Abstract
Depression and schizophrenia are two highly prevalent and severely debilitating neuropsychiatric disorders. Both conventional antidepressant and antipsychotic pharmacotherapies are often inefficient clinically, causing multiple side effects and serious patient compliance problems. Collectively, this calls for the development of novel drug targets for treating depressed and schizophrenic patients. Here, we discuss recent translational advances, research tools and approaches, aiming to facilitate innovative drug discovery in this field. Providing a comprehensive overview of current antidepressants and antipsychotic drugs, we also outline potential novel molecular targets for treating depression and schizophrenia. We also critically evaluate multiple translational challenges and summarize various open questions, in order to foster further integrative cross-discipline research into antidepressant and antipsychotic drug development.
1. Introduction
Neuropsychiatric disorders, especially schizophrenia and depression, are a major cause of human disability and a common risk factor of mortality [1]. Conventional antidepressant and antipsychotic pharmacotherapies are widely used to treat these two highly prevalent and severely debilitating disorders [2]. However, despite the growing drug intake and availability globally, such pharmacotherapies are often inefficient clinically, causing multiple effects and serious patient compliance problems. With the rise of clinical prevalence worldwide, depression and schizophrenia, especially their treatment-resistant forms [3,4], are becoming an urgent unmet biomedical problem, necessitating novel drug targets and broader, translationally-based pharmacotherapy.
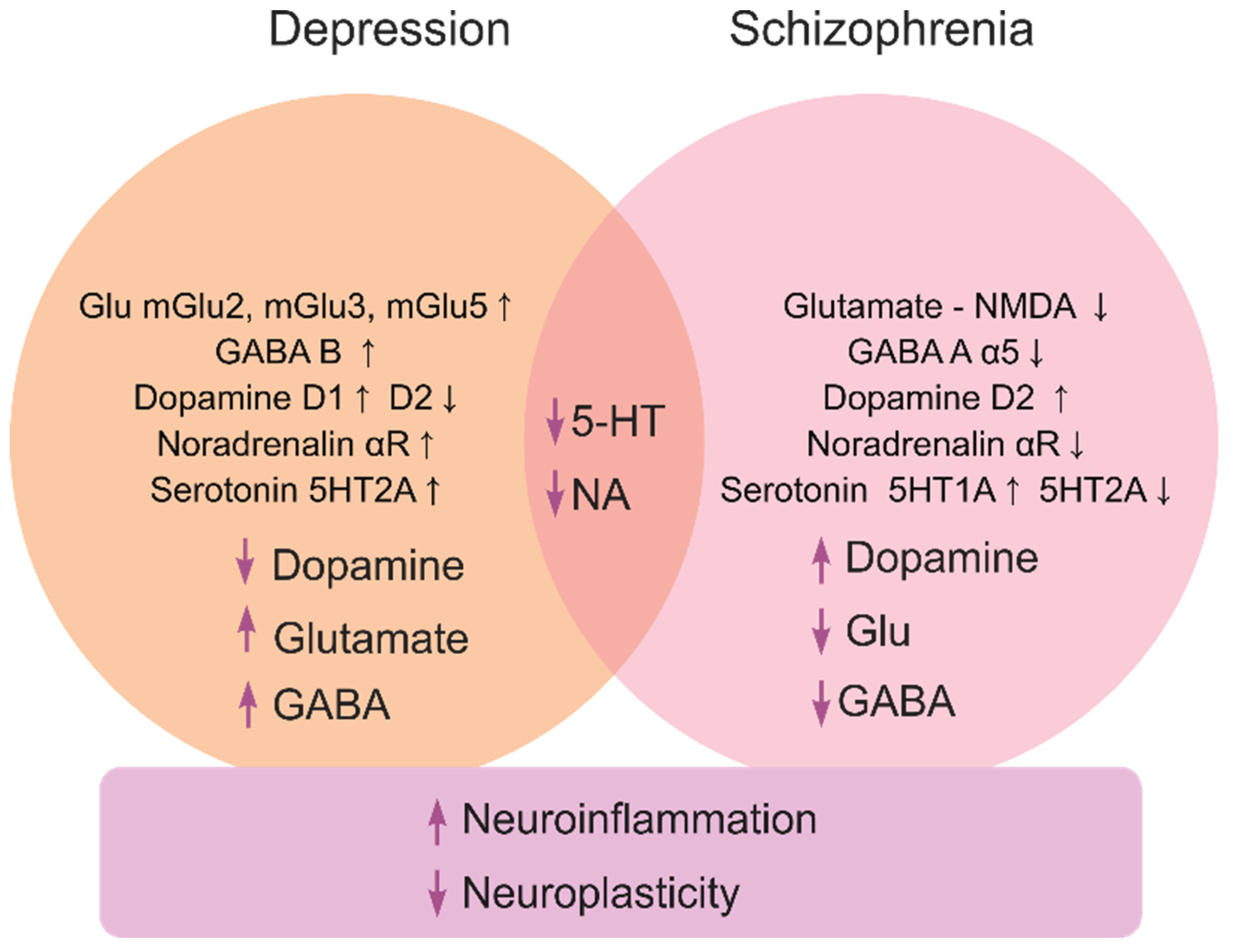
Depression, a highly prevalent mental illness that affects ~5% of the global population, is characterized by low mood, anhedonia, fatigue, attention deficits, suicidal thoughts, motor retardation and neuroendocrine deficits [5,6]. Caused by both genetic and environmental factors [7,8], it often represents a recurrent pathology [9,10,11] with overt monoaminergic, glutamatergic and gamma aminobutyric acid (GABA)-ergic deficits (Figure 1) [2], and multiple genetic risk factors, such as polymorphisms in the dopamine transporter (DAT) [12] and serotonin transporter (SERT) genes [13].
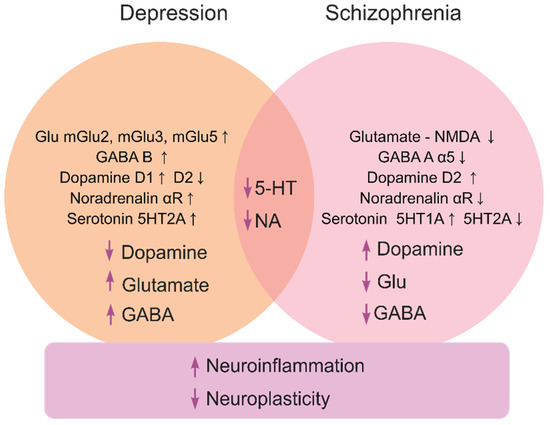
Figure 1.
A brief summary of major molecular mechanisms (at both the receptor and neurotransmitter levels) underlying depression and schizophrenia pathogenesis, and their related processes.
Schizophrenia (psychosis) is a severe psychiatric disorder that affects ~1% of the global population [14]. It typically presents as ‘positive’ (delirium and hallucinations), ‘negative’ (anhedonia, abulia and alogia), cognitive (impaired learning and planning skills) and motor (e.g., dyskinesia, catatonia and hypokinesia) symptoms [15,16]. The pathogenesis of schizophrenia involves multiple neurochemical deficits, especially within the glutamate-, GABA- and monoaminergic signaling systems [2,17,18] (Figure 1). Patients with schizophrenia often have increased levels of dopamine [19] with reduced glutamatergic N-methyl-D-aspartate (NMDA) receptor and (albeit not always) GABA-ergic activity [20]. Risks of psychosis correlate with higher striatal dopamine D2 receptor occupancy [21], further linking dopamine dysregulation and psychosis [22]. While glutamatergic deficits may provoke negative and cognitive symptoms of schizophrenia [23], the disorder is likely linked to disrupted ontogenesis of the glutamatergic and GABAergic neurons [24], and aberrant dorsolateral prefrontal cortex glutamatergic circuitry [25].
2. Pharmacotherapy of Depression
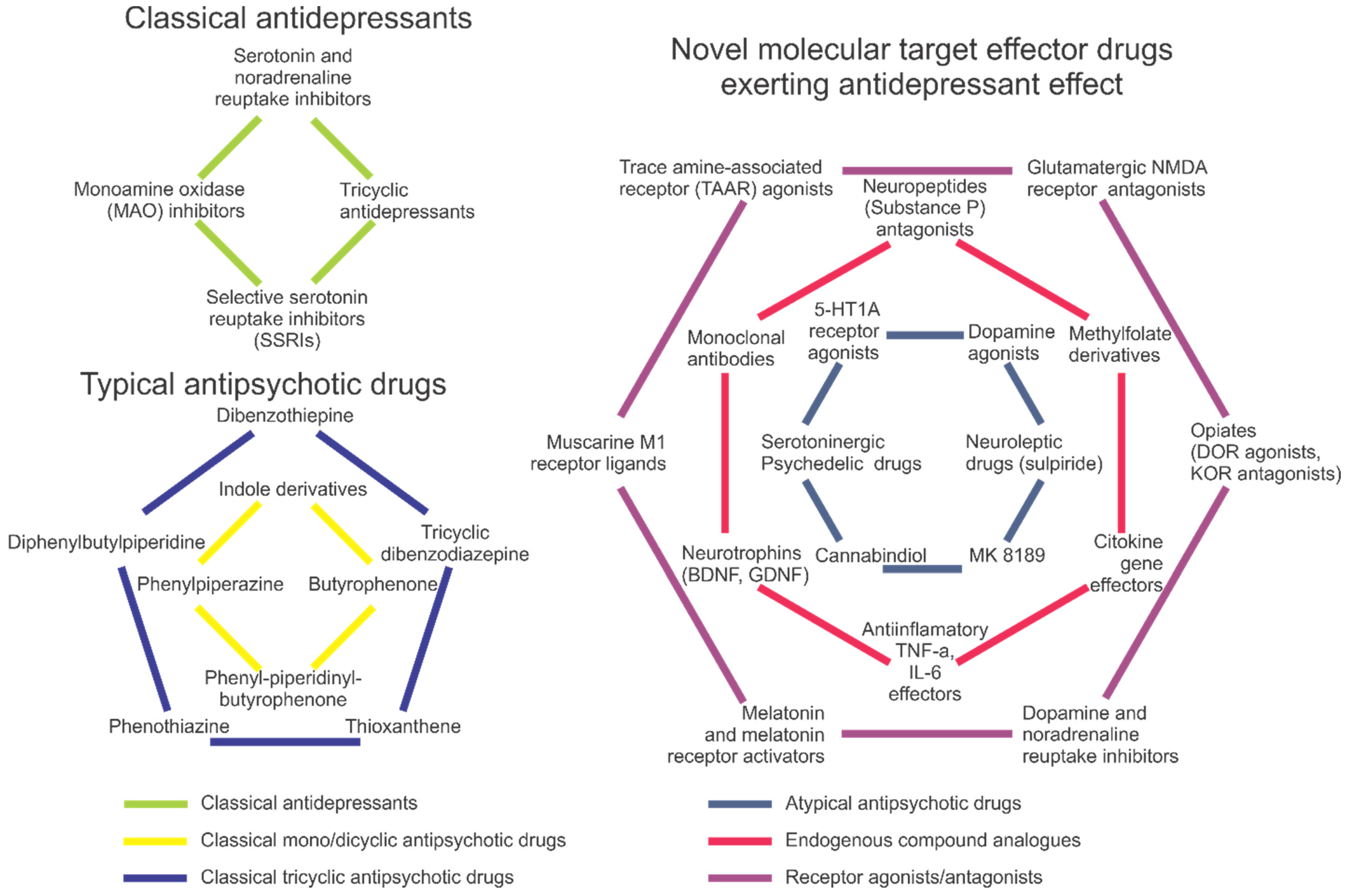
The most commonly prescribed conventional antidepressants include selective serotonin reuptake inhibitors (SSRIs), tricyclic antidepressants and monoamine oxidase (MAO) inhibitors [26,27,28] (Figure 2). They act via several different mechanisms, modulating the uptake, reuptake, synthesis and/or metabolism of neurotransmitters [26], as well as the activity of neuronal receptors and their expression (e.g., stimulating postsynaptic serotonin 5-HT1A, postsynaptic 5-HT1B, 5-HT2B and 5-HT4 receptors, or inhibiting presynaptic 5-HT1A, 5-HT1B, 5-HT2A, 5-HT3 and 5-HT7 receptors [29]); also see [2] for a recent review.
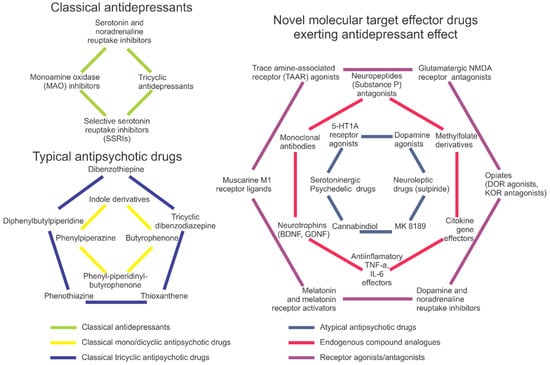
Figure 2.
A brief summary of antidepressant and antipsychotic drugs, including conventional (typical) antidepressants and antipsychotics (left panel) and various novel atypical and newest drugs with antidepressant and antipsychotic properties (right panel).
