Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives
Abstract
1. Introduction
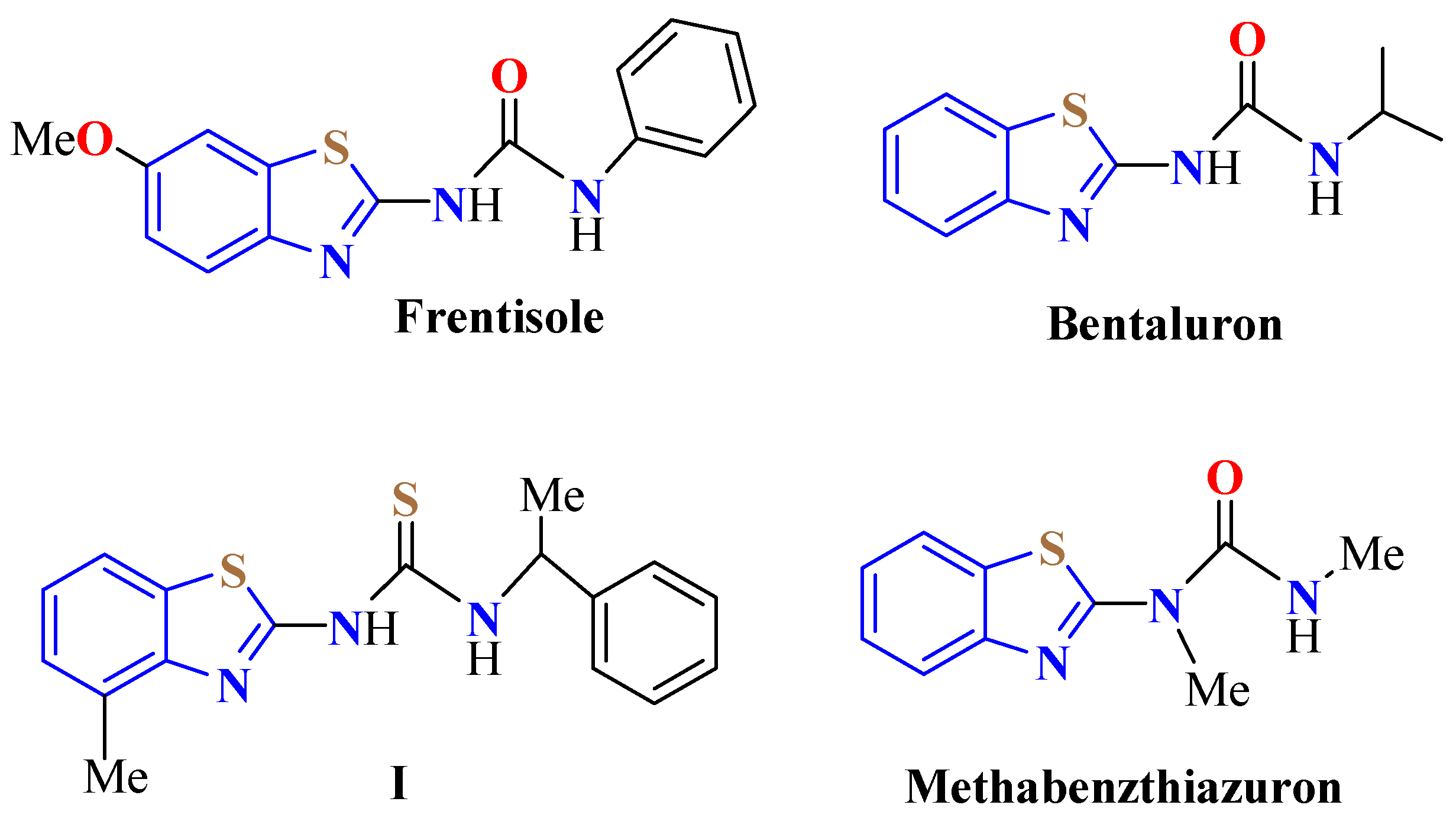
