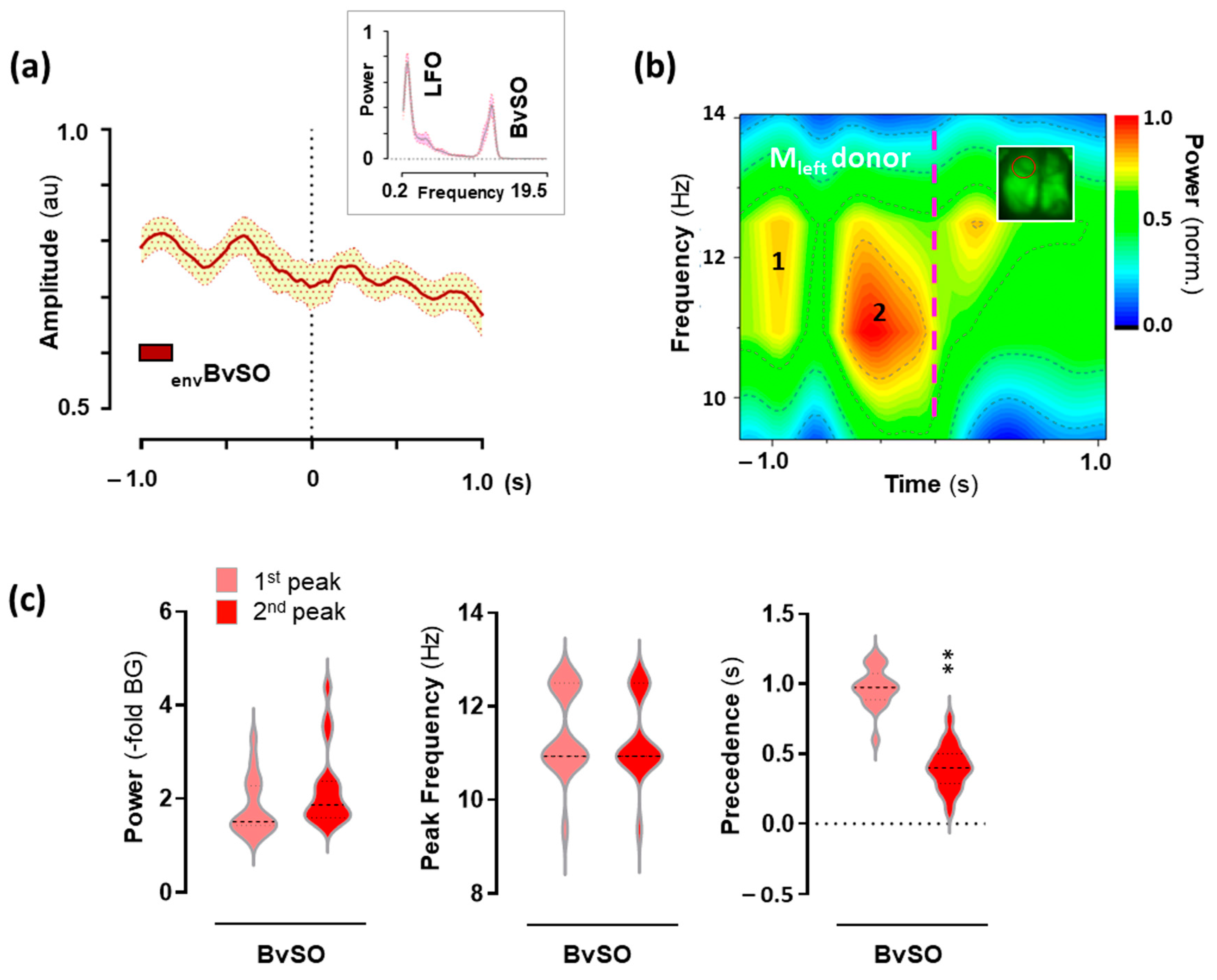
2.4. Hemodynamic Activity in the Motor Cortex Associated with 25CN-NBOH-Induced Shaking Events
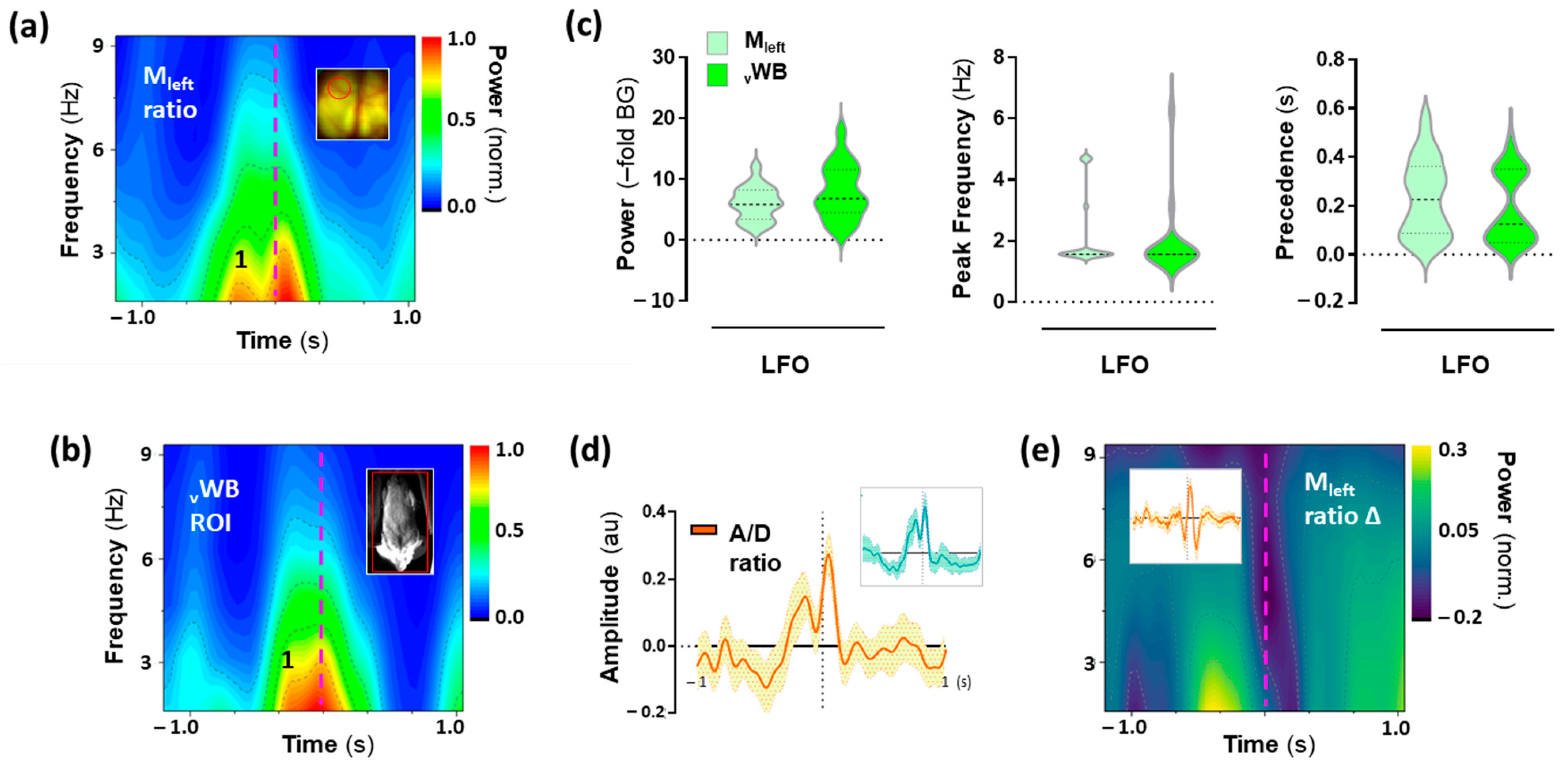
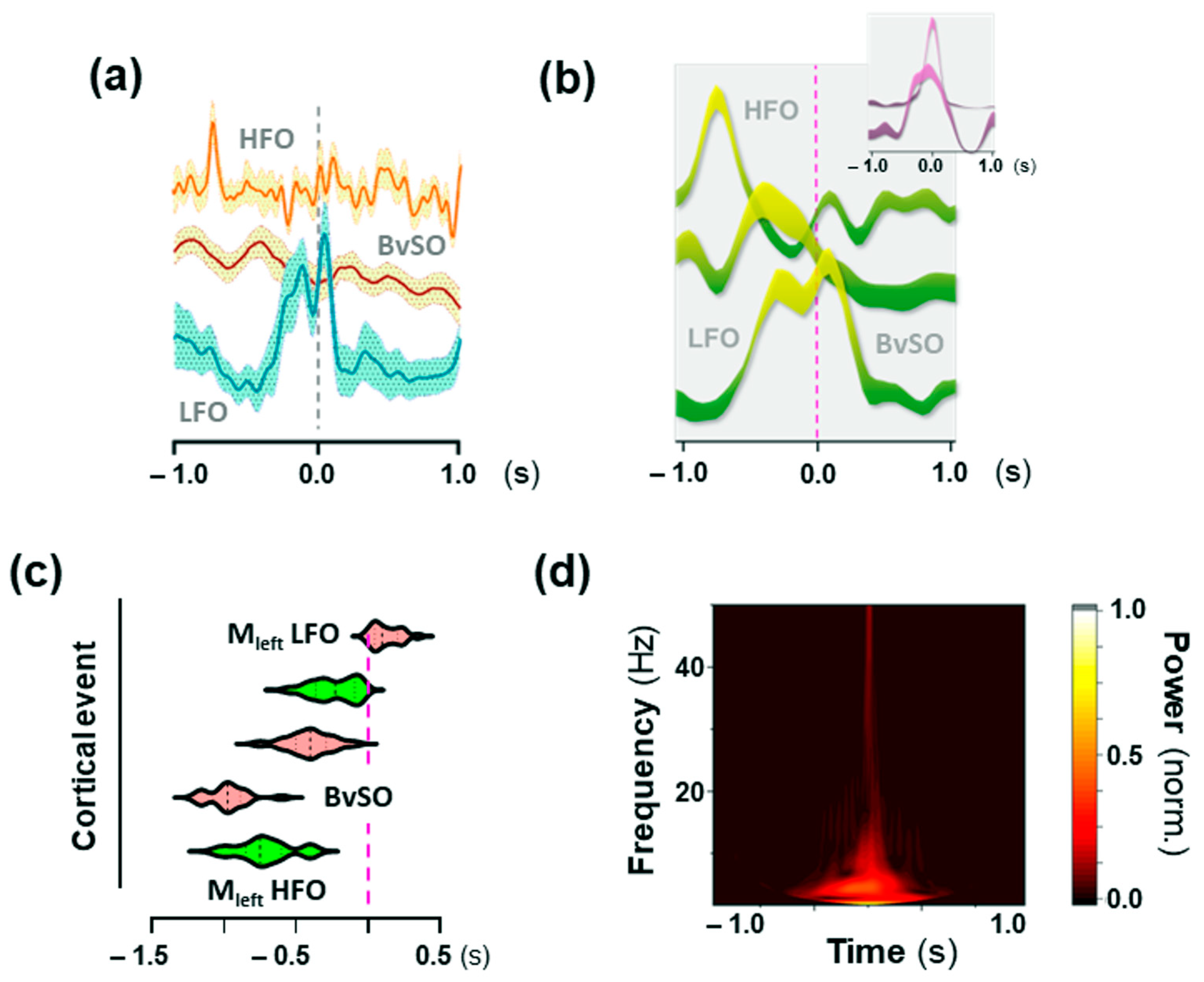
2.5. Low-Frequency Oscillations (LFO) in the Motor Cortex Associated with 25CN-NBOH-Induced Shaking Events
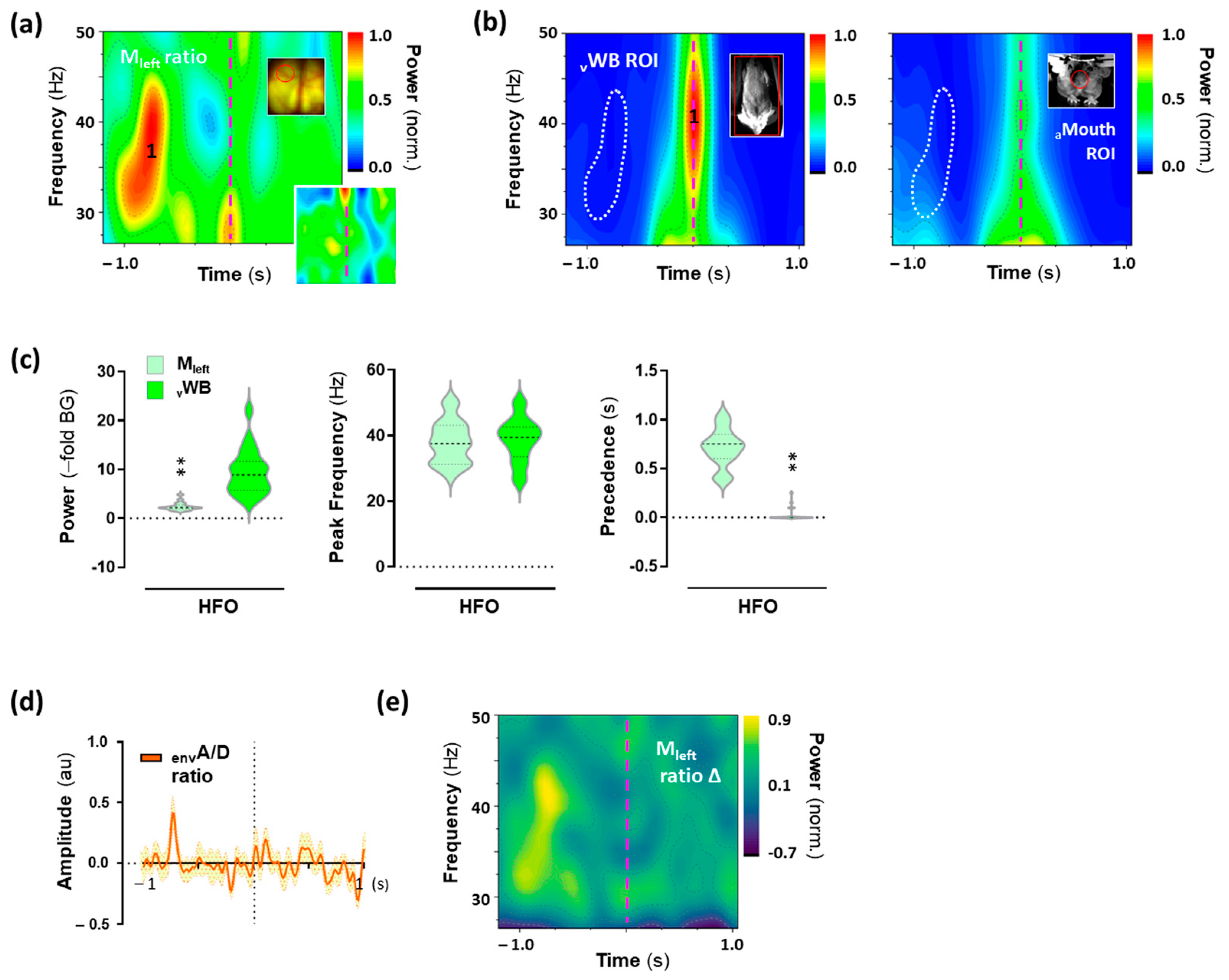
2.6. High-Frequency Oscillations (HFO) in the Motor Cortex Associated with 25CN-NBOH-Induced Shaking Events
3. Discussion
4. Materials and Methods
4.1. Animals and Target Gene Expression
4.2. Surgery and Experimental Habituation
4.3. Treatment and Selection
4.4. Voltage Imaging and Behavioral Monitoring
4.5. Analysis of Motor Behavior
4.6. VSFP Butterfly 1.2 Donor Imaging for Reading out Blood Volume
4.7. VSFP Butterfly 1.2 Voltage Imaging
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchborn, T.; Schröder, H.; Dieterich, D.C.; Grecksch, G.; Höllt, V. Tolerance to LSD and DOB induced Shaking behaviour: Differential adaptations of frontocortical 5-HT2A and glutamate receptor binding sites. Behav. Brain Res. 2015, 281, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Handley, S.L.; Singh, L. Neurotransmitters and shaking behaviour—More than a ‘gut-bath’ for the brain? Trends Pharmacol. Sci. 1986, 7, 324–328. [Google Scholar] [CrossRef]
- Haley, T.J. Pharmacological effects from drugs injected intracerebrally in unanesthetized animals. J. Am. Pharm. Assoc. 1956, 45, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.L.; Umbreit, W.W. Permanent alteration of behavior in mice by chemical and psychological means. Science 1956, 124, 723–724. [Google Scholar] [CrossRef]
- Winter, C.A.; Flataker, L. Effects of lysergic acid diethylamide upon performance of trained rats. Proc. Soc. Exp. Biol. Med. 1956, 92, 285–289. [Google Scholar] [CrossRef]