Glutamate is the main excitatory neurotransmitter in the brain. Glutamatergic neurons, distributed widely throughout the brain, express ionotropic N-methyl-D-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainate receptors, and metabotropic G-protein coupled (mGlu) receptors [2]. In the pathogenesis of schizophrenia, NMDA receptors are often downregulated, causing improper glutamate signaling. To reverse impaired functioning of the NMDA receptors and increase the level of glutamate in the synaptic cleft, these neurons likely initiate compensatory events. For instance, while excitatory amino acid transporters (responsible for the reuptake of the glutamate from the synaptic cleft) are downregulated in schizophrenic patients, they also show upregulated glutaminase that converts glutamine to glutamate, in the thalamus and prefrontal cortex [30]. While NMDA antagonists exert antidepressant effects, the glutamatergic, GABAergic and dopaminergic neuronal connectivity overlap [31], hence supporting the clinical link between schizophrenia and depression (Figure 1).
Based on their structure, profile and specificity of ligand binding, metabotropic glutamate receptors are classified into three main groups. Group 1 encompasses mGluR1 and mGluR5, representing Gq-associated receptors that activate protein kinase C. Group 1 receptor antagonists prevent glutamate from release to the synaptic cleft, thus indirectly reducing its corticolimbic levels, particularly in the amygdala [32]. Thus, the group 1 antagonists exert their antidepressant effects, similar to those of NMDA antagonists. Moreover, mGluR5 antagonists are widely used in animal models of acute and chronic stress. Group 2 includes mGluR2 and mGluR3 Gi-coupled receptors. Their activation prevents glutamate from the release to the synaptic cleft, and agonists promote depressive episodes [33], likely due to action at the projections to the dorsal raphe nucleus serotoninergic neurons. In contrast, antagonists of mGlu2 and mGlu3 receptors show antidepressant effects. Finally, group 3 includes mGluR4, mGluR6, mGluR7 and mGluR8 Gi-coupled receptors that prevent glutamate release to the synaptic cleft, and whose agonists demonstrate antidepressant effects in animal models [34].
However, no current antidepressants directly target the glutamatergic system except lamotrigine, a phenyltriazine that inhibits glutamate release [35]. Thus, the glutamatergic system can represent a potentially promising novel target for the development of antidepressant agents. For instance, since reduced signaling of glutamatergic neurons may serve as a defensive mechanism to mitigate glutamate toxicity, novel pharmaceuticals that lower glutamate transmission may stabilize plastic changes in the nervous system [36]. Reflecting an important CNS role of glutamate, antidepressants often lower plasma levels of glutamate (that are commonly elevated in depressed patients) [37,38,39,40,41].
Paralleling clinical data [42], animal models of depression also present glutamatergic deficits [31,43] corrected by some antidepressant treatments [44,45]. Disrupted glutamatergic signaling [46,47] is further accompanied by aberrant brain-derived neurotrophic factor (BDNF) and transcription factor cyclic AMP response-binding protein (CREB) signaling [48], with excitatory neurotransmission at ionotropic (AMPA, NMDA) glutamate receptors [49,50]. The glutamatergic system also plays a role in neuroplasticity and neurogenesis via (AMPA)/kainate (KA) receptors and mGluR5, critical for neuronal survival [51,52]. As mounting evidence links depression to aberrant glutamate receptor functioning, glutamatergic drugs (e.g., ketamine and other NMDA receptor antagonists) may be promising as potential multi-target antidepressants [53].
Moreover, NMDA receptor antagonists show consistent antidepressant effects in rodent models [54]. For example, ketamine reduces depression-like states in both animal [55] and clinical studies [56,57,58] while also lowering neuroinflammation, microglia activation and cytokine release in the hippocampus in rodent stress models relevant to depression [59]. Likewise, ketamine lowers lipopolysaccharide (LPS)-induced proinflammatory cytokines interleukin (IL) IL-1β and tumor necrosis factor (TNF)-α in microglia [60]. While anti-inflammatory effects of ketamine are reduced by a colony stimulating factor 1 receptor (CSF1R) antagonist PLX3397, its antidepressant action is modulated by transforming growth factor TGF-β1-dependent mechanisms [61]. Ketamine can also regulate inflammation via toll-like receptors and inhibition of extracellular signal-regulated kinases ERK1/2 [62,63], thus likely modulating affective pathogenesis via neuroimmune mechanisms and circuits (Figure 1). Another mechanism of antidepressant effects of ketamine is the modulation of receptor-mediated effects, since ketamine administration increases signal transducer and activator of transcription 3 (STAT3) levels [64] and the expression of BDNF, synapsin I (SYN1) and postsynaptic density protein 95 (PSD95). Clinical data show that ketamine increases plasma BDNF levels [65] and can also exert antidepressant effects through the mammalian target of the rapamycin (mTOR) signaling system [66], hence impacting neuroplasticity, neuronal survival and synaptogenesis (but see [55]).
GABA is a key inhibitory neurotransmitter [67] acting via GABA-A, GABA-B and GABA-C (GABA a-rho) receptors [68]. GABA-A receptors are ligand-gated ion channels regulating the influx of Cl− ions into neurons. They are an incredibly heterogeneous class of pentameric receptors assembled from multiple subunits (6α, 3β, 3γ, 1δ, 1ε, 1θ, 3ρ) [69]. The hippocampus and cortex receive GABAergic inhibitory inputs that are significantly altered in schizophrenia and depression. Reduced signaling of α5 subunits of GABA-A receptors causes hippocampal hyperexcitation due to insufficient inhibition of glutamatergic neurons and disinhibition of glutamatergic pyramidal neurons, causing loss of synchronous cortical activity and impairments in subcortical dopamine production. Activation of these receptors, in turn, exerts a positive effect on dopaminergic signaling and behavioral aspects in schizophrenic patients [70]. Interestingly, altered expression of various GABA-related genes is observed both in schizophrenia and depression. The former shows predominantly under-expression of GABA-related genes that significantly vary (i.e., increase or decrease) with age [71]. In contrast, the latter is mainly associated with overexpression of GABA-related genes [72], likely with deficient BDNF signaling [73]. Taken together, these findings suggest GABA-A receptors as a promising target for complex multimodal antidepressant therapy.
Mounting evidence suggests inflammation, especially neuroinflammation, as a common risk factor for developing depression. Indeed, depressed patients display higher levels of proinflammatory cytokines [74,75,76], especially TNF-α and IL-6 [77,78]. Neuroinflammation evokes depression-like behavior in rodent models, which is reduced by antidepressants [79,80]. For example, mice after chronic stress develop infiltration of microglia, and increased indoleamine-2,3-dioxygenase (a member of the kynurenine pathway) in the raphe, and TNF-α in the prefrontal cortex [81]. In line with this, the monoclonal antibody infliximab, a TNF-α functional antagonist, lowers symptoms of depression in patients with signs of inflammation, but is ineffective in patients with resistant depression [82].
Moreover, antidepressants can alter the expression of various cytokine genes (e.g., IL-4, IL-6 and interferon gamma (IFN-γ) genes) [83,84,85], while some drugs (e.g., imipramine) downregulate microglia (typically activated in rodent hippocampus after stress) [86]. In rodent models of depression, these drugs may also reduce inflammation [87] and proinflammatory cytokines IFN-γ, IL-6 and TNF-α [88]. Since anti-inflammatory effects of SSRIs can play a crucial role in therapy [89], such multimodal effects of antidepressants in depression merit further scrutiny. However, other antidepressants may exert proinflammatory effects as well. For instance, an SSRI, citalopram, induces TNF-α in brain (corrected by a non-steroidal anti-inflammatory drug ibuprofen) [90], whereas a MAO inhibitor phenelzine triggers neuroinflammation through recruitment of NF-kB [91]. Thus, a more comprehensive and nuanced analysis of both anti- and pro-inflammatory effects of antidepressant drugs is warranted.
Pro-inflammatory cytokines can affect a wide range of neurotransmitter systems (neuropeptides, monoamines, GABA and glutamate) and neuroplasticity processes [92]. Neuroplasticity is a key factor in both affective and psychotic pathogenesis (Figure 1), and potent neurotrophins like BDNF have thereby been probed for their putative therapeutic properties [93,94,95]. The importance of neuroplasticity and BDNF is particularly critical in depression treatment [96,97,98]. For instance, stress may downregulate BDNF in the hippocampus [99], whereas BDNF levels are decreased by pro-inflammatory cytokines [100,101,102]. Glial-derived neurotrophic factor (GDNF) is another key regulator of neurogenesis, whose levels decline in depressed patients [103], but are corrected by antidepressants [104]. The neuropeptide substance P is an agonist for neurokinin-1 (NK-1) receptors, widely expressed in brain regions affected by neuroinflammation. Notably, an NK-1 antagonist orvepitant improves depressive symptoms in clinical trials [105].
Overall, depression is commonly accompanied by brain tissue damage, whereas antidepressant treatment tends to improve neuroplasticity (Figure 1). Moreover, depressed patients often suffer from insomnia, likely representing a comorbid state. Antidepressant effects are shown for melatonin, and the melatonin receptor inhibitor agomelatine is the only antidepressant that corrects the melatoninergic system, also acting as a serotonin 5-HT2C antagonist [106]. Melatonin agonists generally decrease pro-inflammatory processes and promote neurotransmission. Furthermore, opioids are also related to the melatoninergic system, showing striking parallels with the fact that, in animals, the opioid system modulators affect depressive symptomatology, as delta opioid receptor (DOR) agonists [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48]) and kappa opioid receptor (KOR) antagonists [54,55,56] exert antidepressant-like effects. Therefore, the link between the melatoninergic system, opioids, neuroinflammation and stress becomes more evident, especially since inflammatory processes can be a core neuropathogenetic factor here, and high concentrations of proinflammatory cytokines may thus diminish concentrations of monoamines and neurotrophins.
Finally, serotonergic psychedelic drugs, currently strictly regulated as hallucinogens in most countries, not only show potential in treatment of psychiatric conditions (e.g., psilocybin in depression [107], also see [108,109]), but also exert immune-modulating effects in vivo as well. Some psychedelic drugs (e.g., psilocybin) have been used to manage treatment-resistant depression. For instance, psilocybin at a single dose reduces depression scores more than a much lower dose given chronically for three weeks [110]. Pramipexole (and, possibly other dopamine agonists) may be useful in treating depression as well, since nearly 80% of treatment-resistant patients show a clinical response to this agent [111]. Similarly, a nutritional adjunctive L-methylfolate (the biologically active form of folic acid, vitamin B9) has also been used [112], increasing clinical responses when co-applied with SSRIs in treatment-resistant depressed patients [113].
3. Approaches to Antipsychotic Therapy
As our understanding of schizophrenia and its molecular biomarkers is rapidly growing [114], dopaminergic deficits are strongly implicated in psychotic pathogenesis, especially in its motor, motivation and volition aspects (Figure 1). In general, schizophrenia is presently treated with neuroleptics and benzodiazepines [115,116,117,118,119] (inhibiting dopamine receptors and locomotion), without involving non-dopaminergic drugs as a primary therapy (Figure 2). From the early beginning, dopamine D2 receptors have been targeted in schizophrenia [120], showing higher density in post-mortem brain samples [121] and increased occupancy in patients with higher risks of psychosis [21,122,123]. Additionally, the D2 receptor-adenosine A2A receptor heterodimers seen in basal ganglia, represent a potential target for novel treatment of schizophrenia [121]. Interestingly, cognitive impairments in schizophrenia are associated with hypofunction of the prefrontal cortex, and transgenic mice overexpressing D2 receptors in the striatum show poorer motivation and cognition (e.g., impaired conditioned associative learning) [124], whereas such aberrant phenotypes are rescued by the D2 receptor gene downregulation [125].
As D2 receptors act via both canonical (G-protein-) and non-canonical (beta-arrestin2 βarr2-dependent) pathways, blocking the β-arrestin signaling may evoke antipsychotic effects [126]. The D2/β-arrestin-biased ligands (e.g., UNC9994) are effective in preclinical studies, having an antagonistic influence on D2-βarr2 in prefrontal cortex GABAergic fast-spiking interneurons, yet antagonizing D2-βarr2 in striatal D2 medium spiny neurons, with a dual action likely to prevent hyperdopaminergia [127]. Such ‘dual’ activity is not limited to D2-, but can involve other (e.g., D3 and A2A) receptors as well. Accordingly, additional mechanisms need to be considered for CNS drug development, as they may affect receptors indirectly (e.g., via endocytosis, due to the fact that D2 agonism can induce endocytosis and mediate ligand-based signaling) [128]. Thus, using the β-arrestin-based antagonism with G protein-dependent signaling may hypothetically help reduce positive psychotic symptoms and/or mitigate antipsychotic drugs’ side effects [129].
While NMDA receptors and aberrant glutamate neurotransmission are strongly implicated in schizophrenia [130], some of its deficits may be caused by epigenetic modifications as well. For instance, RELN and GAD1 genes, as well as NR3B promotors, are epigenetically modified in schizophrenia [131,132,133], whereas the gene responsible for epigenetic genome modifications (DNMT1) is over-expressed in brains of schizophrenic patients [134]. Furthermore, NMDA receptors are downregulated in depressed patients [135], whose positive, cognitive and negative symptoms of schizophrenia are mimicked in healthy volunteers by NMDA antagonists (e.g., phencyclidine) [136], cognitive-impairing effects of which parallel those seen in schizophrenia clinically [137].