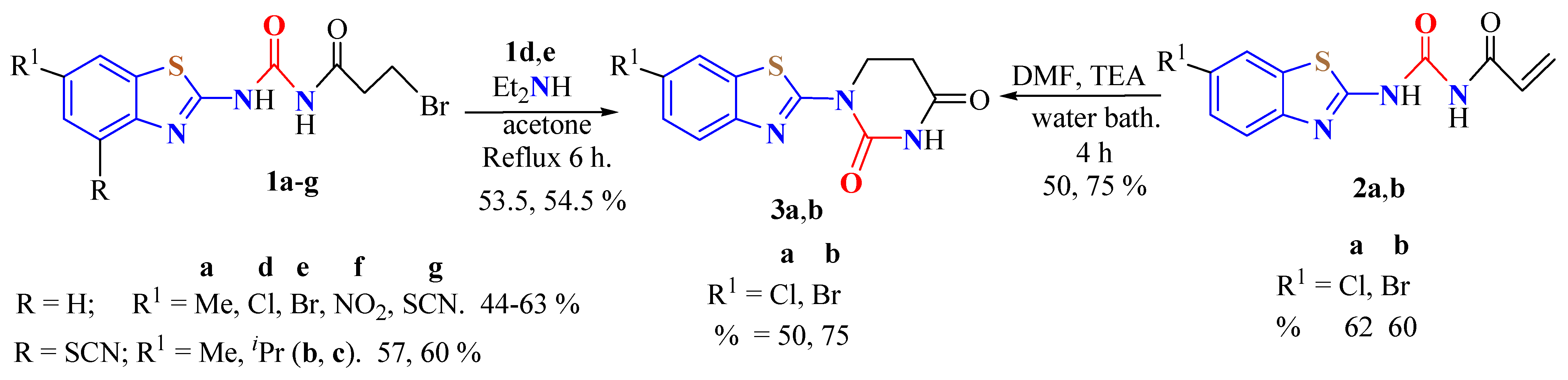
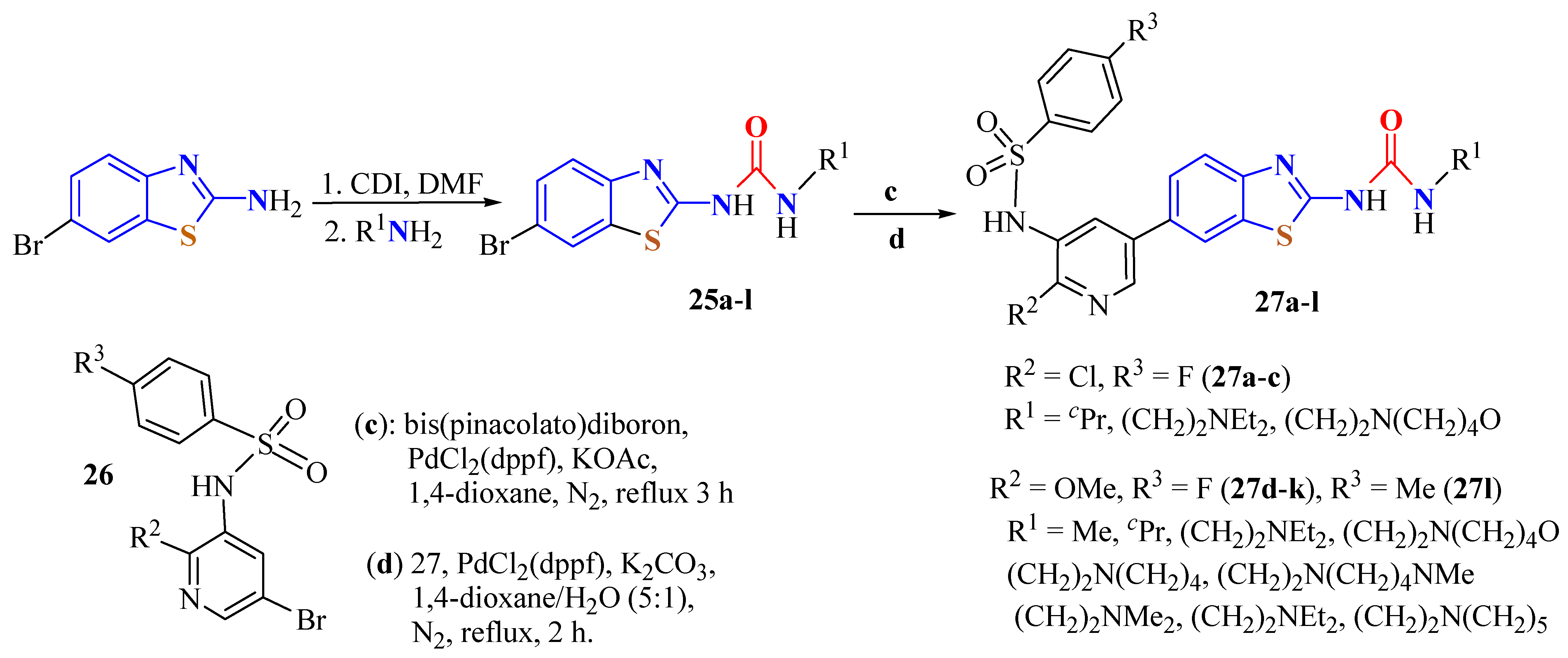
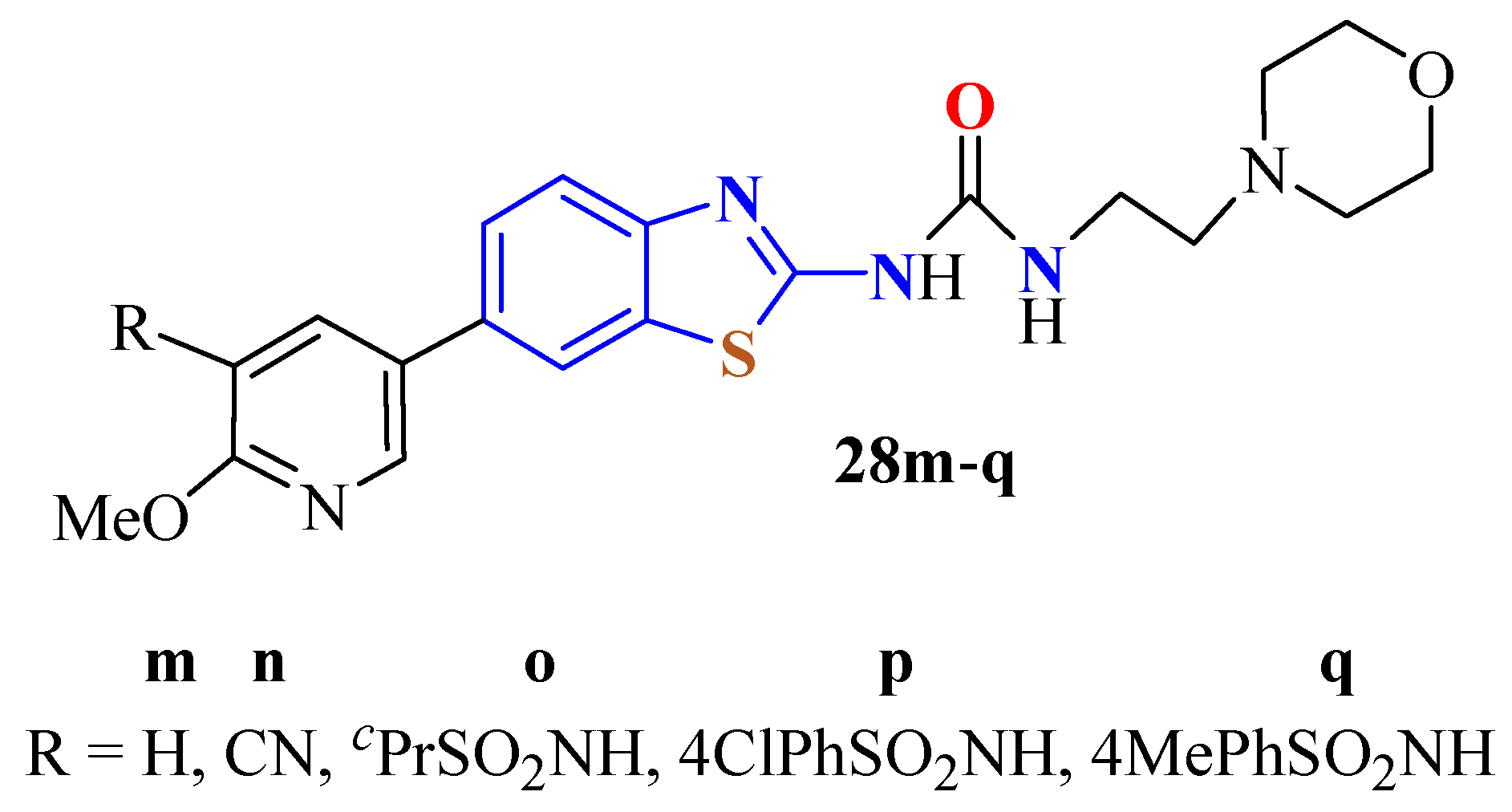