- Niemegeers, C.J.E.; Colpaert, F.C.; Leysen, J.E.; Awouters, F.; Janseen, P.A.J. Mescaline-induced head-twitches in the rat: An in vivo method to evaluate serotonin S2 antagonists. Drug Dev. Res. 1983, 3, 123–135. [Google Scholar] [CrossRef]
- Peroutka, S.; Lebovitz, R.; Snyder, S. Two distinct central serotonin receptors with different physiological functions. Science 1981, 212, 827–829. [Google Scholar] [CrossRef]
- Schreiber, R.; Brocco, M.; Audinot, V.; Gobert, A.; Veiga, S.; Millan, M.J. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT) 2A receptors: Modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J. Pharmacol. Exp. Ther. 1995, 273, 101–112. [Google Scholar]
- Gonzalez-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Koedood, L.; Powell, S.B.; Geyer, M.A. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J. Psychopharmacol. 2011, 25, 1548–1561. [Google Scholar] [CrossRef]
- Dougherty, J.P.; Aloyo, V.J. Pharmacological and behavioral characterization of the 5-HT2A receptor in C57BL/6N mice. Psychopharmacology 2011, 215, 581–593. [Google Scholar] [CrossRef]
- Marek, G.J. Interactions of hallucinogens with the glutamatergic system: Permissive network effects mediated through cortical layer V pyramidal neurons. In Behavioral Neurobiology of Psychedelic Drugs; Halberstadt, A., Vollenweider, F.X., Nichols, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 107–135. [Google Scholar] [CrossRef]
- Bedard, P.; Pycock, C. ‘Wet-dog’shake behaviour in the rat: A possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology 1977, 16, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Lucki, I.; Minugh-Purvis, N. Serotonin-induced head shaking behavior in rats does not involve receptors located in the frontal cortex. Brain Res. 1987, 420, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Khumnark, M.; Hines, R.M.; Hines, D.J. Behavioral arrest and a characteristic slow waveform are hallmark responses to selective 5-HT 2A receptor activation. Sci. Rep. 2021, 11, 1925. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Barajas, C.; Estrada-Sánchez, A.M.; Barton, S.J.; Luedtke, R.R.; Rebec, G.V. Dysregulated corticostriatal activity in open-field behavior and the head-twitch response induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine. Neuropharmacology 2017, 113, 502–510. [Google Scholar] [CrossRef]
- Knöpfel, T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 2012, 13, 687–700. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Carhart-Harris, R.L.; Moran, R.J.; Brookes, M.J.; Williams, T.M.; Errtizoe, D.; Sessa, B.; Papadopoulos, A.; Bolstridge, M.; Singh, K.D. Broadband cortical desynchronization underlies the human psychedelic state. J. Neurosci. 2013, 33, 15171–15183. [Google Scholar] [CrossRef]
- Weiler, N.; Wood, L.; Yu, J.; Solla, S.A.; Shepherd, G.M. Top-down laminar organization of the excitatory network in motor cortex. Nat. Neurosci. 2008, 11, 360–366. [Google Scholar] [CrossRef]
- Akemann, W.; Mutoh, H.; Perron, A.; Park, Y.K.; Iwamoto, Y.; Knöpfel, T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J. Neurophysiol. 2012, 108, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Foehring, R.C.; van Brederode, J.F.; Kinney, G.A.; Spain, W.J. Serotonergic modulation of supragranular neurons in rat sensorimotor cortex. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 8238–8250. [Google Scholar] [CrossRef]
- Michaiel, A.M.; Parker, P.R.; Niell, C.M. A hallucinogenic serotonin-2A receptor agonist reduces visual response gain and alters temporal dynamics in mouse V1. Cell Rep. 2019, 26, 3475–3483.e4. [Google Scholar] [CrossRef]