Interestingly, NMDA antagonists may decrease the GABA-ergic inhibition and thus lead to the release of glutamate and acetylcholine, which in turn induces schizophrenic symptomology [138]. Moreover, modulating the glutamatergic system by the glycine modulatory site (GMS) of the NMDA receptor may help reduce psychotic and cognitive symptoms of schizophrenia, especially by indirect modulation of GMS. For instance, indirect enhancement of synaptic D-serine via the modulation of D-amino acid oxidase consequently normalizes NMDA receptor hypofunction and reduces cognitive impairments [139]. Likewise, an FDA-approved antipsychotic lumateperone is an antagonist for 5-HT2A receptors that also modulates dopamine and glutamate receptors [140,141].
Another promising target group for the treatment of schizophrenia is a family of trace amine-associated receptors (TAARs). For example, TAAR1 agonists modulate presynaptic pathways and regulate dopamine- and glutamatergic neurotransmission in schizophrenia, also reducing negative symptoms and improving cognitive functions in rodent and primate models of this disorder [142]. Specifically, TAAR1 agonists inhibit the dopaminergic pathways in midbrain, enhance glutamatergic circuits in the prefrontal cortex, and also regulate central serotonergic system [142]. Notably, TAAR1 agonists not only treat positive symptoms of schizophrenia, but also ease its negative symptoms and cognitive impairments [143]. For instance, SEP-363856 (a serotonin 5-HT1A receptor and TAAR1 modulator) shows promising results decreasing schizophrenic symptoms clinically [144].
The interplay between the monoaminergic and the cholinergic systems in schizophrenia is also observed, since schizophrenic patients show a loss of 75% of muscarinic M1 receptors [145]. Drugs binding to M1 receptors improve cognitive functions in rodents, and some of them show promise in clinical practice (e.g., KarXT, acting via muscarinic receptors, reduces cognitive and positive symptoms) [146]. Furthermore, serotonergic 5-HT2A hyperactivity [147] caused by stress, especially in the anterior cingulate cortex and dorsolateral frontal lobe, leads to synaptic atrophy and loss of the gray matter. A novel atypical antipsychotic, pimavanserin, is an agonist at 5-HT2A receptors that reduces psychotic symptoms, especially in Alzheimer patients [148]. Likewise, pharmacogenetic factors also contribute to the pathogenesis and development of personalized medicines for schizophrenia. For example, since inhibitory GABA interneurons contribute to pathogenesis of schizophrenia [149], the glutamate decarboxylase (GAD) and the GABA membrane transporter-1 (GAT) genes are downregulated in schizophrenic patients [150].
As with depression, neuroimmune mechanisms play a key role in pathogenesis of psychoses. For example, microglia promote the degradation of gray matter in schizophrenic patients and reduce neuroprotection by BDNF [151]. In turn, activated microglia (via proinflammatory cytokines) induce neuronal apoptosis [152] and neuroinflammation [153], as, for example, is often seen in postmortem brain samples from schizophrenic patients [154]. Moreover, while LPS induces morphological changes and activates microglia and macrophages in the brain [155], immune-based therapeutics have been tested in clinical trials, targeting p38 MAP kinase (losmapimod) [156], COX2 (celecoxib), adjunctive to reboxetine [157] and TNF (infliximab) [82]. Likewise, while stress activates glucocorticoids and consequently reactivates microglia [158], schizophrenic patients display a hyper-functioning neuroendocrine hypothalamo-pituitary-adrenal (HPA) axis [159,160,161] those deficits may precede the first-episode psychosis [160,162,163]. Furthermore, calprotectin, a neuroinflammatory glial marker, is increased in schizophrenic patients [164]. Finally, some patients with schizophrenia display elevated levels of proline and the proline dehydrogenase (PRODH) gene over-expression [165], hence implicating abnormal proline metabolism in schizophrenia. In line with this, administration of proline to zebrafish (Danio rerio) triggers schizophrenia-like states in this aquatic model, whereas a neuroleptic sulpiride (but not haloperidol) protects from them [166].
Another interesting candidate novel antipsychotic drug is ulotaront, a mixed TAAR1 and 5-HT1A receptor agonist that is chronically efficient in patients with acute schizophrenia [145]. MK-8189 is a potent and highly selective inhibitor of PDE10A (an important regulator of striatal signaling that, when inhibited, can normalize dysfunctional activity) currently being developed as a novel therapeutic for schizophrenia [167]. Furthermore, cannabidiol (CBD) has been tested as an adjunct treatment to antipsychotics. For example, individuals with schizophrenia receiving CBD (1000 mg) for six weeks have fewer positive psychotic symptoms than placebo [168], thus implying some beneficial effects of CBD in patients with schizophrenia.
4. In Silico-Driven Search for Novel Therapeutic Agents
Modern drug development actively employs computer-aided drug design (CADD) methods in the search for novel therapeutic agents and drug targets. CADD-based approaches are traditionally divided into target- and ligand-based drug designs [169]. Target-based drug design (e.g., docking) utilizes 3D structures of drug targets related to the treatment of respective disorders. Ligand-based drug design, based on the knowledge of structures and experimental data on ligands tested in interactions with the drug targets, most commonly includes the similarity estimation and structure–activity relationships ((Q)SAR) models. Since both CADD methods require knowing molecular targets for their respective disorders, the discovery of novel drug targets is a necessary prerequisite for the search for new effective drugs, typically performed using bioinformatics and systems biology (e.g., OMICS) data [169,170].
Over the last decade, there has been a rapid increase in CADD-based studies of depression, including docking studies of ligands for serotonin reuptake [171,172], MAO A and MAO B [173,174], dual action on MAO-B/AChE [175], glycogen synthase kinase [176], sodium hNaV1.2 or hNaV1.7 channels [177], serotonin receptors (5HT1A, 5-HT2A, 5-HT2C and 5-HT4) [171,178,179,180,181], adenosine A1/A2A receptors [182], T-type calcium channels [183], tryptophan 2,3-dioxygenase [184] and sigma receptor [185]. Similarly, application of docking in psychoses involved ligands for serotonin 5HT2 and dopamine D2 receptors [186], α4β2 and α7 nicotinic acetylcholine receptors [187,188], phosphodiesterase 10A [189], MAO A and B [190], a syntaxin-binding protein (STXBP1) [191], NMDA type subunit 1 (GRIN1) [192], fatty acid binding protein 7 (FABP7) [193,194], metabotropic glutamate mGluR5 receptor [195], ionotropic GABA-A receptor [196], glycine transporter type 1 (GlyT1) [197] and kynurenine aminotransferase II (KATII) [198].
CADD strategy may also involve natural compounds and probing pharmacological effects of their extracts, combining a network pharmacology approach and docking. In general, network pharmacology utilizes the systems biology methods to analyze biological networks (e.g., metabolic or signaling pathways, protein–protein interactions) in order to infer drug actions and interactions with various targets [199]. Multiple recent studies have revealed drug targets of phytocomponents from extracts with antidepressant effects [193,194,200,201,202], and a similar approach has been used to search for drug targets related to the treatment of schizophrenia by known schizophrenia drugs [203]. Some studies also combine docking with (Q)SAR methods, e.g., identifying monoamine neurotransmitters reuptake inhibitors as antidepressants [204] or a selective positive allosteric modulation of α1-containing GABA-A receptors [196]. The use of only QSAR models, albeit less common than docking studies, has linked antidepressant effects to MAO A [205], serotonin 5-HT2A receptor [206] and norepinephrine/dopamine reuptake activity [207], and antipsychotic effects - to 5-HT6 [208], D2, 5-HT2A [209] and sigma-2 receptors [210].
There are freely available web services and applications that facilitate the search for possible ligand–target interactions based on the structural formula of compounds. These useful tools are based on similarity estimation (e.g., SwissTargetPrediction [211]), SAR models (e.g., PASS Online [212,213], Super-PRED [214]) and docking (e.g., [215], 1-Click Docking [216]); also see [217] for details. For example, the PASS Online database can predict not only the action on molecular targets, but also the associated pharmacological effects. Briefly, if there is a simultaneous prediction of molecular mechanisms of action of the compound and the corresponding pharmacological effect, the chance to corroborate this effect in the experiment increases significantly, since this confirms the action of the substance at different (molecular, cellular, tissue/organ, and the whole organism) levels of biological organization.
Such knowledge of mechanism–effect relationships, extracted from the literature, is implemented in the PharmaExpert software developed for interpreting the PASS prediction results and containing >15,000 such relationships [169]. The PASS and PASS Online (version 2022) databases predict antidepressant effects with the invariant accuracy of prediction (IAP, equivalent to an area-under-the-curve/AUC value and calculated by the leave-one-out cross-validation (LOO CV) procedure) of 0.897, yielding 90 related mechanisms of actions with the mean accuracy of prediction of ~0.977 (Supplementary Table S1). Evaluation by the PASS software of pharmacological potential of phytocomponents from St John’s wort (Hypericum perforatum) and chaff-flower (Achyranthes aspera), the two well-known medicinal plants with established antidepressant effect, has also been performed [218,219]. Computational analyses of St John’s wort extract activity assessed the predicted biological activity spectra for 93 phytocomponents, revealing several likely phytocomponents that may be responsible for its pharmacological (e.g., antidepressant) effects [210]. Studying eight phytocomponents from chaff-flower predicts their likely antidepressant profile, with estimated probabilities exceeding those of conventional antidepressants. Notably, such simultaneous prediction of both antidepressant effects and the putative mechanisms of action markedly facilitates CNS drug screening, as for the chaff-flower extract that was experimentally tested in animal models and did show antidepressant-like effects [211].
Antipsychotic profile is predicted with the accuracy of 0.910, and 69 related mechanisms of actions are predicted with mean accuracy of prediction 0.983 (Supplementary Table S2). Because the accuracy of prediction of pharmacological effects is less than that for molecular mechanisms of action, simultaneous prediction of the pharmacological effect and the associated mechanisms of action is important for experimental validation. The latest version of PASS Online (http://way2drug.com/all/, accessed on 1 April 2023) enables selecting pharmacological effects and appropriate mechanisms of action based on PharmaExpert data, as seen for key activities predicted by the PASS Online database related to antidepressant and antipsychotic activity (Table 1 and Table 2).

Table 1.
Selected active compounds and their invariant accuracy of prediction (IAP) for antidepressant profile and related key mechanisms of action, as predicted by the PASS Online 2022 database.

Table 2.
Selected active compounds and their invariant accuracy of prediction (IAP) for antipsychotic profile and related key mechanisms of action, as predicted by the PASS Online 2022 database.
Furthermore, predicting biological activity spectra for substances and the knowledge of mechanism–effect relationships for antidepressant and antipsychotic effects provide an opportunity to study not only individual drugs but also drug combinations and complex phytocomponents. This may help reveal the most promising candidates that act via distinct pathogenetic mechanisms, hence leading to synergistic therapeutic effects with, possibly, fewer side effects.
5. Conclusions
Complementing traditional targets for pharmacological treatment of depression and schizophrenia (Figure 1), novel putative drug targets continue to emerge, implicating a wide range of CNS mechanisms and molecular circuits (Figure 2). However, in addition to numerous questions that remain open in this field (Table 3), other challenges continue to factor in. For example, comorbidity states and poorly identified, often overlapping clinical and preclinical symptoms markedly complicate the development of new drugs and their practical use for the treatment of depression and schizophrenia. Likewise, as already mentioned, there seem to be several common, overlapping molecular targets for both antidepressants and antipsychotics (Figure 1). As such, it is logical to expect that novel CNS drugs can be developed that target both disorders simultaneously via those common ‘shared’ molecular targets (Table 3). For example, from a conceptual standpoint, it is plausible that novel ‘combined action’ antidepressant or antipsychotic drugs may be developed based on simultaneous targeting of more than one aberrant signaling system (e.g., GABA + serotonin, TAAR + serotonin, dopamine + glutamate). However, if they do this, potential risks and benefits of using such a pharmacotherapeutic strategy are not fully understood, warranting further pre-clinical (and, eventually, clinical) testing.

Table 3.
Selected potential open questions related to developing novel antidepressant and antipsychotic therapies.
Because neuroplasticity plays an increasingly recognized role in the pathogenesis of depression and schizophrenia (Figure 1), modulation of CNS remodeling may become a promising target for CNS drug discovery. Likewise, given a key role of neuroinflammation in depression, novel antidepressant drug candidates may emerge that can exert ‘combined’ (e.g., neurotropic + anti-neuroinflammatory, or antidepressant + antipsychotic) activity (Table 3), consistent with the idea of complex, polytarget neuropharmacotherapy for CNS pathogenesis. However, again, if they do exert such effects, their potential risks and benefits for CNS pharmacotherapy warrant further studies.