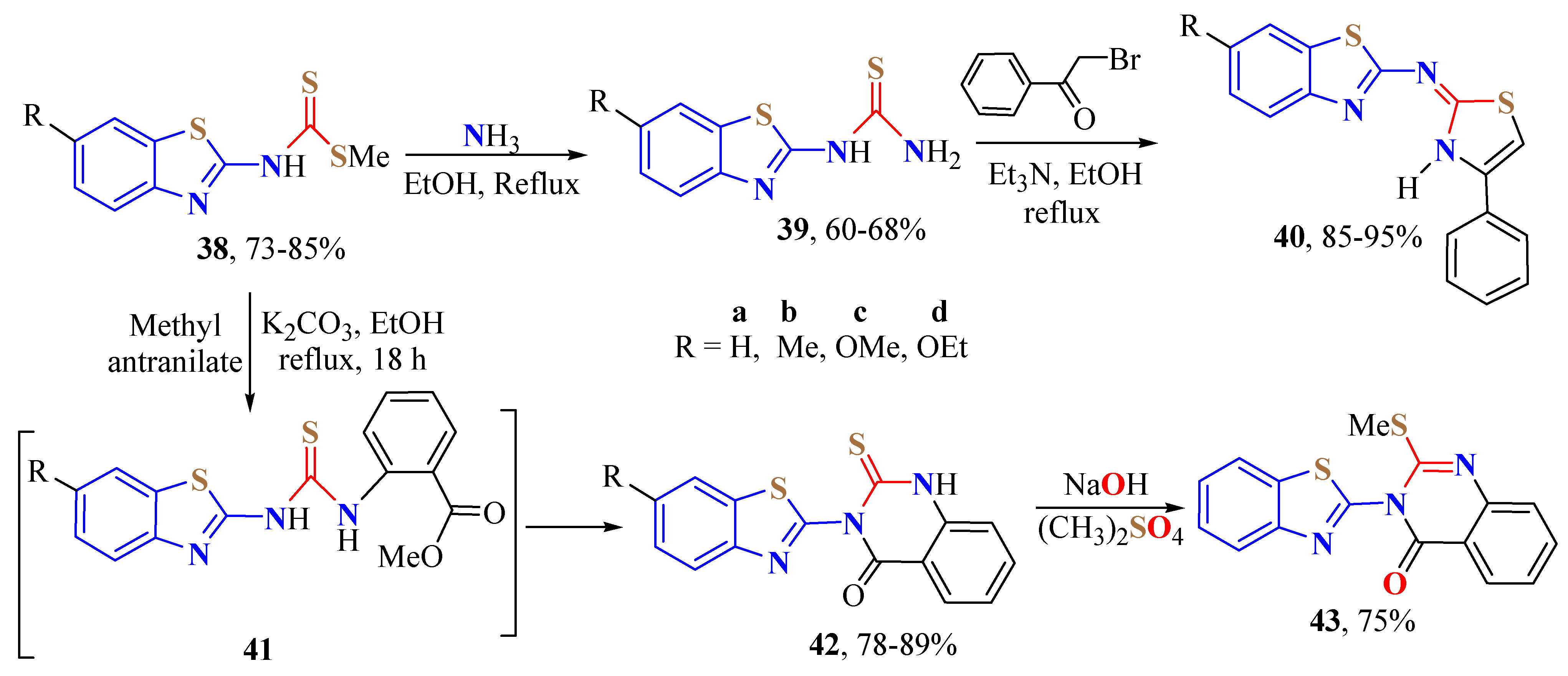
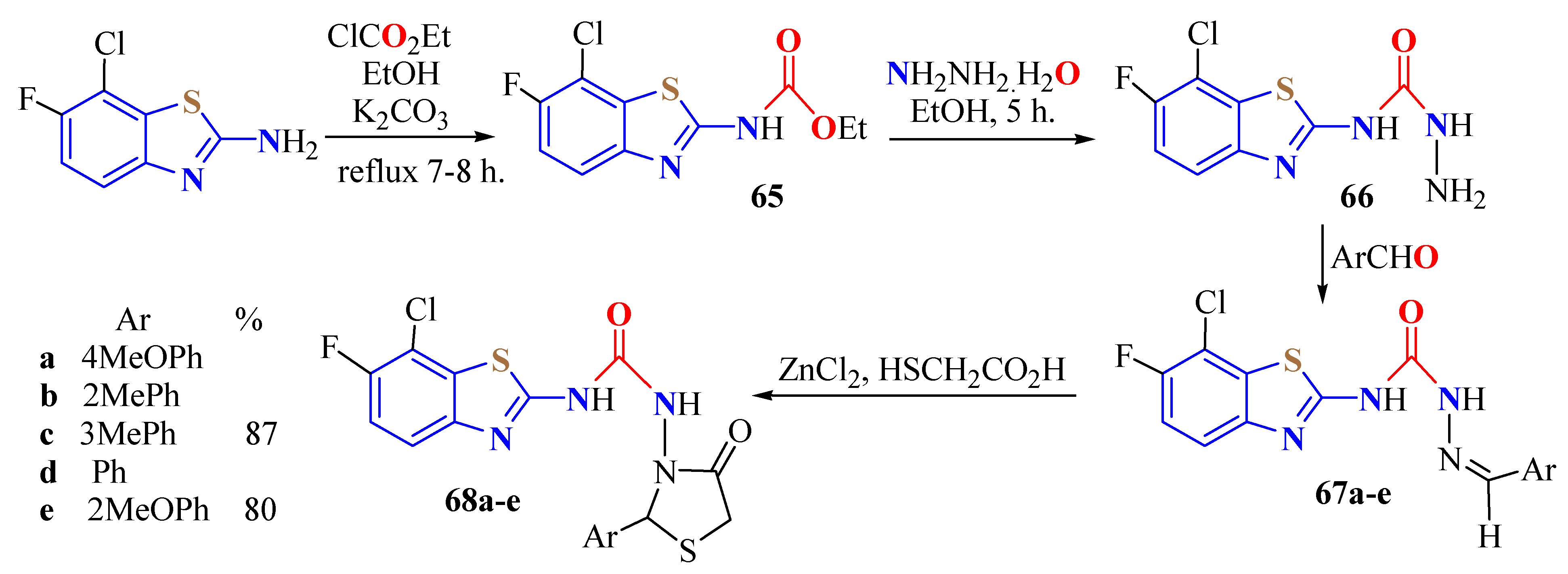
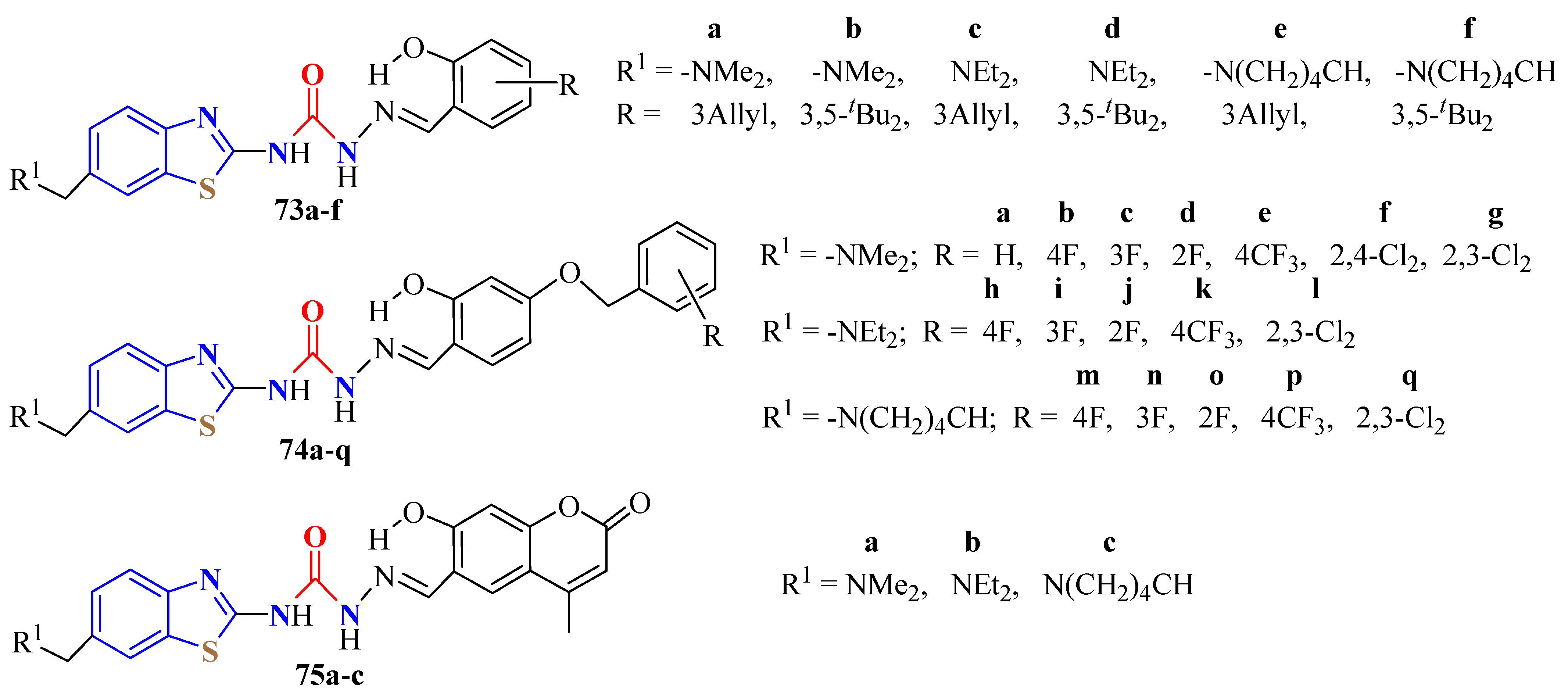
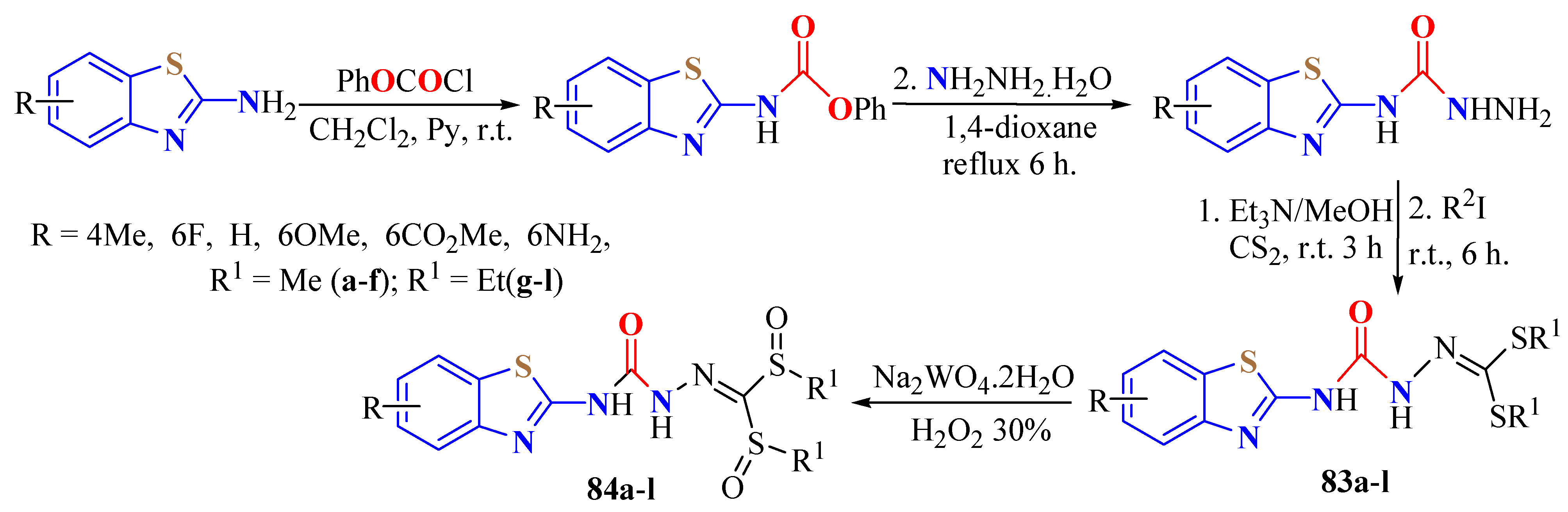