- Watakabe, A.; Komatsu, Y.; Sadakane, O.; Shimegi, S.; Takahata, T.; Higo, N.; Tochitani, S.; Hashikawa, T.; Naito, T.; Osaki, H. Enriched expression of serotonin 1B and 2A receptor genes in macaque visual cortex and their bidirectional modulatory effects on neuronal responses. Cereb. Cortex 2009, 19, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.A.; McCorvy, J.D.; Leth-Petersen, S.; Bundgaard, C.; Liebscher, G.; Kenakin, T.P.; Bräuner-Osborne, H.; Kehler, J.; Kristensen, J.L. Detailed characterization of the in vitro pharmacological and pharmacokinetic properties of N-(2-hydroxybenzyl)-2, 5-dimethoxy-4-cyanophenylethylamine (25CN-NBOH), a highly selective and brain-penetrant 5-HT2A receptor agonist. J. Pharmacol. Exp. 2017, 361, 441–453. [Google Scholar] [CrossRef]
- Cohen, M.L. Serotonin receptors in vascular smooth muscle. In The Serotonin Receptors; Sanders-Bush, E., Ed.; Springer: Manasquan, NJ, USA, 1988; pp. 295–316. [Google Scholar] [CrossRef]
- Martin, G.R. Vascular receptors for 5-hydroxytryptamine: Distribution, function and classification. Pharmacol. Ther. 1994, 62, 283–324. [Google Scholar] [CrossRef]
- Knöpfel, T.; Gallero-Salas, Y.; Song, C. Genetically encoded voltage indicators for large scale cortical imaging come of age. Curr. Opin. Chem. Biol. 2015, 27, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Buchborn, T.; Lyons, T.; Knöpfel, T. Tolerance and tachyphylaxis to head twitches induced by the 5-HT2A agonist 25CN-NBOH in mice. Front. Pharmacol. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Buñag, R.D.; Walaszek, E.J. Differential antagonism by BAS-phenol of responses to the indolealkylamines. J. Pharmacol. Exp. Ther. 1962, 136, 59–67. [Google Scholar] [PubMed]