Moreover, neuroimmune factors may also determine drug resistance, as a separate but related trait, as well. For example, high levels of cytokines TNF-α and IL-6 correlate with resistance to SSRIs [226]. However, neuroinflammation can also be a side effect of CNS drugs, for instance, as some antidepressants may trigger neuroinflammation and activate microglia [227]. Pro-inflammatory cytokines, in turn, may modulate central glutamatergic and monoaminergic systems, neurotrophins, neurohormones, and cellular immune cascades [92,228,229]. Thus, the link between the immune and the nervous systems emerges as an important potential target for novel CNS drugs. Immunological bases of depression are actively investigated both clinically and in animal models [230], showing marked symptomatic similarity between species [231,232,233,234,235,236,237,238].
Likewise, the melanocortin system, stress resilience and sleep/wake patterns are also important for normal brain functioning, and their deficits can trigger both depression and psychoses. As oxidative stress triggers neuroinflammation, developing novel antioxidants may also be promising for treating psychiatric disorders. Mitochondrial deficits cause neuronal network damage and, hence, trigger affective and schizophrenic symptoms [239]. Apoptosis is also observed in some brain areas in schizophrenic patients [240], necessitating further studies of antipsychotic (and, possibly, antidepressant) potential of antiapoptotic drugs (Table 3).
Lentivirus and adeno-associated viruses are another strategy for probing antidepressant and antipsychotic mechanisms in the brain via targeted activation or inactivation of gene expression of specific drug receptors, transporters [227,228,229] or nanocarriers (e.g., clozapine targeting 5-HT1A and D2 receptors due to its low selectivity) [241]. Considering individual characteristics of patients is also critically important for the success of psychopharmacotherapy. For example, sex differences (Table 3) are widely discussed in the context of psychiatric diseases [242,243], and some antipsychotics and antidepressants show sex differences in clinical pharmacokinetics [226,244] as well as in animal models [245,246].
Further broadening the spectrum of potential molecular drug targets beyond obvious well-established neurochemical systems is becoming critically important as well. For instance, in addition to its well-established physiological role as a regulator of Ca++ metabolism and bone growth, vitamin D has emerged as a potent neurosteroid hormone (Table 3) whose deficiency and aberrant signaling via nuclear vitamin D receptors (VDRs) have been linked to both depression and psychoses clinically, as well as in animal models [247,248,249]. This raises the possibility that novel CNS drugs can possibly be developed based on targeting the CNS vitamin D/VDR signaling system.
Finally, further broadening the spectrum of model organisms beyond traditional (e.g., rodent) models is necessary for innovating antidepressant and antipsychotic drug discovery (Table 3). For example, mounting evidence shows that relatively novel ‘alternative’ model organisms like zebrafish can be used in neuroscience not only to generate genetic, pharmacological or other experimental models of human CNS disorders, but to screen for a wide spectrum of CNS drugs as well, including both antidepressants [5,6] and antipsychotics [166]. Characterized by robust face, predictive and construct validity, such models offer genetic tractability, high genetic and physiological homology and an unparalleled high-throughput drug screening capacity [5] that can collectively foster the search and development of novel antidepressant and antipsychotic drugs. Addressing this and other remaining problems and questions (Table 3) can be expected to advance innovative antidepressant and antipsychotic drug discovery and promote further personalizing pharmacotherapy for depression and schizophrenia.
Supplementary Materials
The supplementary materials are available online at https://www.mdpi.com/article/10.3390/ijms24119482/s1.
Author Contributions
Conceptualization, Y.M.K., K.B.Y., V.V.P. and A.V.K.; methodology, investigation, Y.M.K., A.A.L., V.V.P. and M.S.d.A.; data curation, Y.M.K.; writing—original draft preparation, Y.M.K., T.O.K. and M.S.d.A.; writing—review and editing, T.O.K., M.S.d.A., A.A.L., V.V.P., H.S.H., K.B.Y. and A.V.K.; visualization, H.S.H.; supervision, K.B.Y. and A.V.K.; project administration, K.B.Y. and A.V.K.; funding acquisition, K.B.Y., A.A.L., V.V.P. and A.V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Republic of Armenia State Committee of Science (N 10-14/23-I/YSMU) and the European Union-funded H2020 COBRAIN project (857600). The funders had no role in the design, analyses and interpretation of the submitted study, or decision to publish.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Computer-aided prediction of biological activity in this pharmacotherapeutic field (A.A.L. and V.V.P.) was overviewed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021–2030) (project 122030100170-5). The research partially used the facilities and equipment of the Resource Fund of Applied Genetics MIPT (support grant 075-15-2021-684). A.V.K. is supported by St. Petersburg State University 2023 budget assignment funds. T.O.K. is supported by Neurobiology program of Sirius University of Science and Technology 2023 research budget funds. The authors thank Hasmik Harutyunyan (COBRAIN Center, Yerevan State Medical University) for help with graphical illustrations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Depression, W. Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017; Volume 24. [Google Scholar]
- Wierońska, J.M.; Pilc, A. Depression and schizophrenia viewed from the perspective of amino acidergic neurotransmission: Antipodes of psychiatric disorders. Pharmacol. Ther. 2019, 193, 75–82. [Google Scholar] [CrossRef]
- Pandarakalam, J.P. Challenges of treatment-resistant depression. Psychiatr. Danub. 2018, 30, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Nucifora, F.C., Jr.; Woznica, E.; Lee, B.J.; Cascella, N.; Sawa, A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 2019, 131, 104257. [Google Scholar] [CrossRef]
- de Abreu, M.S.; Friend, A.J.; Demin, K.A.; Amstislavskaya, T.G.; Bao, W.; Kalueff, A.V. Zebrafish models: Do we have valid paradigms for depression? J. Pharmacol. Toxicol. Methods 2018, 94, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Demin, K.A.; Kolesnikova, T.O.; Khatsko, S.L.; Zhu, X.; Yuan, X.; Song, C.; Meshalkina, D.A.; Leonard, B.E.; Tian, L.; et al. Animal inflammation-based models of depression and their application to drug discovery. Expert Opin. Drug Discov. 2017, 12, 995–1009. [Google Scholar] [CrossRef]
- Venzala, E.; Garcia-Garcia, A.L.; Elizalde, N.; Tordera, R.M. Social vs. environmental stress models of depression from a behavioural and neurochemical approach. Eur. Neuropsychopharmacol. 2013, 23, 697–708. [Google Scholar] [CrossRef]
- Rutter, M. Commentary: Nature-nurture interplay in emotional disorders. J. Child Psychol. Psychiatry 2003, 44, 934–944. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol. Psychiatry 2004, 9, 326–357. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.N.; McIntyre, R.S. What are the implications of the STAR* D trial for primary care? A review and synthesis. Prim. Care Companion J. Clin. Psychiatry 2008, 10, 91. [Google Scholar] [CrossRef]
- Wong, M.-L.; Licinio, J. From monoamines to genomic targets: A paradigm shift for drug discovery in depression. Nat. Rev. Drug Discov. 2004, 3, 136–151. [Google Scholar] [CrossRef]
- Haeffel, G.J.; Getchell, M.; Koposov, R.A.; Yrigollen, C.M.; Deyoung, C.G.; Klinteberg, B.A.; Oreland, L.; Ruchkin, V.V.; Grigorenko, E.L. Association between polymorphisms in the dopamine transporter gene and depression: Evidence for a gene-environment interaction in a sample of juvenile detainees. Psychol. Sci. 2008, 19, 62–69. [Google Scholar] [CrossRef]
- Risch, N.; Herrell, R.; Lehner, T.; Liang, K.Y.; Eaves, L.; Hoh, J.; Griem, A.; Kovacs, M.; Ott, J.; Merikangas, K.R. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA 2009, 301, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Edition, F. Diagnostic and statistical manual of mental disorders. Am. Psychiatr. Assoc. 2013, 21, 591–643. [Google Scholar]
- Walther, S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015, 233, 293–298. [Google Scholar] [CrossRef]
- Gawel, K.; Banono, N.S.; Michalak, A.; Esguerra, C.V. A critical review of zebrafish schizophrenia models: Time for validation? Neurosci. Biobehav. Rev. 2019, 107, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T. Glutamate and schizophrenia: Beyond the dopamine hypothesis. Cell. Mol. Neurobiol. 2006, 26, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Margolis, R.L.; Reading, S.A.; Pletnikov, M.; Coyle, J.T. Neurobiology of schizophrenia. Neuron 2006, 52, 139–153. [Google Scholar] [CrossRef]
- Dean, B. Neurochemistry of schizophrenia: The contribution of neuroimaging postmortem pathology and neurochemistry in schizophrenia. Curr. Top. Med. Chem. 2012, 12, 2375–2392. [Google Scholar] [CrossRef]
- Salavati, B.; Rajji, T.K.; Price, R.; Sun, Y.; Graff-Guerrero, A.; Daskalakis, Z.J. Imaging-based neurochemistry in schizophrenia: A systematic review and implications for dysfunctional long-term potentiation. Schizophr. Bull. 2015, 41, 44–56. [Google Scholar] [CrossRef]
- Girgis, R.R.; Slifstein, M.; Brucato, G.; Kegeles, L.S.; Colibazzi, T.; Lieberman, J.A.; Abi-Dargham, A. Imaging synaptic dopamine availability in individuals at clinical high-risk for psychosis: A [(11)C]-(+)-PHNO PET with methylphenidate challenge study. Mol. Psychiatry 2021, 26, 2504–2513. [Google Scholar] [CrossRef]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: Version III--the final common pathway. Schizophr. Bull. 2009, 35, 549–562. [Google Scholar] [CrossRef]
- Howes, O.; McCutcheon, R.; Stone, J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015, 29, 97–115. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectr. 2018, 23, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.A.; Moghaddam, B. Cognitive dysfunction in schizophrenia: Convergence of γ-aminobutyric acid and glutamate alterations. Arch. Neurol. 2006, 63, 1372–1376. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Carmassi, C.; Mucci, F.; Marazziti, D. Depression, serotonin and tryptophan. Curr. Pharm. Des. 2016, 22, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, K.; Miyazaki, K.W.; Doya, K. The role of serotonin in the regulation of patience and impulsivity. Mol. Neurobiol. 2012, 45, 213–224. [Google Scholar] [CrossRef]
- Cowen, P.J. Serotonin and depression: Pathophysiological mechanism or marketing myth? Trends Pharmacol. Sci. 2008, 29, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Slifirski, G.; Krol, M.; Turlo, J. 5-HT receptors and the development of new antidepressants. Int. J. Mol. Sci. 2021, 22, 9015. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; MacDonald, M.L.; Elswick, D.E.; Sweet, R.A. The glutamate hypothesis of schizophrenia: Evidence from human brain tissue studies. Ann. N. Y. Acad. Sci. 2015, 1338, 38–57. [Google Scholar] [CrossRef] [PubMed]
- Trullas, R.; Skolnick, P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990, 185, 1–10. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Bauer, E.P.; Farb, C.R.; Schafe, G.E.; LeDoux, J.E. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J. Neurosci. 2002, 22, 5219–5229. [Google Scholar] [CrossRef]
- Schoepp, D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001, 299, 12–20. [Google Scholar]
- Khoodoruth, M.A.S.; Estudillo-Guerra, M.A.; Pacheco-Barrios, K.; Nyundo, A.; Chapa-Koloffon, G.; Ouanes, S. Glutamatergic System in Depression and Its Role in Neuromodulatory Techniques Optimization. Front. Psychiatry 2022, 13, 886918. [Google Scholar] [CrossRef]