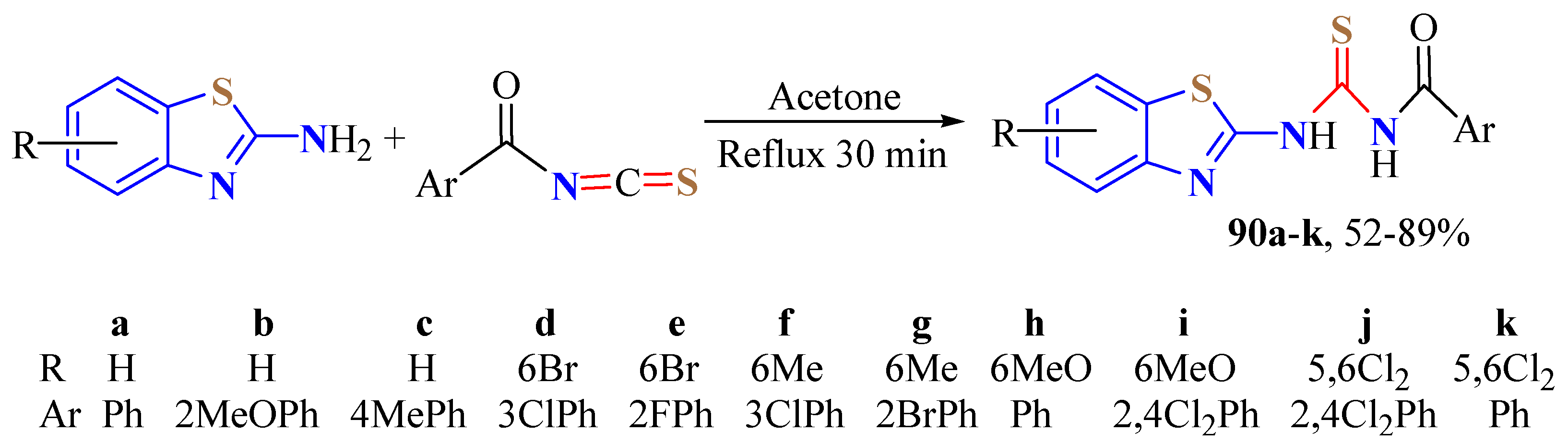
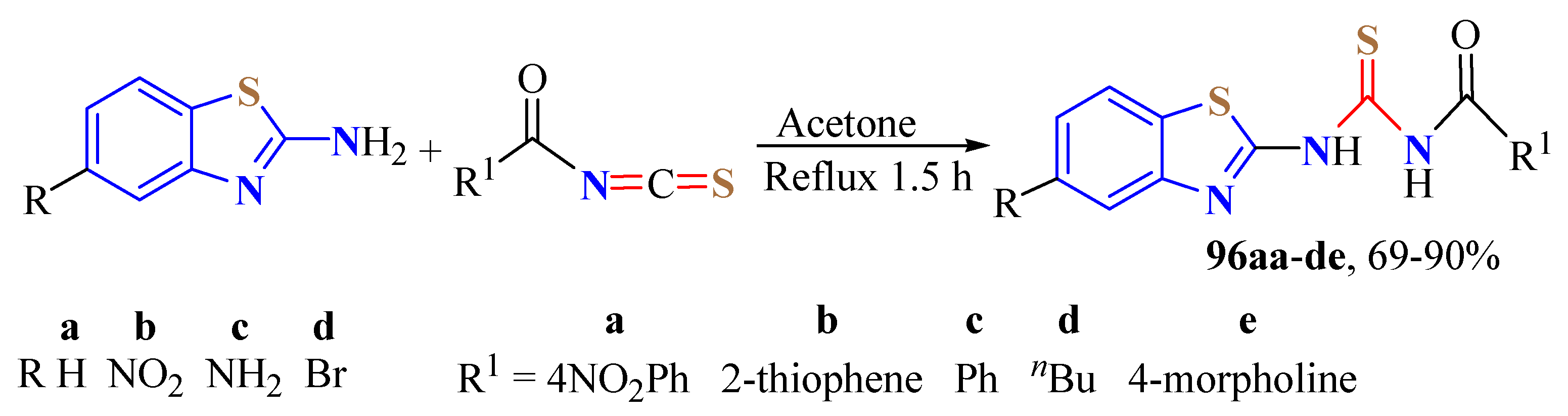
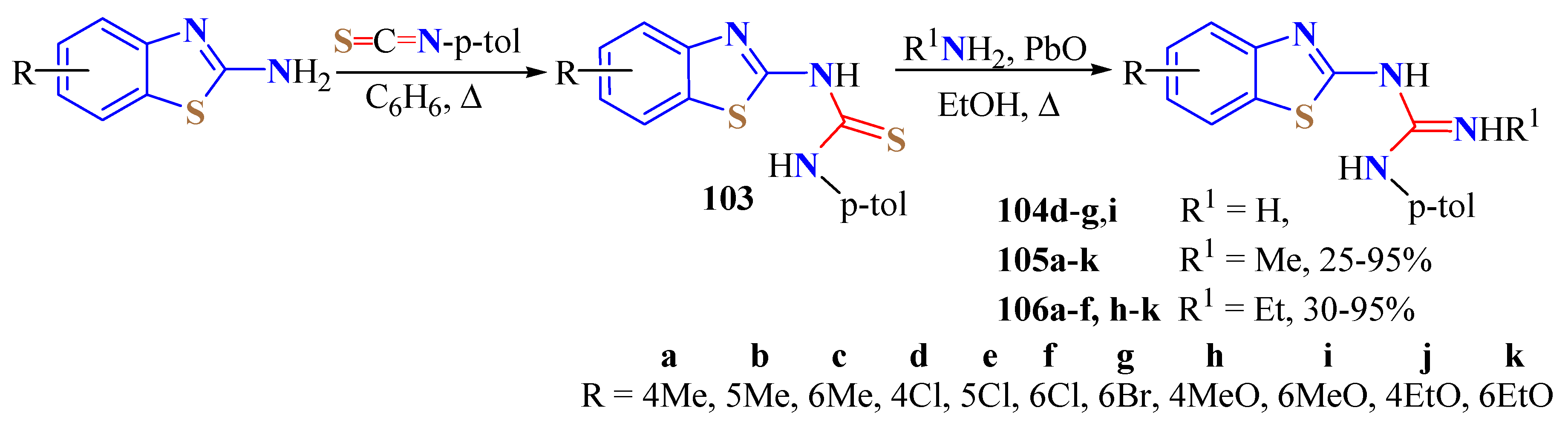