- Dolder, P.C.; Schmid, Y.; Steuer, A.E.; Kraemer, T.; Rentsch, K.M.; Hammann, F.; Liechti, M.E. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin. Pharmacokinet. 2017, 56, 1219–1230. [Google Scholar] [CrossRef]
- Huang, J.T.; Ho, B.T. The pressor action of 2, 5-dimethoxy-4-methylamphetamine in rats. J. Pharm. Pharmacol. 1972, 24, 656–657. [Google Scholar] [CrossRef]
- Chaouche-Teyara, K.; Fournier, B.; Safar, M.; Dabiré, H. Systemic and Regional Haemodynamic Effects of 1–(2, 5–Dimethoxy-4–IODO-Phenyl)-2–Aminopropane (DOI) and α-Methyl-5–HT, in the Anaesthetised Rat. Clin. Exp. Hypertens. 1994, 16, 779–798. [Google Scholar] [CrossRef]
- Ramage, A.G.; Shepheard, S.L.; Jordan, D.; Koss, M.C. Can the 5-HT2/1c agonist DOI cause differential sympatho-excitation in nerves supplying the heart in anaesthetized cats? J. Auton. Nerv. Syst. 1993, 42, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Tadepalli, A.S.; Friedman, E.; Gershon, S. Cardiovascular actions of 2, 5-dimethoxy-4-methylamphetamine (DOM) in the cat. Eur. J. Pharmacol. 1975, 31, 305–312. [Google Scholar] [CrossRef]
- Buchborn, T.; Lyons, T.; Song, C.; Feilding, A.; Knöpfel, T. The serotonin 2A receptor agonist 25CN-NBOH increases murine heart rate and neck-arterial blood flow in a temperature-dependent manner. J. Psychopharmacol. 2020, 34, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Badhwar, A.; Stanimirovic, D.B.; Hamel, E.; Haqqani, A.S. The proteome of mouse cerebral arteries. J. Cereb. Blood Flow Metab. 2014, 34, 1033–1046. [Google Scholar] [CrossRef]
- de la Fuente Revenga, M.; Shin, J.M.; Vohra, H.Z.; Hideshima, K.S.; Schneck, M.; Poklis, J.L.; González-Maeso, J. Fully automated head-twitch detection system for the study of 5-HT 2A receptor pharmacology in vivo. Sci. Rep. 2019, 9, 14247. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Characterization of the head-twitch response induced by hallucinogens in mice. Psychopharmacology 2013, 227, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, A.K.; Mills, Z.G.; Hu, D.L. Wet mammals shake at tuned frequencies to dry. J. R. Soc. Interface 2012, 9, 3208–3218. [Google Scholar] [CrossRef]
- Fultz, N.E.; Bonmassar, G.; Setsompop, K.; Stickgold, R.A.; Rosen, B.R.; Polimeni, J.R.; Lewis, L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science 2019, 366, 628–631. [Google Scholar] [CrossRef]
- Andrade, R.; Weber, E.T. Htr2a gene and 5-HT2A receptor expression in the cerebral cortex studied using genetically modified mice. Front. Neurosci. 2010, 4, 36. [Google Scholar] [CrossRef]
- Jakab, R.L.; Goldman-Rakic, P.S. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl. Acad. Sci. USA 1998, 95, 735–740. [Google Scholar] [CrossRef]
- Shukla, R.; Watakabe, A.; Yamamori, T. mRNA expression profile of serotonin receptor subtypes and distribution of serotonergic terminations in marmoset brain. Front. Neural Circuits 2014, 8, 52. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Vavladeli, A.; Pala, A.; Galan, K.; Crochet, S.; Petersen, S.S.; Petersen, C.C. Diverse long-range axonal projections of excitatory layer 2/3 neurons in mouse barrel cortex. Front. Neuroanat. 2018, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Cho, R.H.; Li, Y.Q.; Nomura, S.; Mizuno, N. Predominant information transfer from layer III pyramidal neurons to corticospinal neurons. J. Comp. Neurol. 2000, 423, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Tfelt-Hansen, P.; Nilsson, E.; Edvinsson, L. Contractile responses to ergotamine and dihydroergotamine in the perfused middle cerebral artery of rat. J. Headache Pain 2007, 8, 83–89. [Google Scholar] [CrossRef]