- Verrotti, A.; Striano, P.; Iapadre, G.; Zagaroli, L.; Bonanni, P.; Coppola, G.; Elia, M.; Mecarelli, O.; Franzoni, E.; De Liso, P. The pharmacological management of Lennox-Gastaut syndrome and critical literature review. Seizure 2018, 63, 17–25. [Google Scholar] [CrossRef]
- Gorman, J.M.; Docherty, J.P. A hypothesized role for dendritic remodeling in the etiology of mood and anxiety disorders. J. Neuropsychiatry Clin. Neurosci. 2010, 22, 256–264. [Google Scholar] [CrossRef]
- Altamura, C.A.; Mauri, M.C.; Ferrara, A.; Moro, A.R.; D’Andrea, G.; Zamberlan, F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am. J. Psychiatry 1993, 150, 1731–1733. [Google Scholar]
- Altamura, C.; Maes, M.; Dai, J.; Meltzer, H.Y. Plasma concentrations of excitatory amino acids, serine, glycine, taurine and histidine in major depression. Eur. Neuropsychopharmacol. 1995, 5 (Suppl. S1), 71–75. [Google Scholar] [CrossRef]
- Mauri, M.C.; Ferrara, A.; Boscati, L.; Bravin, S.; Zamberlan, F.; Alecci, M.; Invernizzi, G. Plasma and platelet amino acid concentrations in patients affected by major depression and under fluvoxamine treatment. Neuropsychobiology 1998, 37, 124–129. [Google Scholar] [CrossRef]
- Mitani, H.; Shirayama, Y.; Yamada, T.; Maeda, K.; Ashby, C.R., Jr.; Kawahara, R. Correlation between plasma levels of glutamate, alanine and serine with severity of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1155–1158. [Google Scholar] [CrossRef]
- Kucukibrahimoglu, E.; Saygin, M.Z.; Caliskan, M.; Kaplan, O.K.; Unsal, C.; Goren, M.Z. The change in plasma GABA, glutamine and glutamate levels in fluoxetine- or S-citalopram-treated female patients with major depression. Eur. J. Clin. Pharmacol. 2009, 65, 571–577. [Google Scholar] [CrossRef]
- Racagni, G.; Popoli, M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin. Neurosci. 2008, 10, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Musazzi, L.; Racagni, G.; Popoli, M. Stress, glucocorticoids and glutamate release: Effects of antidepressant drugs. Neurochem. Int. 2011, 59, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Skolnick, P.; Layer, R.T.; Popik, P.; Nowak, G.; Paul, I.A.; Trullas, R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: Implications for the pharmacotherapy of depression. Pharmacopsychiatry 1996, 29, 23–26. [Google Scholar] [CrossRef]
- Nowak, G.; Trullas, R.; Layer, R.T.; Skolnick, P.; Paul, I.A. Adaptive changes in the N-methyl-D-aspartate receptor complex after chronic treatment with imipramine and 1-aminocyclopropanecarboxylic acid. J. Pharmacol. Exp. Ther. 1993, 265, 1380–1386. [Google Scholar]
- Li, C.T.; Yang, K.C.; Lin, W.C. Glutamatergic Dysfunction and Glutamatergic Compounds for Major Psychiatric Disorders: Evidence From Clinical Neuroimaging Studies. Front. Psychiatry 2018, 9, 767. [Google Scholar] [CrossRef]
- Ohgi, Y.; Futamura, T.; Hashimoto, K. Glutamate Signaling in Synaptogenesis and NMDA Receptors as Potential Therapeutic Targets for Psychiatric Disorders. Curr. Mol. Med. 2015, 15, 206–221. [Google Scholar] [CrossRef]
- Martin, J.L.; Finsterwald, C. Cooperation between BDNF and glutamate in the regulation of synaptic transmission and neuronal development. Commun. Integr. Biol. 2011, 4, 14–16. [Google Scholar] [CrossRef]
- Kornmeier, J.; Sosic-Vasic, Z. Parallels between spacing effects during behavioral and cellular learning. Front. Hum. Neurosci. 2012, 6, 203. [Google Scholar] [CrossRef]
- Javitt, D.C.; Schoepp, D.; Kalivas, P.W.; Volkow, N.D.; Zarate, C.; Merchant, K.; Bear, M.F.; Umbricht, D.; Hajos, M.; Potter, W.Z.; et al. Translating glutamate: From pathophysiology to treatment. Sci. Transl. Med. 2011, 3, 102mr2. [Google Scholar] [CrossRef]
- McDonald, J.W.; Johnston, M.V. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res. Rev. 1990, 15, 41–70. [Google Scholar] [CrossRef]
- Jansson, L.C.; Åkerman, K.E. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J. Neural Transm. 2014, 121, 819–836. [Google Scholar] [CrossRef]
- Peyrovian, B.; Rosenblat, J.D.; Pan, Z.; Iacobucci, M.; Brietzke, E.; McIntyre, R.S. The glycine site of NMDA receptors: A target for cognitive enhancement in psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Krystal, J.H.; Sanacora, G.; Duman, R.S. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol. Psychiatry 2013, 73, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Diazgranados, N.; Ibrahim, L.; Brutsche, N.E.; Newberg, A.; Kronstein, P.; Khalife, S.; Kammerer, W.A.; Quezado, Z.; Luckenbaugh, D.A.; Salvadore, G.; et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 2010, 67, 793–802. [Google Scholar] [CrossRef]
- Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Green, C.E.; Perez, A.M.; Iqbal, S.; Pillemer, S.; Foulkes, A.; Shah, A.; et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry 2013, 170, 1134–1142. [Google Scholar] [CrossRef]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 2013, 38, 1609–1616. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Y.; Chen, K.; Long, Z.; Zou, J. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biol. Pharm. Bull. 2017, 40, 1260–1267. [Google Scholar] [CrossRef]
- Shibakawa, Y.S.; Sasaki, Y.; Goshima, Y.; Echigo, N.; Kamiya, Y.; Kurahashi, K.; Yamada, Y.; Andoh, T. Effects of ketamine and propofol on inflammatory responses of primary glial cell cultures stimulated with lipopolysaccharide. Br. J. Anaesth. 2005, 95, 803–810. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, C.; Chang, L.; Sakamoto, A.; Suzuki, T.; Fujita, Y.; Qu, Y.; Wang, S.; Pu, Y.; Tan, Y.; et al. Essential role of microglial transforming growth factor-beta1 in antidepressant actions of (R)-ketamine and the novel antidepressant TGF-beta1. Transl. Psychiatry 2020, 10, 32. [Google Scholar] [CrossRef]
- Chang, Y.; Lee, J.J.; Hsieh, C.Y.; Hsiao, G.; Chou, D.S.; Sheu, J.R. Inhibitory effects of ketamine on lipopolysaccharide-induced microglial activation. Mediat. Inflamm. 2009, 2009, 705379. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.P.; Zhou, Y.; Wang, W.; Tang, J.; Wang, W.; Zhang, H.; Xu, L.X.; Li, Y.Q. Ketamine depresses toll-like receptor 3 signaling in spinal microglia in a rat model of neuropathic pain. Neurosignals 2011, 19, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.F.; Zhang, C.; Zhang, L.; Li, H.; Weinshilboum, R.M. Ketamine and Active Ketamine Metabolites Regulate STAT3 and the Type I Interferon Pathway in Human Microglia: Molecular Mechanisms Linked to the Antidepressant Effects of Ketamine. Front. Pharmacol. 2019, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Haile, C.N.; Murrough, J.W.; Iosifescu, D.V.; Chang, L.C.; Al Jurdi, R.K.; Foulkes, A.; Iqbal, S.; Mahoney, J.J., 3rd; De La Garza, R., 2nd; Charney, D.S.; et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int. J. Neuropsychopharmacol. 2014, 17, 331–336. [Google Scholar] [CrossRef]
- Cui, W.; Ning, Y.; Hong, W.; Wang, J.; Liu, Z.; Li, M.D. Crosstalk Between Inflammation and Glutamate System in Depression: Signaling Pathway and Molecular Biomarkers for Ketamine’s Antidepressant Effect. Mol. Neurobiol. 2019, 56, 3484–3500. [Google Scholar] [CrossRef]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef]
- Vargas, R. The GABAergic system: An overview of physiology, physiopathology and therapeutics. Int. J. Clin. Pharmacol. Pharmacother. 2018, 3, 142. [Google Scholar] [CrossRef]
- Rudolph, U.; Crestani, F.; Möhler, H. GABAA receptor subtypes: Dissecting their pharmacological functions. Trends Pharmacol. Sci. 2001, 22, 188–194. [Google Scholar] [CrossRef]
- Marques, T.R.; Ashok, A.H.; Angelescu, I.; Borgan, F.; Myers, J.; Lingford-Hughes, A.; Nutt, D.J.; Veronese, M.; Turkheimer, F.E.; Howes, O.D. GABA-A receptor differences in schizophrenia: A positron emission tomography study using [11C] Ro154513. Mol. Psychiatry 2021, 26, 2616–2625. [Google Scholar] [CrossRef]
- Hoftman, G.D.; Volk, D.W.; Bazmi, H.H.; Li, S.; Sampson, A.R.; Lewis, D.A. Altered cortical expression of GABA-related genes in schizophrenia: Illness progression vs developmental disturbance. Schizophr. Bull. 2015, 41, 180–191. [Google Scholar] [CrossRef]
- Guilloux, J.-P.; Douillard-Guilloux, G.; Kota, R.; Wang, X.; Gardier, A.; Martinowich, K.; Tseng, G.C.; Lewis, D.A.; Sibille, E. Molecular evidence for BDNF-and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 2012, 17, 1130–1142. [Google Scholar] [CrossRef]
- Oh, H.; Piantadosi, S.C.; Rocco, B.R.; Lewis, D.A.; Watkins, S.C.; Sibille, E. The Role of Dendritic Brain-Derived Neurotrophic Factor Transcripts on Altered Inhibitory Circuitry in Depression. Biol. Psychiatry 2019, 85, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Miller, B.J. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol. Psychiatry 2015, 78, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Engler, H.; Brendt, P.; Wischermann, J.; Wegner, A.; Rohling, R.; Schoemberg, T.; Meyer, U.; Gold, R.; Peters, J.; Benson, S.; et al. Selective increase of cerebrospinal fluid IL-6 during experimental systemic inflammation in humans: Association with depressive symptoms. Mol. Psychiatry 2017, 22, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Schwieler, L.; Larsson, M.K.; Skogh, E.; Kegel, M.E.; Orhan, F.; Abdelmoaty, S.; Finn, A.; Bhat, M.; Samuelsson, M.; Lundberg, K.; et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia--significance for activation of the kynurenine pathway. J. Psychiatry Neurosci. 2015, 40, 126–133. [Google Scholar] [CrossRef]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctot, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef]
- Enache, D.; Pariante, C.M.; Mondelli, V. Markers of central inflammation in major depressive disorder: A systematic review and meta-analysis of studies examining cerebrospinal fluid, positron emission tomography and post-mortem brain tissue. Brain Behav. Immun. 2019, 81, 24–40. [Google Scholar] [CrossRef]
- Kubera, M.; Obuchowicz, E.; Goehler, L.; Brzeszcz, J.; Maes, M. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 744–759. [Google Scholar] [CrossRef]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Couch, Y.; Anthony, D.C.; Dolgov, O.; Revischin, A.; Festoff, B.; Santos, A.I.; Steinbusch, H.W.; Strekalova, T. Microglial activation, increased TNF and SERT expression in the prefrontal cortex define stress-altered behaviour in mice susceptible to anhedonia. Brain Behav. Immun. 2013, 29, 136–146. [Google Scholar] [CrossRef]
- Raison, C.L.; Rutherford, R.E.; Woolwine, B.J.; Shuo, C.; Schettler, P.; Drake, D.F.; Haroon, E.; Miller, A.H. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry 2013, 70, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Fazzino, F.; Urbina, M.; Cedeno, N.; Lima, L. Fluoxetine treatment to rats modifies serotonin transporter and cAMP in lymphocytes, CD4+ and CD8+ subpopulations and interleukins 2 and 4. Int. Immunopharmacol. 2009, 9, 463–467. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Deak, T.; Owens, S.M.; Kohno, T.; Fleshner, M.; Watkins, L.R.; Maier, S.F. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J. Neurosci. 1998, 18, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, Y.; Liu, H.; Zhou, Z.; Zhai, Y.; Yang, J. Chronic unpredictable stress accelerates atherosclerosis through promoting inflammation in apolipoprotein E knockout mice. Thromb. Res. 2010, 126, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Iwata, M.; Ishida, H.; Kaneko, K.; Shirayama, Y. Learned helplessness activates hippocampal microglia in rats: A potential target for the antidepressant imipramine. Pharmacol. Biochem. Behav. 2016, 150–151, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.; Sheridan, J.F. Antidepressant imipramine diminishes stress-induced inflammation in the periphery and central nervous system and related anxiety- and depressive- like behaviors. Brain Behav. Immun. 2016, 57, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Alboni, S.; Benatti, C.; Montanari, C.; Tascedda, F.; Brunello, N. Chronic antidepressant treatments resulted in altered expression of genes involved in inflammation in the rat hypothalamus. Eur. J. Pharmacol. 2013, 721, 158–167. [Google Scholar] [CrossRef]