2. (T)UBT Derivatives
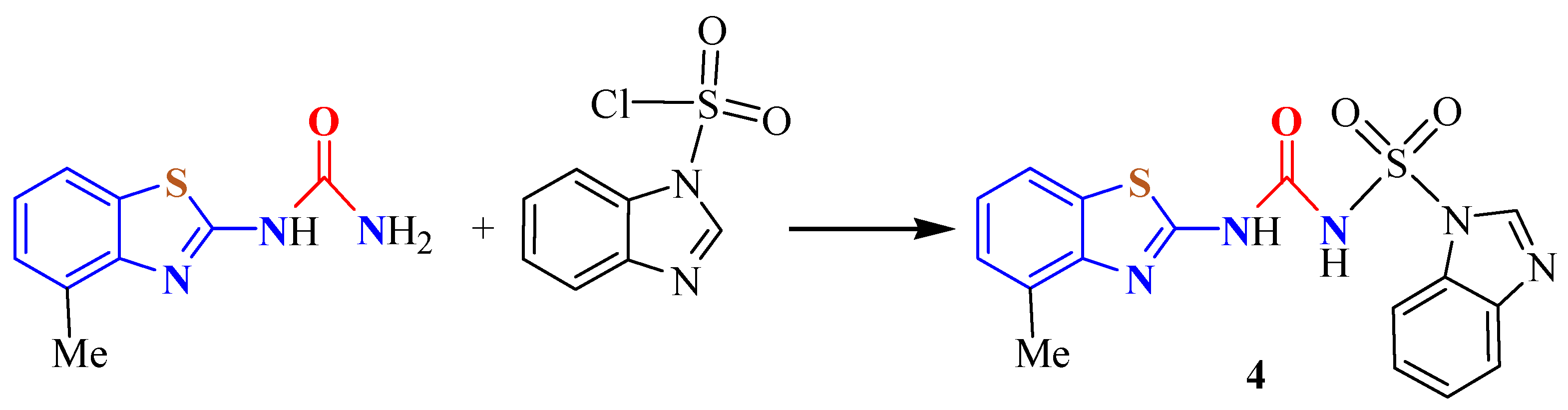
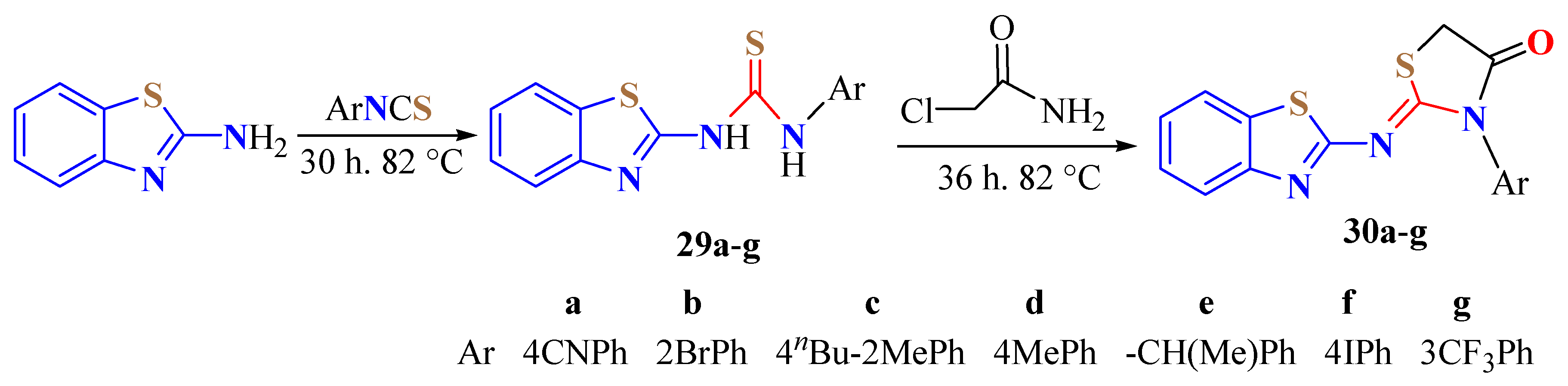