- Kermorgant, M.; Pavy-Le Traon, A.; Senard, J.M.; Arvanitis, D.N. Serotonergic receptor 5-HT 2A in the cardiosympathovagal system. In 5-HT2A Receptors in the Central Nervous System; Guiard, B.P., Di Giovanni, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 137–145. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, C.; Roseman, L.; Haridas, S.; Rosas, F.E.; Luan, L.; Kettner, H.; Martell, J.; Erritzoe, D.; Tagliazucchi, E.; Pallavicini, C. Human brain effects of DMT assessed via EEG-fMRI. Proc. Natl. Acad. Sci. USA 2023, 120, e2218949120. [Google Scholar] [CrossRef]
- Scholkmann, F.; Vollenweider, F.X. Psychedelics and fNIRS neuroimaging: Exploring new opportunities. Neurophotonics 2022, 10, 013506. [Google Scholar] [CrossRef] [PubMed]
- Spain, A.; Howarth, C.; Khrapitchev, A.A.; Sharp, T.; Sibson, N.R.; Martin, C. Neurovascular and neuroimaging effects of the hallucinogenic serotonin receptor agonist psilocin in the rat brain. Neuropharmacology 2015, 99, 210–220. [Google Scholar] [CrossRef]
- Madisen, L.; Garner, A.R.; Shimaoka, D.; Chuong, A.S.; Klapoetke, N.C.; Li, L.; Van Der Bourg, A.; Niino, Y.; Egolf, L.; Monetti, C. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 2015, 85, 942–958. [Google Scholar] [CrossRef]
- Empson, R.M.; Goulton, C.; Scholtz, D.; Gallero-Salas, Y.; Zeng, H.; Knöpfel, T. Validation of optical voltage reporting by the genetically encoded voltage indicator VSFP-Butterfly from cortical layer 2/3 pyramidal neurons in mouse brain slices. Physiol. Rep. 2015, 3, e12468. [Google Scholar] [CrossRef]
- Song, C.; Do, Q.B.; Antic, S.D.; Knöpfel, T. Transgenic strategies for sparse but strong expression of genetically encoded voltage and calcium indicators. Int. J. Mol. Sci. 2017, 18, 1461. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Piscopo, D.M.; Niell, C.M.; Knöpfel, T. Cortical signatures of wakeful somatosensory processing. Sci. Rep. 2018, 8, 11977. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, D.; Song, C.; Knöpfel, T. State-dependent modulation of slow wave motifs towards awakening. Front. Cell. Neurosci. 2017, 11, 108. [Google Scholar] [CrossRef]