- O’Brien, S.M.; Scully, P.; Fitzgerald, P.; Scott, L.V.; Dinan, T.G. Plasma cytokine profiles in depressed patients who fail to respond to selective serotonin reuptake inhibitor therapy. J. Psychiatr. Res. 2007, 41, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Warner-Schmidt, J.L.; Vanover, K.E.; Chen, E.Y.; Marshall, J.J.; Greengard, P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc. Natl. Acad. Sci. USA 2011, 108, 9262–9267. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, H.; Bae, H. Phenelzine (monoamine oxidase inhibitor) increases production of nitric oxide and proinflammatory cytokines via the NF-kappaB pathway in lipopolysaccharide-activated microglia cells. Neurochem. Res. 2012, 37, 2117–2124. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: Neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Kavalali, E.T.; Monteggia, L.M. Targeting Homeostatic Synaptic Plasticity for Treatment of Mood Disorders. Neuron 2020, 106, 715–726. [Google Scholar] [CrossRef]
- Maya Vetencourt, J.F.; Sale, A.; Viegi, A.; Baroncelli, L.; De Pasquale, R.; O’Leary, O.F.; Castren, E.; Maffei, L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 2008, 320, 385–388. [Google Scholar] [CrossRef]
- Castren, E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry 2013, 70, 983–989. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef]
- Zhang, J.-C.; Wu, J.; Fujita, Y.; Yao, W.; Ren, Q.; Yang, C.; Li, S.-X.; Shirayama, Y.; Hashimoto, K. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int. J. Neuropsychopharmacol. 2015, 18, 1–12. [Google Scholar] [CrossRef]
- Lapchak, P.; Araujo, D.; Hefti, F. Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience 1993, 53, 297–301. [Google Scholar] [CrossRef]
- Guan, Z.; Fang, J. Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav. Immun. 2006, 20, 64–71. [Google Scholar] [CrossRef]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef]
- Mahmoudi Asl, A.; Mehdizadeh, M.; Kulisevsky, J.; Sabet, A.; Taghavi Azar Sharabiani, P.; Mehdizadeh, H.; Ashayeri, H.; Taghizadeh, G. Reliability, Validity, and Diagnostic Accuracy of Parkinson’s Disease-Cognitive Rating Scale in Iranian Patients with Idiopathic Parkinson’s Disease. Disabil. Rehabil. 2022, 44, 2091–2098. [Google Scholar] [CrossRef]
- Ratti, E.; Bettica, P.; Alexander, R.; Archer, G.; Carpenter, D.; Evoniuk, G.; Gomeni, R.; Lawson, E.; Lopez, M.; Millns, H.; et al. Full central neurokinin-1 receptor blockade is required for efficacy in depression: Evidence from orvepitant clinical studies. J. Psychopharmacol. 2013, 27, 424–434. [Google Scholar] [CrossRef]
- Tchekalarova, J.; Kortenska, L.; Ivanova, N.; Atanasova, M.; Marinov, P. Agomelatine treatment corrects impaired sleep-wake cycle and sleep architecture and increases MT 1 receptor as well as BDNF expression in the hippocampus during the subjective light phase of rats exposed to chronic constant light. Psychopharmacology 2020, 237, 503–518. [Google Scholar] [CrossRef]
- Li, N.-X.; Hu, Y.-R.; Chen, W.-N.; Zhang, B. Dose effect of psilocybin on primary and secondary depression: A preliminary systematic review and meta-analysis. J. Affect. Disord. 2022, 296, 26–34. [Google Scholar] [CrossRef]
- Gonda, X.; Dome, P.; Neill, J.C.; Tarazi, F.I. Novel antidepressant drugs: Beyond monoamine targets. CNS Spectr. 2021, 28, 6–15. [Google Scholar] [CrossRef]
- Demin, K.A.; Kupriyanova, O.V.; Shevyrin, V.A.; Derzhavina, K.A.; Krotova, N.A.; Ilyin, N.P.; Kolesnikova, T.O.; Galstyan, D.S.; Kositsyn, Y.M.; Khaybaev, A.-A.S. Acute behavioral and Neurochemical Effects of Novel N-Benzyl-2-Phenylethylamine Derivatives in Adult Zebrafish. ACS Chem. Neurosci. 2022, 13, 1902–1922. [Google Scholar] [CrossRef]
- Goodwin, G.M.; Aaronson, S.T.; Alvarez, O.; Arden, P.C.; Baker, A.; Bennett, J.C.; Bird, C.; Blom, R.E.; Brennan, C.; Brusch, D.; et al. Single-Dose Psilocybin for a Treatment-Resistant Episode of Major Depression. N. Engl. J. Med. 2022, 387, 1637–1648. [Google Scholar] [CrossRef]
- Fawcett, J.; Rush, A.J.; Vukelich, J.; Diaz, S.H.; Dunklee, L.; Romo, P.; Yarns, B.C.; Escalona, R. Clinical experience with high-dosage pramipexole in patients with treatment-resistant depressive episodes in unipolar and bipolar depression. Am. J. Psychiatry 2016, 173, 107–111. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Bottiglieri, T.; Roffman, J.; Cassiello, C.; Stahl, S.M.; Fava, M. Effect of adjunctive L-methylfolate 15 mg among inadequate responders to SSRIs in depressed patients who were stratified by biomarker levels and genotype: Results from a randomized clinical trial. J. Clin. Psychiatry 2014, 75, 5464. [Google Scholar] [CrossRef]
- Macaluso, M. L-Methylfolate in Antidepressant Non-responders: The Impact of Body Weight and Inflammation. Front. Psychiatry 2022, 13, 840116. [Google Scholar] [CrossRef]
- Lai, C.Y.; Scarr, E.; Udawela, M.; Everall, I.; Chen, W.J.; Dean, B. Biomarkers in schizophrenia: A focus on blood based diagnostics and theranostics. World J. Psychiatry 2016, 6, 102–117. [Google Scholar] [CrossRef]
- Dold, M.; Samara, M.T.; Li, C.; Tardy, M.; Leucht, S. Haloperidol versus first-generation antipsychotics for the treatment of schizophrenia and other psychotic disorders. Cochrane Database Syst. Rev. 2015, 1, CD009831. [Google Scholar] [CrossRef]
- Brasseur, R. Clinical trial with bromperidol in psychotic states. Acta Psychiatr. Belg. 1978, 78, 110–117. [Google Scholar]
- Nestoros, J.N.; Suranyl-Cadotte, B.E.; Spees, R.C.; Schwartz, G.; Vasavan Nair, N. Diazepam in high doses is effective in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 1982, 6, 513–516. [Google Scholar] [CrossRef]
- Currier, G.W.; Chou, J.C.-Y.; Feifel, D.; Bossie, C.A.; Turkoz, I.; Mahmoud, R.A.; Gharabawi, G.M. Acute treatment of psychotic agitation: A randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. J. Clin. Psychiatry 2004, 65, 387. [Google Scholar] [CrossRef]
- Kousgaard, S.J.; Licht, R.W.; Nielsen, R.E. Effects of intramuscular midazolam and lorazepam on acute agitation in non-Elderly Subjects—A systematic review. Pharmacopsychiatry 2017, 50, 129–135. [Google Scholar] [CrossRef]
- Madras, B.K. History of the discovery of the antipsychotic dopamine D2 receptor: A basis for the dopamine hypothesis of schizophrenia. J. Hist. Neurosci. 2013, 22, 62–78. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Rodriguez, D.; Romero-Fernandez, W.; Kapla, J.; Jaiteh, M.; Ranganathan, A.; Lazarova, T.; Fuxe, K.; Carlsson, J. Mapping the Interface of a GPCR Dimer: A Structural Model of the A(2A) Adenosine and D(2) Dopamine Receptor Heteromer. Front. Pharmacol. 2018, 9, 829. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Stahl, S.M. The dopamine hypothesis of schizophrenia: A review. Schizophr. Bull. 1976, 2, 19–76. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology 1988, 1, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bach, M.-E.; Simpson, E.H.; Kahn, L.; Marshall, J.J.; Kandel, E.R.; Kellendonk, C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc. Natl. Acad. Sci. USA 2008, 105, 16027–16032. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.; Simpson, E.; Kellendonk, C.; Herzberg, W.; Lipatova, O.; Fairhurst, S.; Kandel, E.R.; Malapani, C.; Balsam, P.D. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J. Neurosci. 2007, 27, 7731–7739. [Google Scholar] [CrossRef] [PubMed]
- Masri, B.; Salahpour, A.; Didriksen, M.; Ghisi, V.; Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Antagonism of dopamine D2 receptor/beta-arrestin 2 interaction is a common property of clinically effective antipsychotics. Proc. Natl. Acad. Sci. USA 2008, 105, 13656–13661. [Google Scholar] [CrossRef]
- Urs, N.M.; Peterson, S.M.; Caron, M.G. New Concepts in Dopamine D(2) Receptor Biased Signaling and Implications for Schizophrenia Therapy. Biol. Psychiatry 2017, 81, 78–85. [Google Scholar] [CrossRef]
- De Vries, L.; Finana, F.; Cathala, C.; Ronsin, B.; Cussac, D. Innovative Bioluminescence Resonance Energy Transfer Assay Reveals Differential Agonist-Induced D2 Receptor Intracellular Trafficking and Arrestin-3 Recruitment. Mol. Pharmacol. 2019, 96, 308–319. [Google Scholar] [CrossRef]
- Simpson, E.H.; Gallo, E.F.; Balsam, P.D.; Javitch, J.A.; Kellendonk, C. How changes in dopamine D2 receptor levels alter striatal circuit function and motivation. Mol. Psychiatry 2022, 27, 436–444. [Google Scholar] [CrossRef]
- Mohn, A.R.; Gainetdinov, R.R.; Caron, M.G.; Koller, B.H. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 1999, 98, 427–436. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Cheng, K.H.; Russo, A.; Smith, C.L.; Faraone, S.V.; Wilcox, M.; Shafa, R.; Glatt, S.J.; Nguyen, G.; Ponte, J.F. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: A preliminary report. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 134, 60–66. [Google Scholar] [CrossRef]
- Ruzicka, W.; Zhubi, A.; Veldic, M.; Grayson, D.; Costa, E.; Guidotti, A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol. Psychiatry 2007, 12, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Mill, J.; Tang, T.; Kaminsky, Z.; Khare, T.; Yazdanpanah, S.; Bouchard, L.; Jia, P.; Assadzadeh, A.; Flanagan, J.; Schumacher, A. Epigenomic profiling reveals DNA-methylation changes associated with major psychosis. Am. J. Hum. Genet. 2008, 82, 696–711. [Google Scholar] [CrossRef]
- Veldic, M.; Guidotti, A.; Maloku, E.; Davis, J.M.; Costa, E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc. Natl. Acad. Sci. USA 2005, 102, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Gibbons, A.S.; Boer, S.; Uezato, A.; Meador-Woodruff, J.; Scarr, E.; McCullumsmith, R.E. Changes in cortical N-methyl-D-aspartate receptors and post-synaptic density protein 95 in schizophrenia, mood disorders and suicide. Aust. N. Z. J. Psychiatry 2016, 50, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T. Targeting serotonin 5-HT2A receptors to better treat schizophrenia: Rationale and current approaches. CNS Drugs 2020, 34, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Zhou, Y. NMDAR Hypofunction Animal Models of Schizophrenia. Front. Mol. Neurosci. 2019, 12, 185. [Google Scholar] [CrossRef]
- Farber, N.B. The NMDA receptor hypofunction model of psychosis. Ann. N. Y. Acad. Sci. 2003, 1003, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.C.; Luo, D.Z.; Gau, S.S.; Chang, C.Y.; Lai, W.S. Directly and Indirectly Targeting the Glycine Modulatory Site to Modulate NMDA Receptor Function to Address Unmet Medical Needs of Patients With Schizophrenia. Front. Psychiatry 2021, 12, 742058. [Google Scholar] [CrossRef]
- Greenwood, J.; Acharya, R.B.; Marcellus, V.; Rey, J.A. Lumateperone: A Novel Antipsychotic for Schizophrenia. Ann. Pharmacother. 2021, 55, 98–104. [Google Scholar] [CrossRef]
- Davis, R.E.; Correll, C.U. ITI-007 in the treatment of schizophrenia: From novel pharmacology to clinical outcomes. Expert Rev. Neurother. 2016, 16, 601–614. [Google Scholar] [CrossRef]
- Dedic, N.; Dworak, H.; Zeni, C.; Rutigliano, G.; Howes, O.D. Therapeutic Potential of TAAR1 Agonists in Schizophrenia: Evidence from Preclinical Models and Clinical Studies. Int. J. Mol. Sci. 2021, 22, 13185. [Google Scholar] [CrossRef] [PubMed]
- Halff, E.F.; Rutigliano, G.; Garcia-Hidalgo, A.; Howes, O.D. Trace amine-associated receptor 1 (TAAR1) agonism as a new treatment strategy for schizophrenia and related disorders. Trends Neurosci. 2022, 46, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Koblan, K.S.; Kent, J.; Hopkins, S.C.; Krystal, J.H.; Cheng, H.; Goldman, R.; Loebel, A. A Non-D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N. Engl. J. Med. 2020, 382, 1497–1506. [Google Scholar] [CrossRef]
- Scarr, E.; Cowie, T.; Kanellakis, S.; Sundram, S.; Pantelis, C.; Dean, B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol. Psychiatry 2009, 14, 1017–1023. [Google Scholar] [CrossRef]