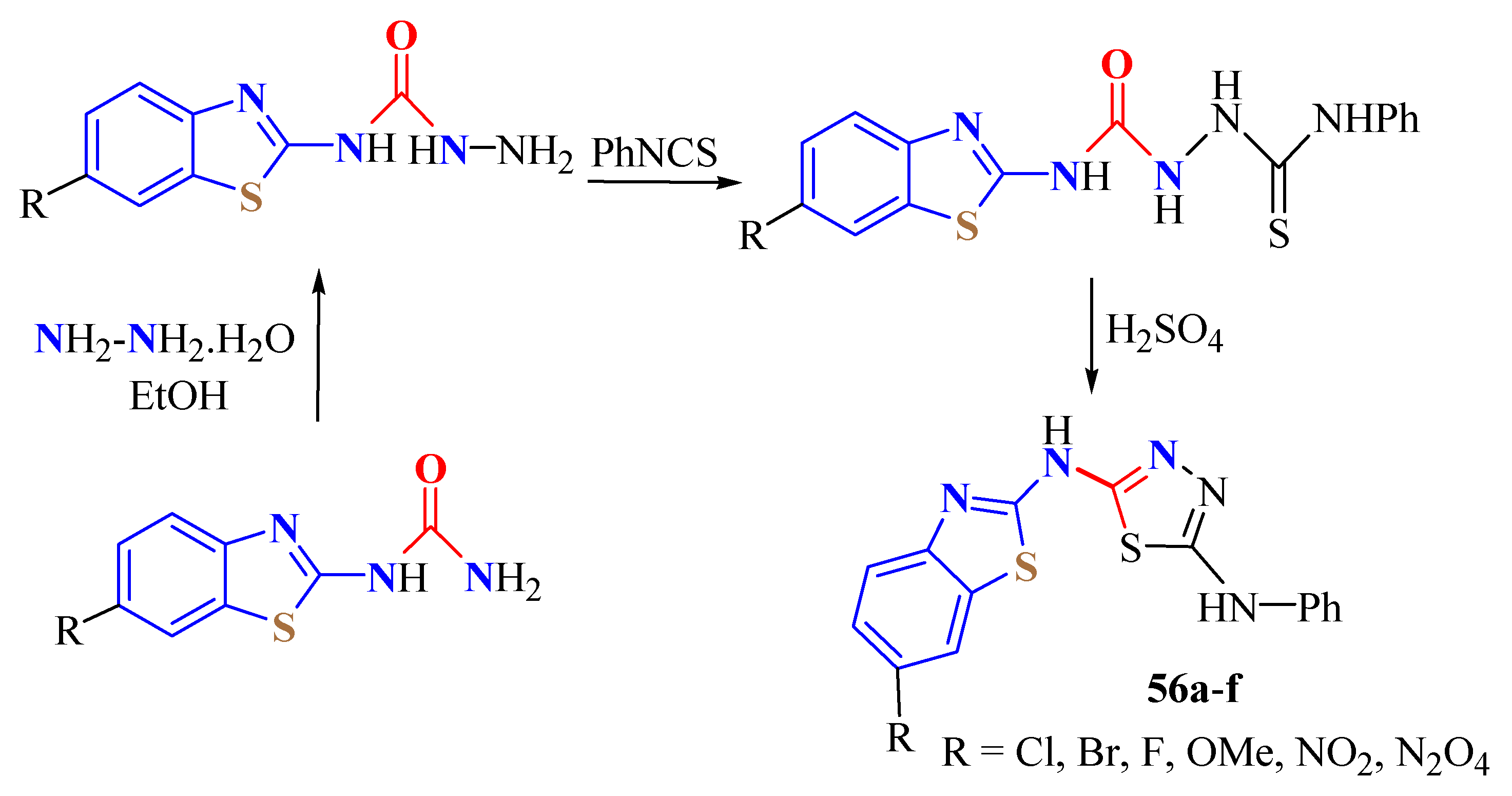
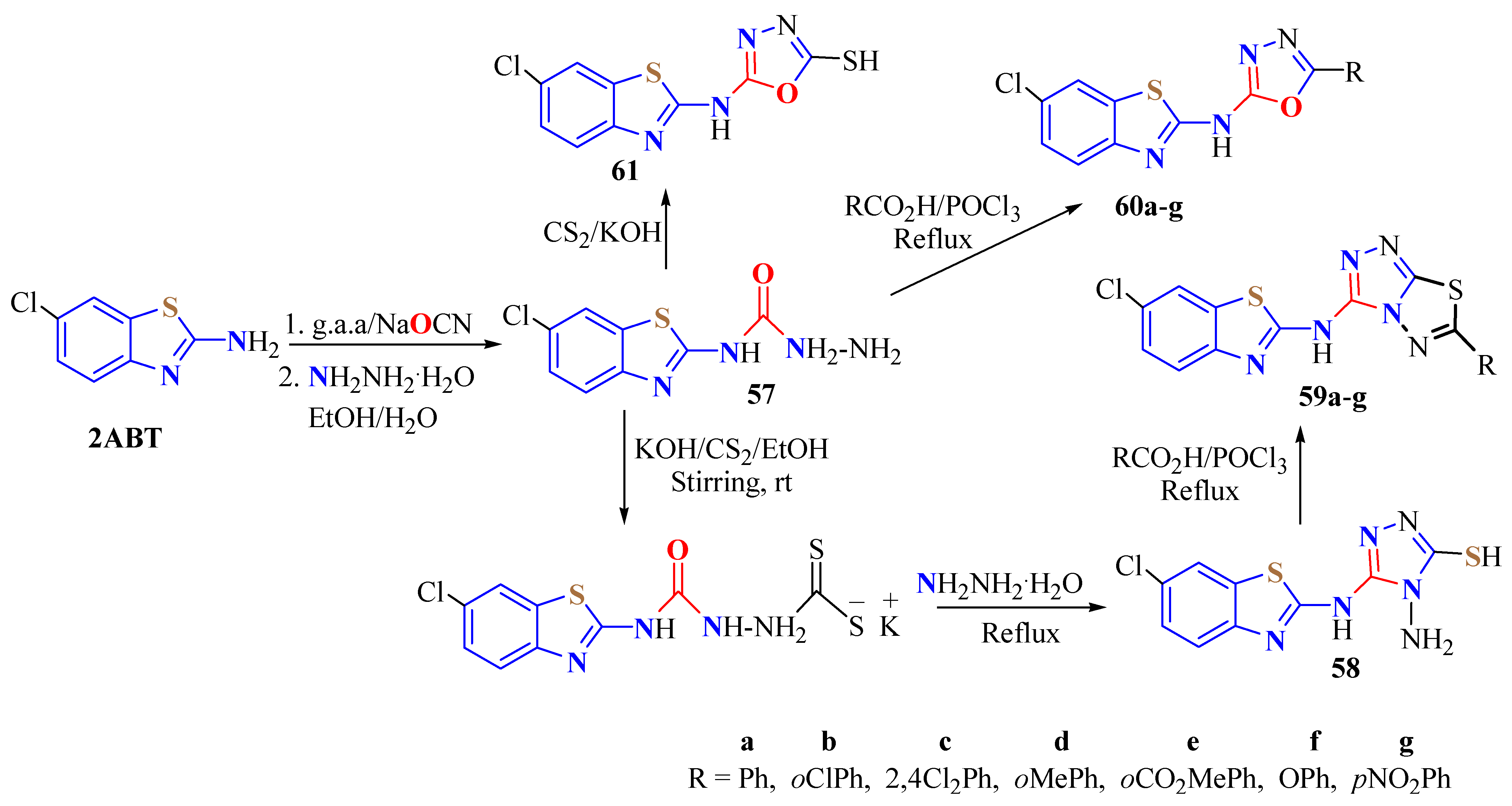
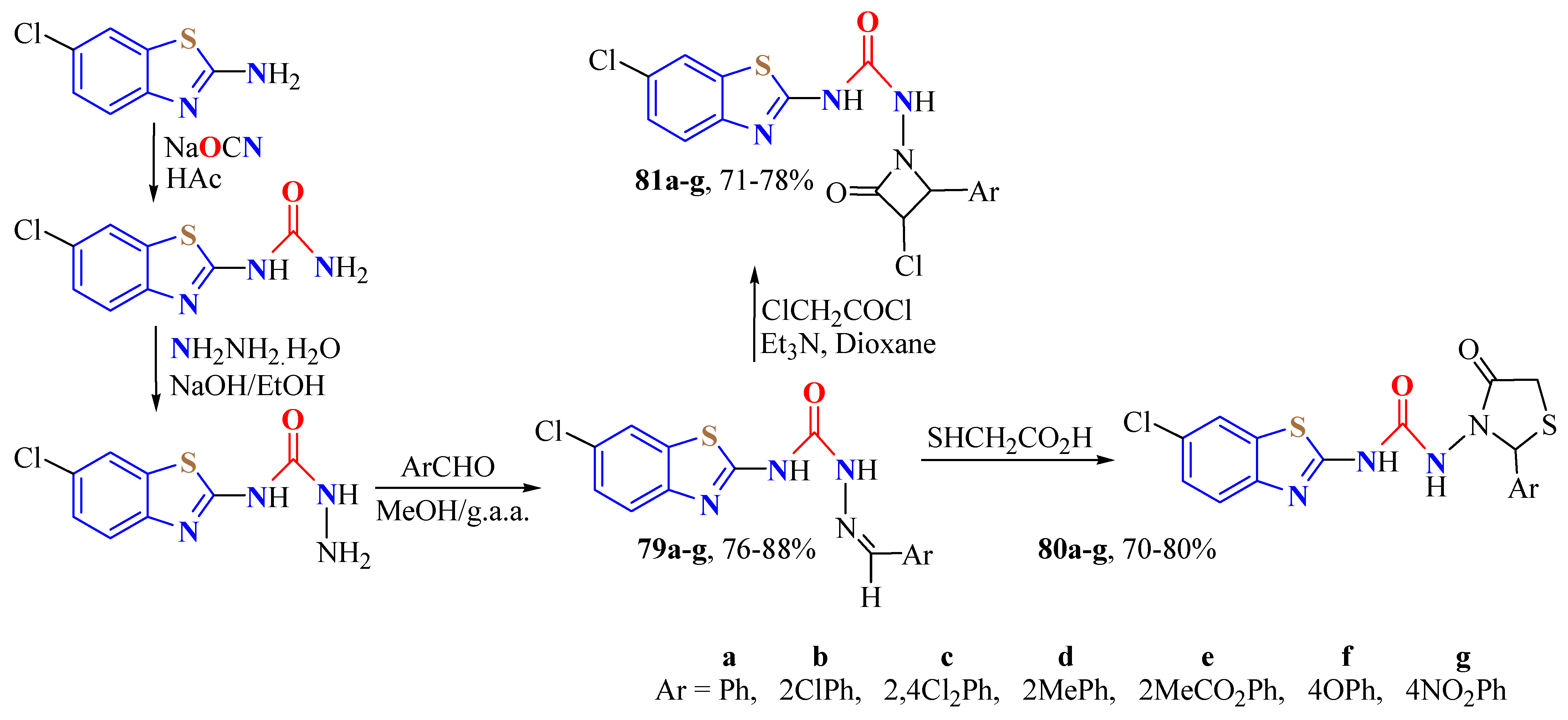
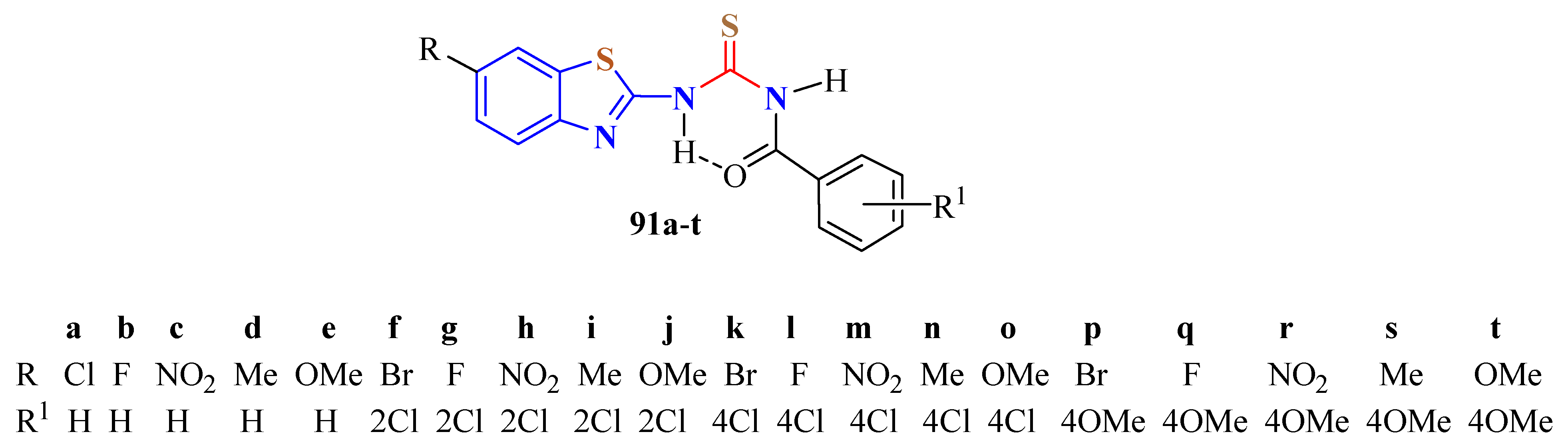