- Ray, T.S. Psychedelics and the human receptorome. PLoS ONE 2010, 5, e9019. [Google Scholar] [CrossRef]
- Märcher Rørsted, E.; Jensen, A.A.; Kristensen, J.L. 25CN-NBOH: A Selective Agonist for in vitro and in vivo Investigations of the Serotonin 2A Receptor. ChemMedChem 2021, 16, 3263–3270. [Google Scholar] [CrossRef]
- Mutoh, H.; Mishina, Y.; Gallero-Salas, Y.; Knöpfel, T. Comparative performance of a genetically-encoded voltage indicator and a blue voltage sensitive dye for large scale cortical voltage imaging. Front. Cell. Neurosci. 2015, 9, 147. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchborn, T.; Lyons, T.; Song, C.; Feilding, A.; Knöpfel, T. Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging. Int. J. Mol. Sci. 2023, 24, 9463. https://doi.org/10.3390/ijms24119463
Buchborn T, Lyons T, Song C, Feilding A, Knöpfel T. Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging. International Journal of Molecular Sciences. 2023; 24(11):9463. https://doi.org/10.3390/ijms24119463
Chicago/Turabian StyleBuchborn, Tobias, Taylor Lyons, Chenchen Song, Amanda Feilding, and Thomas Knöpfel. 2023. "Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging" International Journal of Molecular Sciences 24, no. 11: 9463. https://doi.org/10.3390/ijms24119463
APA StyleBuchborn, T., Lyons, T., Song, C., Feilding, A., & Knöpfel, T. (2023). Cortical Correlates of Psychedelic-Induced Shaking Behavior Revealed by Voltage Imaging. International Journal of Molecular Sciences, 24(11), 9463. https://doi.org/10.3390/ijms24119463