- Dean, B.; Scarr, E. Muscarinic M1 and M4 receptors: Hypothesis driven drug development for schizophrenia. Psychiatry Res. 2020, 288, 112989. [Google Scholar] [CrossRef]
- Eggers, A.E. A serotonin hypothesis of schizophrenia. Med. Hypotheses 2013, 80, 791–794. [Google Scholar] [CrossRef]
- Srinivasan, S.; Tampi, R.R.; Balaram, K.; Kapoor, A. Pimavanserin for the treatment of psychosis in Alzheimer’s disease: A literature review. World J. Psychiatry 2020, 10, 162–174. [Google Scholar] [CrossRef]
- Di Cristo, G. Development of cortical GABAergic circuits and its implications for neurodevelopmental disorders. Clin. Genet. 2007, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Volk, D.W.; Eggan, S.M.; Mirnics, K.; Pierri, J.N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J. Neurosci. 2003, 23, 6315–6326. [Google Scholar] [CrossRef]
- Racki, V.; Petric, D.; Kucic, N.; Grzeta, N.; Jurdana, K.; Roncevic-Grzeta, I. Cortical gray matter loss in schizophrenia: Could microglia be the culprit? Med. Hypotheses 2016, 88, 18–21. [Google Scholar] [CrossRef]
- Réus, G.Z.; Fries, G.R.; Stertz, L.; Badawy, M.; Passos, I.; Barichello, T.; Kapczinski, F.; Quevedo, J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 2015, 300, 141–154. [Google Scholar] [CrossRef]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.-G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; zu Schwabedissen, L.M. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm. 2011, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, E.; Crow, T.J.; Walker, M.A.; Black, G.; Chance, S.A. Reduced neuron density, enlarged minicolumn spacing and altered ageing effects in fusiform cortex in schizophrenia. Psychiatry Res. 2009, 166, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef]
- Inamdar, A.; Merlo-Pich, E.; Gee, M.; Makumi, C.; Mistry, P.; Robertson, J.; Steinberg, E.; Zamuner, S.; Learned, S.; Alexander, R.; et al. Evaluation of antidepressant properties of the p38 MAP kinase inhibitor losmapimod (GW856553) in Major Depressive Disorder: Results from two randomised, placebo-controlled, double-blind, multicentre studies using a Bayesian approach. J. Psychopharmacol. 2014, 28, 570–581. [Google Scholar] [CrossRef]
- Muller, N.; Schwarz, M.J.; Dehning, S.; Douhe, A.; Cerovecki, A.; Goldstein-Muller, B.; Spellmann, I.; Hetzel, G.; Maino, K.; Kleindienst, N.; et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol. Psychiatry 2006, 11, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; McCutcheon, R. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: A reconceptualization. Transl. Psychiatry 2017, 7, e1024. [Google Scholar] [CrossRef]
- Walder, D.J.; Walker, E.F.; Lewine, R.J. Cognitive functioning, cortisol release, and symptom severity in patients with schizophrenia. Biol. Psychiatry 2000, 48, 1121–1132. [Google Scholar] [CrossRef]
- Mondelli, V.; Dazzan, P.; Hepgul, N.; Di Forti, M.; Aas, M.; D’Albenzio, A.; Di Nicola, M.; Fisher, H.; Handley, R.; Marques, T.R. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: The role of stress and of antipsychotic treatment. Schizophr. Res. 2010, 116, 234–242. [Google Scholar] [CrossRef]
- Chiappelli, J.; Shi, Q.; Kodi, P.; Savransky, A.; Kochunov, P.; Rowland, L.M.; Nugent, K.L.; Hong, L.E. Disrupted glucocorticoid—Immune interactions during stress response in schizophrenia. Psychoneuroendocrinology 2016, 63, 86–93. [Google Scholar] [CrossRef]
- Ryan, M.C.; Sharifi, N.; Condren, R.; Thakore, J.H. Evidence of basal pituitary–adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology 2004, 29, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, V.; Pariante, C.M.; Navari, S.; Aas, M.; D’Albenzio, A.; Di Forti, M.; Handley, R.; Hepgul, N.; Marques, T.R.; Taylor, H. Higher cortisol levels are associated with smaller left hippocampal volume in first-episode psychosis. Schizophr. Res. 2010, 119, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Kandanearatchi, A.; Beasley, C.; Williams, B.; Khan, N.; Fagerhol, M.K.; Everall, I.P. Calprotectin in microglia from frontal cortex is up-regulated in schizophrenia: Evidence for an inflammatory process? Eur. J. Neurosci. 2006, 24, 3561–3566. [Google Scholar] [CrossRef] [PubMed]
- Bender, H.-U.; Almashanu, S.; Steel, G.; Hu, C.-A.; Lin, W.-W.; Willis, A.; Pulver, A.; Valle, D. Functional consequences of PRODH missense mutations. Am. J. Hum. Genet. 2005, 76, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; Vuaden, F.C.; Piato, A.L.; Bonan, C.D.; Wyse, A.T. Behavioral changes induced by long-term proline exposure are reversed by antipsychotics in zebrafish. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 36, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Layton, M.E.; Kern, J.C.; Hartingh, T.J.; Shipe, W.D.; Raheem, I.; Kandebo, M.; Hayes, R.P.; Huszar, S.; Eddins, D.; Ma, B. Discovery of MK-8189, a Highly Potent and Selective PDE10A Inhibitor for the Treatment of Schizophrenia. J. Med. Chem. 2023, 66, 1157–1171. [Google Scholar] [CrossRef]
- McGuire, P.; Robson, P.; Cubala, W.J.; Vasile, D.; Morrison, P.D.; Barron, R.; Taylor, A.; Wright, S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am. J. Psychiatry 2018, 175, 225–231. [Google Scholar] [CrossRef]
- Lagunin, A.A.; Goel, R.K.; Gawande, D.Y.; Pahwa, P.; Gloriozova, T.A.; Dmitriev, A.V.; Ivanov, S.M.; Rudik, A.V.; Konova, V.I.; Pogodin, P.V. Chemo-and bioinformatics resources for in silico drug discovery from medicinal plants beyond their traditional use: A critical review. Nat. Prod. Rep. 2014, 31, 1585–1611. [Google Scholar] [CrossRef]
- Van den Broeck, W.M. Drug Targets, Target Identification, Validation, and Screening. In The Practice of Medicinal Chemistry; Elsevier: Amsterdam, The Netherlands, 2015; pp. 45–70. [Google Scholar]
- Ayipo, Y.O.; Alananzeh, W.A.; Ahmad, I.; Patel, H.; Mordi, M.N. Structural modelling and in silico pharmacology of β-carboline alkaloids as potent 5-HT1A receptor antagonists and reuptake inhibitors. J. Biomol. Struct. Dyn. 2022, 26, 1–17. [Google Scholar] [CrossRef]
- Jaramillo, D.N.; Millán, D.; Guevara-Pulido, J. Design, synthesis and cytotoxic evaluation of a selective serotonin reuptake inhibitor (SSRI) by virtual screening. Eur. J. Pharm. Sci. 2023, 183, 106403. [Google Scholar] [CrossRef]
- Javid, N.; Jalil, S.; Munir, R.; Zia-ur-Rehman, M.; Sahar, A.; Arshad, S.; Iqbal, J. 2, 1-Benzothiazine–(quinolin/thiophen) yl hydrazone frameworks as new monoamine oxidase inhibitory agents; synthesis, in vitro and in silico investigation. RSC Adv. 2023, 13, 1701–1710. [Google Scholar] [CrossRef]
- Seong, S.H.; Kim, B.-R.; Cho, M.L.; Kim, T.-S.; Im, S.; Han, S.; Jeong, J.-W.; Jung, H.A.; Choi, J.S. Phytoestrogen Coumestrol Selectively Inhibits Monoamine Oxidase-A and Amyloid β Self-Aggregation. Nutrients 2022, 14, 3822. [Google Scholar] [CrossRef] [PubMed]
- El-Damasy, A.K.; Park, J.E.; Kim, H.J.; Lee, J.; Bang, E.-K.; Kim, H.; Keum, G. Identification of New N-methyl-piperazine Chalcones as Dual MAO-B/AChE Inhibitors. Pharmaceuticals 2023, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, D.; Mellado, G.; Espinoza, N.; Gárate, J.A.; Morales, C.; Castro-Alvarez, A.; Matos, M.J.; Mellado, M.; Mella, J. In silico approaches to develop new phenyl-pyrimidines as glycogen synthase kinase 3 (GSK-3) inhibitors with halogen-bonding capabilities: 3D-QSAR CoMFA/CoMSIA, molecular docking and molecular dynamics studies. J. Biomol. Struct. Dyn. 2023, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Yu, M.-C. Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy) phenyl] ethyl] isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process. Int. J. Mol. Sci. 2023, 24, 1805. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Ibrahim, M.A.; Rizwan-ul-Hasan, S.; Khaliq, S.; Gabr, G.A.; Khan, A.; Sidhom, P.A.; Tikmani, P.; Shawky, A.M.; Ahmad, S. Interactions of Apigenin and Safranal with the 5HT1A and 5HT2A Receptors and Behavioral Effects in Depression and Anxiety: A Molecular Docking, Lipid-Mediated Molecular Dynamics, and In Vivo Analysis. Molecules 2022, 27, 8658. [Google Scholar] [CrossRef]
- da Rocha, M.J.; Pires, C.S.; Presa, M.H.; Besckow, E.M.; Nunes, G.D.A.; Gomes, C.S.; Penteado, F.; Lenardão, E.J.; Bortolatto, C.F.; Brüning, C.A. Involvement of the serotonergic system in the antidepressant-like effect of 1-(phenylselanyl)-2-(p-tolyl) indolizine in mice. Psychopharmacology 2023, 240, 373–389. [Google Scholar] [CrossRef]
- Ayipo, Y.O.; Alananzeh, W.A.; Yahayaa, S.N.; Mordi, M.N. Molecular Modelling and Virtual Screening to Identify New Piperazine Derivatives as Potent Human 5-HT1A Antagonists and Reuptake Inhibitors. Comb. Chem. High Throughput Screen. 2022. [Google Scholar] [CrossRef]
- Jha, P.; Chaturvedi, S.; Bhat, R.; Jain, N.; Mishra, A.K. Insights of ligand binding in modeled h5-HT1A receptor: Homology modeling, docking, MM-GBSA, screening and molecular dynamics. J. Biomol. Struct. Dyn. 2022, 40, 11625–11637. [Google Scholar] [CrossRef]
- Camargo, A.; Bettio, L.E.; Rosa, P.B.; Rosa, J.M.; Altê, G.A.; Rodrigues, A.L.S. The antidepressant-like effect of guanosine involves the modulation of adenosine A1 and A2A receptors. Purinergic Signal. 2022. [Google Scholar] [CrossRef]
- Rangel-Galván, M.; Castro, M.E.; Perez-Aguilar, J.M.; Caballero, N.A.; Melendez, F.J. Conceptual DFT, QTAIM, and Molecular Docking Approaches to Characterize the T-Type Calcium Channel Blocker Anandamide. Front. Chem. 2022, 10, 920661. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Bano, S.; Badawy, A.A.-B. Inflammation and serotonin deficiency in major depressive disorder: Molecular docking of antidepressant and anti-inflammatory drugs to tryptophan and indoleamine 2, 3-dioxygenases. Biosci. Rep. 2022, 42, BSR20220426. [Google Scholar] [CrossRef] [PubMed]
- Elkholy, N.; Abdelwaly, A.; Mohamed, K.; Amata, E.; Lombino, J.; Cosentino, G.; Intagliata, S.; Helal, M.A. Discovery of 3-(2-aminoethyl)-thiazolidine-2, 4-diones as a novel chemotype of sigma-1 receptor ligands. Chem. Biol. Drug Des. 2022, 100, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Gawai, A.A.; Biyani, K.; Das, S.; Tapadiya, G.; Mokale, S.; Dhawale, S.A. Design, Synthesis, Molecular Docking, and Preliminary pharmacological screening of some new benzo [d] thiazol-2-ylamino containing chromen-2-one derivatives with atypical antipsychotic profile. Curr. Comput. Aided Drug Des. 2023, 19, 465–475. [Google Scholar]
- Batista, V.S.; Gonçalves, A.M.; Nascimento-Júnior, N.M. Pharmacophore Mapping Combined with dbCICA Reveal New Structural Features for the Development of Novel Ligands Targeting α4β2 and α7 Nicotinic Acetylcholine Receptors. Molecules 2022, 27, 8236. [Google Scholar] [CrossRef]
- Nag, S.; Miranda-Azpiazu, P.; Jia, Z.; Datta, P.; Arakawa, R.; Moein, M.M.; Yang, Z.; Tu, Y.; Lemoine, L.; Ågren9(6):, H. Development of 11C-Labeled ASEM Analogues for the Detection of Neuronal Nicotinic Acetylcholine Receptors (α7-nAChR). ACS Chem. Neurosci. 2022, 13, 352–362. [Google Scholar] [CrossRef]
- Sharma, B.; Bhattacherjee, D.; Zyryanov, G.V.; Purohit, R. An insight from computational approach to explore novel, high-affinity phosphodiesterase 10A inhibitors for neurological disorders. J. Biomol. Struct. Dyn. 2022, 11, 1–13. [Google Scholar] [CrossRef]
- Hitge, R.; Petzer, J.P.; Petzer, A. The inhibition of monoamine oxidase by 2H-1, 4-benzothiazin-3 (4H)-ones. Bioorganic Med. Chem. Lett. 2022, 77, 129038. [Google Scholar] [CrossRef]
- Alharbi, A.H. Bio-Computational Evaluation of Compounds of Bacopa Monnieri as a Potential Treatment for Schizophrenia. Molecules 2022, 27, 7050. [Google Scholar] [CrossRef]
- Bhardwaj, T.; Ahmad, I.; Somvanshi, P. Systematic analysis to identify novel disease indications and plausible potential chemical leads of glutamate ionotropic receptor NMDA type subunit 1, GRIN1. J. Mol. Recognit. 2023, 36, e2997. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; He, J.; Huang, D.; Xi, Y.; Xiao, T.; Ouyang, Q.; Zhang, S.; Wan, S.; Chen, X. Potential mechanisms underlying the therapeutic roles of sinisan formula in depression: Based on network pharmacology and molecular docking study. Front. Psychiatry 2022, 13, 1063489. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Taouil, A.; Awwa, M.; Clement, T.; Zhu, C.; Kim, J.; Rendina, D.; Jayanetti, K.; Maharaj, A.; Wang, L. SAR study on Novel truxillic acid monoester-Based inhibitors of fatty acid binding proteins as Next-Generation antinociceptive agents. Bioorganic Chem. 2022, 129, 106184. [Google Scholar] [CrossRef] [PubMed]