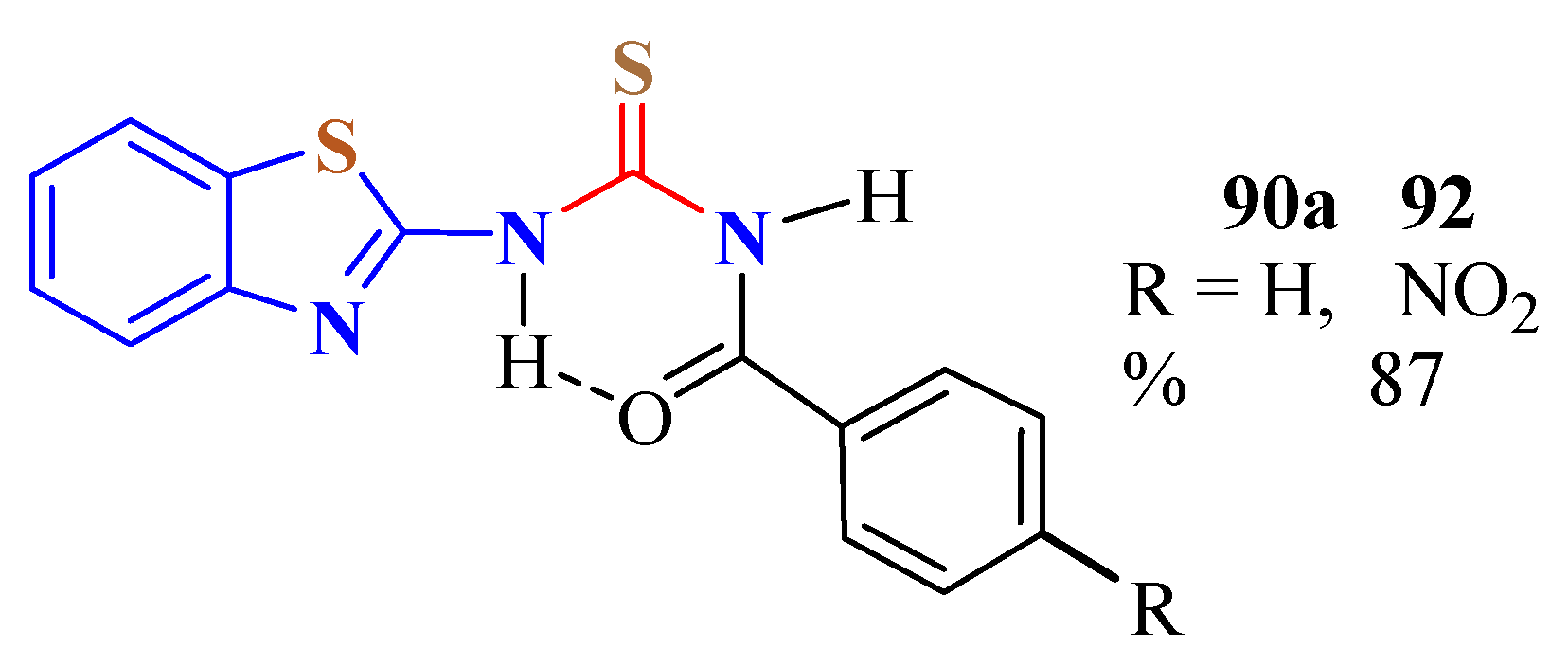
3. BT-Semi(Thio)Carbazide Derivatives
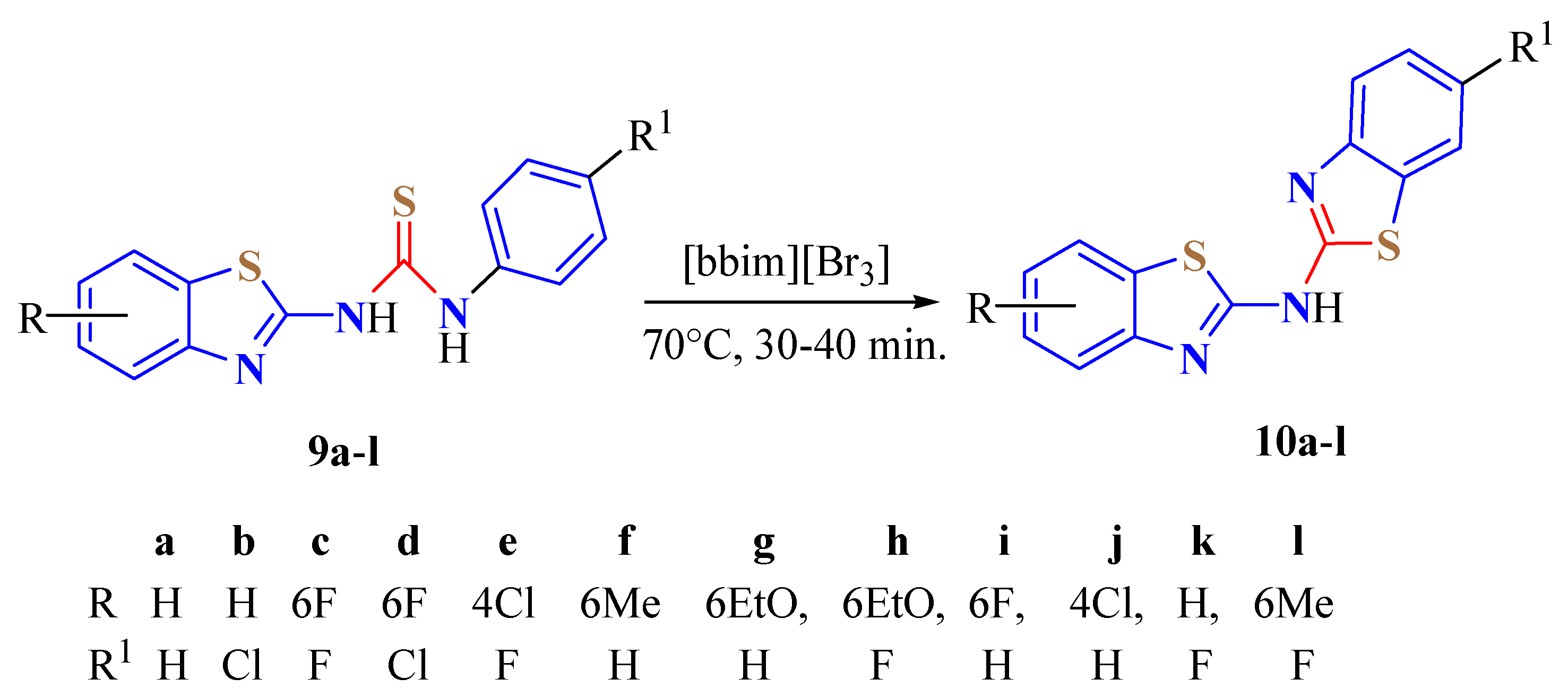