- Uba, A.I.; Chea, J.; Hoag, H.; Hryb, M.; Bui-Linh, C.; Wu, C. Binding of a positive allosteric modulator CDPPB to metabotropic glutamate receptor type 5 (mGluR5) probed by all-atom molecular dynamics simulations. Life Sci. 2022, 309, 121014. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, V.; Petković, M.; Živković, J.; Nikolić, G.M.; Veselinović, A.M. Development of Novel Therapeutics for Schizophrenia Treatment Based on a Selective Positive Allosteric Modulation of α1-Containing GABAARs—In Silico Approach. Curr. Issues Mol. Biol. 2022, 44, 3398–3412. [Google Scholar] [CrossRef]
- El Fadili, M.; Er-Rajy, M.; Kara, M.; Assouguem, A.; Belhassan, A.; Alotaibi, A.; Mrabti, N.N.; Fidan, H.; Ullah, R.; Ercisli, S. QSAR, ADMET In silico pharmacokinetics, molecular docking and molecular dynamics studies of novel bicyclo (aryl methyl) benzamides as potent GlyT1 inhibitors for the treatment of schizophrenia. Pharmaceuticals 2022, 15, 670. [Google Scholar] [CrossRef]
- Noorbakhsh, A.; Hosseininezhadian Koushki, E.; Farshadfar, C.; Ardalan, N. Designing a natural inhibitor against human kynurenine aminotransferase type II and a comparison with PF-04859989: A computational effort against schizophrenia. J. Biomol. Struct. Dyn. 2022, 40, 7038–7051. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Wen, L.; Fan, Y.; Xiong, W.; Liu, Y.; Zhang, T.; Wei, G.; Altamirano, A.; Zhang, T.-E.; Yan, Z. Exploring the Mechanism of Action of Trachelospermi Caulis et Folium for Depression Based on Experiments: Combining Network Pharmacology and Molecular Docking. Comput. Math. Methods Med. 2022, 2022, 3945063. [Google Scholar] [CrossRef]
- Nguyen, L.T.H.; Nguyen, N.P.K.; Tran, K.N.; Shin, H.-M.; Yang, I.-J. Network Pharmacology and Experimental Validation to Investigate the Antidepressant Potential of Atractylodes lancea (Thunb.) DC. Life 2022, 12, 1925. [Google Scholar] [CrossRef]
- Zhao, Q.; Pan, W.; Shi, H.; Qi, F.; Liu, Y.; Yang, T.; Si, H.; Si, G. Network pharmacology and molecular docking analysis on the mechanism of Baihe Zhimu decoction in the treatment of postpartum depression. Medicine 2022, 101, e29323. [Google Scholar] [CrossRef]
- Podder, A.; Latha, N. New insights into schizophrenia disease genes interactome in the human brain: Emerging targets and therapeutic implications in the postgenomics era. OMICS J. Integr. Biol. 2014, 18, 754–766. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Min, X.; Acharya, S.; Khadka, D.B.; Yoon, G.; Kim, K.-M.; Cheon, S.H. Design, synthesis, and systematic evaluation of 4-arylpiperazine-and 4-benzylpiperidine napthyl ethers as inhibitors of monoamine neurotransmitters reuptake. Bioorg. Med. Chem. 2018, 26, 5538–5546. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Ashraf, G.M.; Ganash, M.; Shen, B. Physicochemical, Interaction & Topological Descriptors vs. hMAO-A Inhibition of Aplysinopsin Analogs: A Boulevard to the Discovery of Semi-synthetic Antidepression Agents. Curr. Drug Metab. 2021, 22, 905–915. [Google Scholar] [PubMed]
- Santos, G.R.; Chiari, L.P.; da Silva, A.P.; Lipinski, C.F.; Oliveira, A.A.; Honorio, K.M.; de Sousa, A.G.; da Silva, A.B. A partial least squares and artificial neural network study for a series of arylpiperazines as antidepressant agents. J. Mol. Model. 2021, 27, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jing, C.; Yu, P.; Lu, M.; Xu, X.; Pei, Q.; Yan, F. Profiling the structural determinants of aminoketone derivatives as hNET and hDAT reuptake inhibitors by field-based QSAR based on molecular docking. Technol. Health Care 2021, 29, 257–273. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Elsherif, M.A.; Junaid, K.; Ejaz, H.; Alam, P.; Samad, A.; Jawarkar, R.D.; Masand, V.H. Perceiving the Concealed and Unreported Pharmacophoric Features of the 5-Hydroxytryptamine Receptor Using Balanced QSAR Analysis. Pharmaceuticals 2022, 15, 834. [Google Scholar] [CrossRef]
- Rathore, A.; Asati, V.; Mishra, M.; Das, R.; Kashaw, V.; Kashaw, S.K. Computational approaches for the design of novel dopamine D2 and serotonin 5-HT2A receptor dual antagonist towards schizophrenia. Silico Pharmacol. 2022, 10, 7. [Google Scholar] [CrossRef]
- Yu, Y.; Dong, H.; Peng, Y.; Welsh, W.J. QSAR-Based Computational Approaches to Accelerate the Discovery of Sigma-2 Receptor (S2R) Ligands as Therapeutic Drugs. Molecules 2021, 26, 5270. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Poroikov, V.; Filimonov, D.; Gloriozova, T.; Lagunin, A.; Druzhilovskiy, D.; Rudik, A.; Stolbov, L.; Dmitriev, A.; Tarasova, O.; Ivanov, S. Computer-aided prediction of biological activity spectra for organic compounds: The possibilities and limitations. Russ. Chem. Bull. 2019, 68, 2143–2154. [Google Scholar] [CrossRef]
- Filimonov, D.; Lagunin, A.; Gloriozova, T.; Rudik, A.; Druzhilovskii, D.; Pogodin, P.; Poroikov, V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.-O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on drug classification and target prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Potemkin, V.; Potemkin, A.; Grishina, M. Internet resources for drug discovery and design. Curr. Top. Med. Chem. 2018, 18, 1955–1975. [Google Scholar] [CrossRef]
- Murtazalieva, K.; Druzhilovskiy, D.; Goel, R.; Sastry, G.; Poroikov, V. How good are publicly available web services that predict bioactivity profiles for drug repurposing? SAR QSAR Environ. Res. 2017, 28, 843–862. [Google Scholar] [CrossRef]
- Lagunin, A.; Filimonov, D.; Poroikov, V. Multi-targeted natural products evaluation based on biological activity prediction with PASS. Curr. Pharm. Des. 2010, 16, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Gawande, D.; Lagunin, A.; Poroikov, V. Pharmacological repositioning of Achyranthes aspera as an antidepressant using pharmacoinformatic tools PASS and PharmaExpert: A case study with wet lab validation. SAR QSAR Environ. Res. 2018, 29, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Dong, B.; Sakata, K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl. Psychiatry 2011, 1, e40. [Google Scholar] [CrossRef]
- Marcon, M.; Mocelin, R.; Benvenutti, R.; Costa, T.; Herrmann, A.P.; de Oliveira, D.L.; Koakoski, G.; Barcellos, L.J.G.; Piato, A. Environmental enrichment modulates the response to chronic stress in zebrafish. J. Exp. Biol. 2018, 221, jeb176735. [Google Scholar] [CrossRef]
- Zhu, X.; Grace, A.A. Prepubertal Environmental Enrichment Prevents Dopamine Dysregulation and Hippocampal Hyperactivity in MAM Schizophrenia Model Rats. Biol. Psychiatry 2021, 89, 298–307. [Google Scholar] [CrossRef]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2016, 18, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, S.; Bartz-Johannessen, C.; Sinkeviciute, I.; Reitan, S.K.; Kroken, R.A.; Løberg, E.-M.; Larsen, T.K.; Rettenbacher, M.; Johnsen, E.; Sommer, I.E. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: Results from the BeSt InTro study. NPJ Schizophr. 2021, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Furey, M.L.; Drevets, W.C. Antidepressant efficacy of the antimuscarinic drug scopolamine: A randomized, placebo-controlled clinical trial. Arch. Gen. Psychiatry 2006, 63, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Lanquillon, S.; Krieg, J.C.; Bening-Abu-Shach, U.; Vedder, H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000, 22, 370–379. [Google Scholar] [CrossRef]
- Kopschina Feltes, P.; Doorduin, J.; Klein, H.C.; Juarez-Orozco, L.E.; Dierckx, R.A.; Moriguchi-Jeckel, C.M.; de Vries, E.F. Anti-inflammatory treatment for major depressive disorder: Implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. J. Psychopharmacol. 2017, 31, 1149–1165. [Google Scholar] [CrossRef]
- Furtado, M.; Katzman, M.A. Examining the role of neuroinflammation in major depression. Psychiatry Res. 2015, 229, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Lotrich, F.E. Inflammatory cytokine-associated depression. Brain Res. 2015, 1617, 113–125. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef]
- Yirmiya, R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996, 711, 163–174. [Google Scholar] [CrossRef]
- Capuron, L.; Ravaud, A.; Neveu, P.; Miller, A.; Maes, M.; Dantzer, R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry 2002, 7, 468–473. [Google Scholar] [CrossRef]
- Vollmer-Conna, U.A.; Fazou, C.; Cameron, B.; Li, H.; Brennan, C.; Luck, L.; Davenport, T.; Wakefield, D.; Hickie, I.; Lloyd, A. Production of pro-inflammatory cytokines correlates with the symptoms of acute sickness behaviour in humans. Psychol. Med. 2004, 34, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, W.R.; Hegel, M.; Unützer, J.; Fan, M.-Y.; Sateia, M.J.; Lyness, J.M.; Phillips, C.; Perlis, M.L. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep 2008, 31, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Demyttenaere, K.; De Fruyt, J.; Stahl, S.M. The many faces of fatigue in major depressive disorder. Int. J. Neuropsychopharmacol. 2005, 8, 93–105. [Google Scholar] [CrossRef] [PubMed]
- George, L.K.; Blazer, D.G.; Hughes, D.C.; Fowler, N. Social support and the outcome of major depression. Br. J. Psychiatry 1989, 154, 478–485. [Google Scholar] [CrossRef]
- Bluthé, R.-M.; Beaudu, C.; Kelley, K.W.; Dantzer, R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995, 677, 171–176. [Google Scholar] [CrossRef]
- Remus, J.L.; Dantzer, R. Inflammation models of depression in rodents: Relevance to psychotropic drug discovery. Int. J. Neuropsychopharmacol. 2016, 19, pyw028. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shachar, D. The bimodal mechanism of interaction between dopamine and mitochondria as reflected in Parkinson’s disease and in schizophrenia. J. Neural Transm. 2020, 127, 159–168. [Google Scholar] [CrossRef]
- Jarskog, L.F. Apoptosis in schizophrenia: Pathophysiologic and therapeutic considerations. Curr. Opin. Psychiatry 2006, 19, 307–312. [Google Scholar] [CrossRef]
- Łukasiewicz, S. Development of a New Polymeric Nanocarrier Dedicated to Controlled Clozapine Delivery at the Dopamine D2-Serotonin 5-HT1A Heteromers. Polymers 2021, 13, 1000. [Google Scholar] [CrossRef]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
- Kokras, N.; Dalla, C. Preclinical sex differences in depression and antidepressant response: Implications for clinical research. J. Neurosci. Res. 2017, 95, 731–736. [Google Scholar] [CrossRef] [PubMed]
- Kokras, N.; Dalla, C.; Papadopoulou-Daifoti, Z. Sex differences in pharmacokinetics of antidepressants. Expert Opin. Drug Metab. Toxicol. 2011, 7, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Puga-Olguín, A.; Rodríguez-Landa, J.F.; de Jesús Rovirosa-Hernández, M.; Germán-Ponciano, L.J.; Caba, M.; Meza, E.; Guillén-Ruiz, G.; Olmos-Vázquez, O.J. Long-term ovariectomy increases anxiety-and despair-like behaviors associated with lower Fos immunoreactivity in the lateral septal nucleus in rats. Behav. Brain Res. 2019, 360, 185–195. [Google Scholar] [CrossRef]
- Kokras, N.; Pastromas, N.; Papasava, D.; de Bournonville, C.; Cornil, C.; Dalla, C. Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology 2018, 87, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.; Wong, K.; Cachat, J.; Elegante, M.; Gilder, T.; Mohnot, S.; Wu, N.; Minasyan, A.; Tuohimaa, P.; Kalueff, A.V. Neurosteroid vitamin D system as a nontraditional drug target in neuropsychopharmacology. Behav. Pharmacol. 2010, 21, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Eremin, K.O.; Tuohimaa, P. Mechanisms of neuroprotective action of vitamin D(3). Biochemistry 2004, 69, 738–741. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).