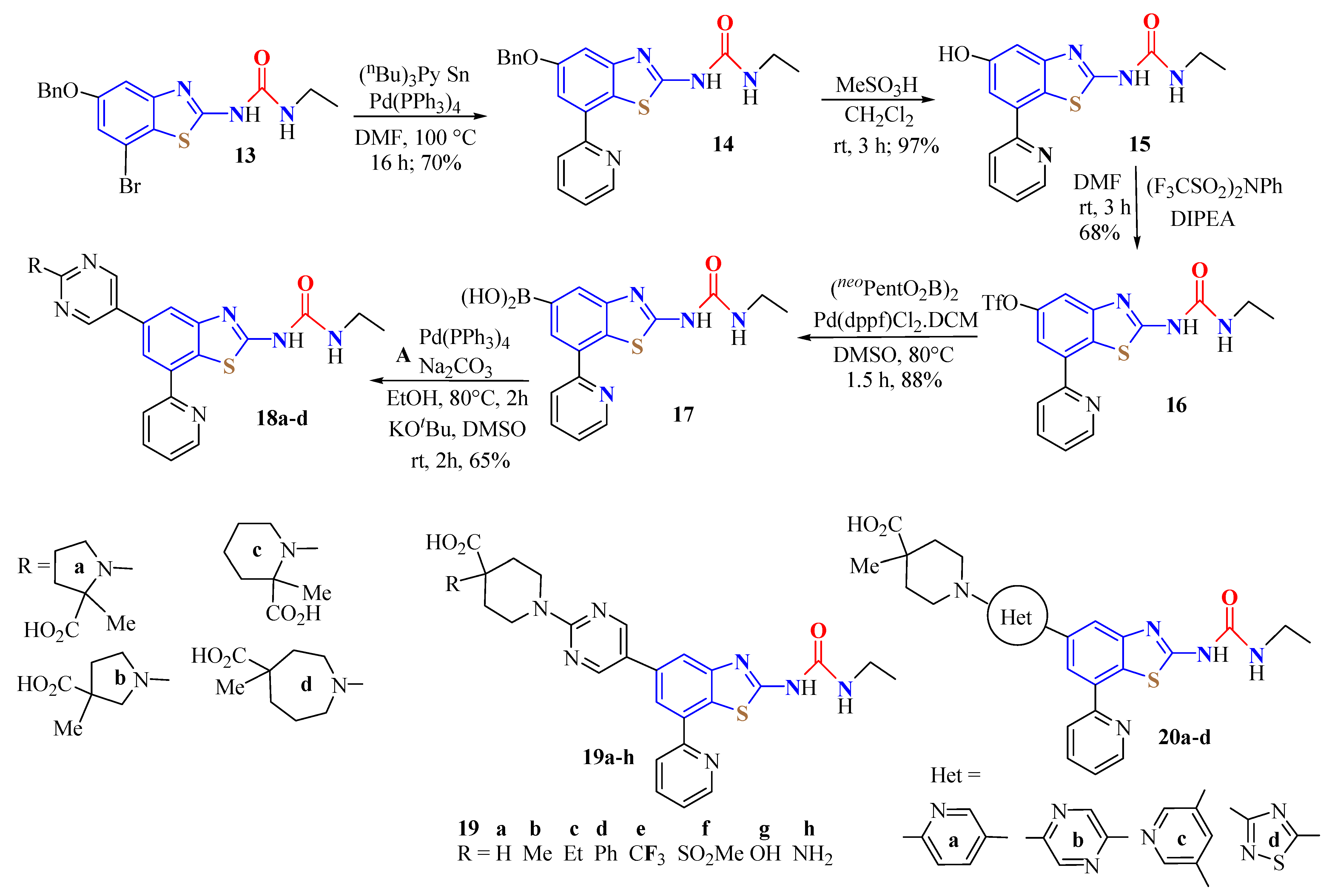
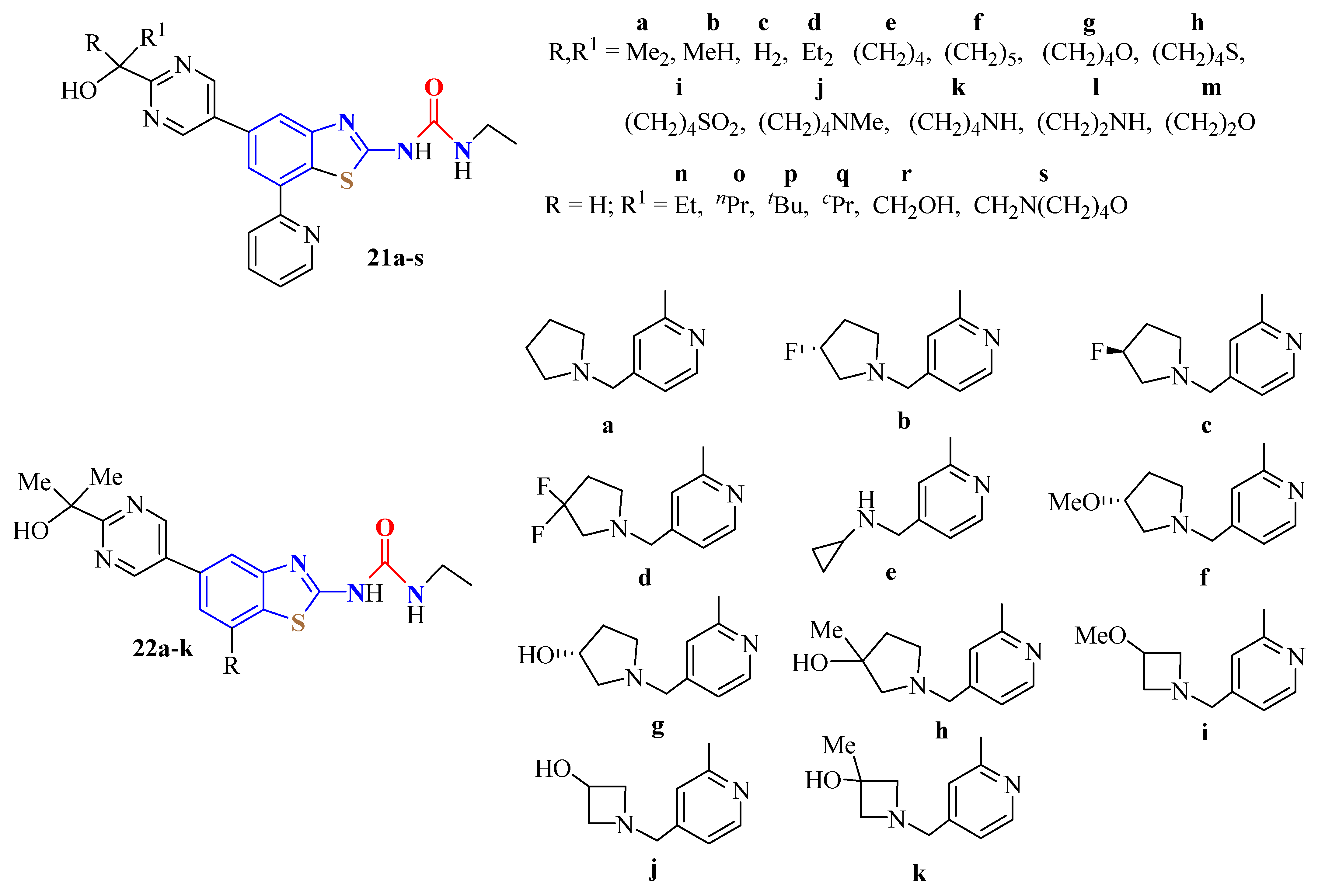
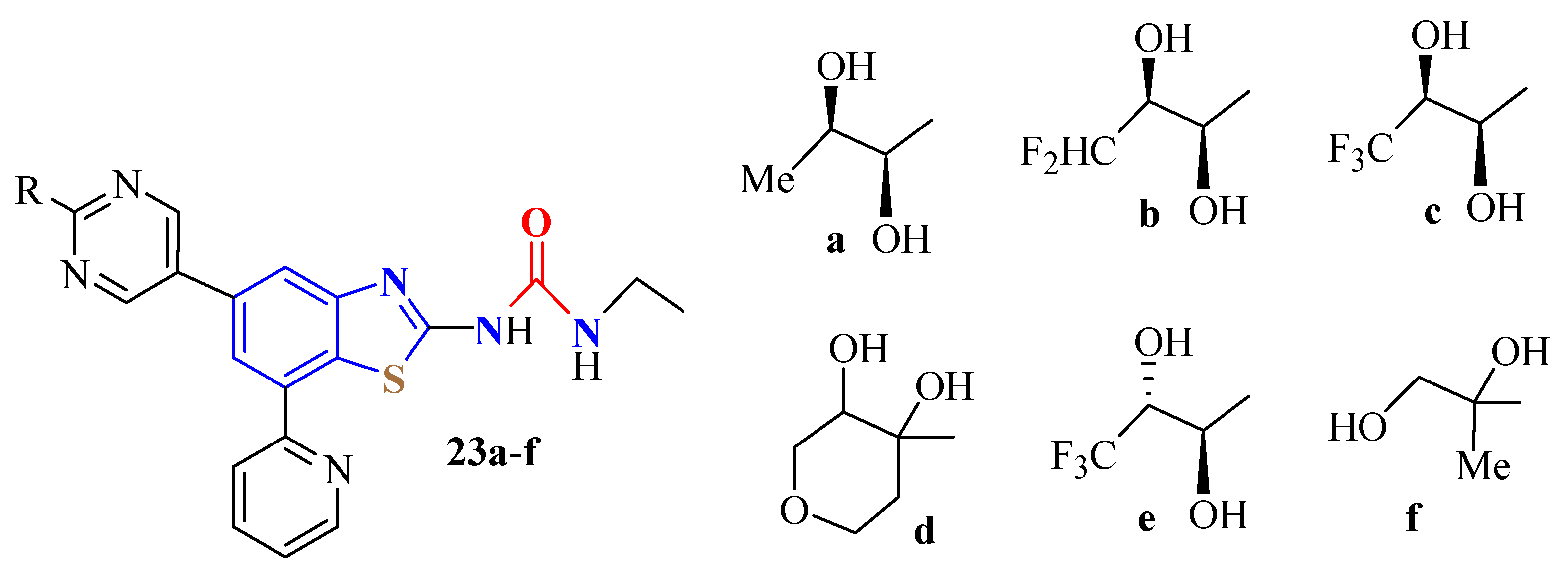
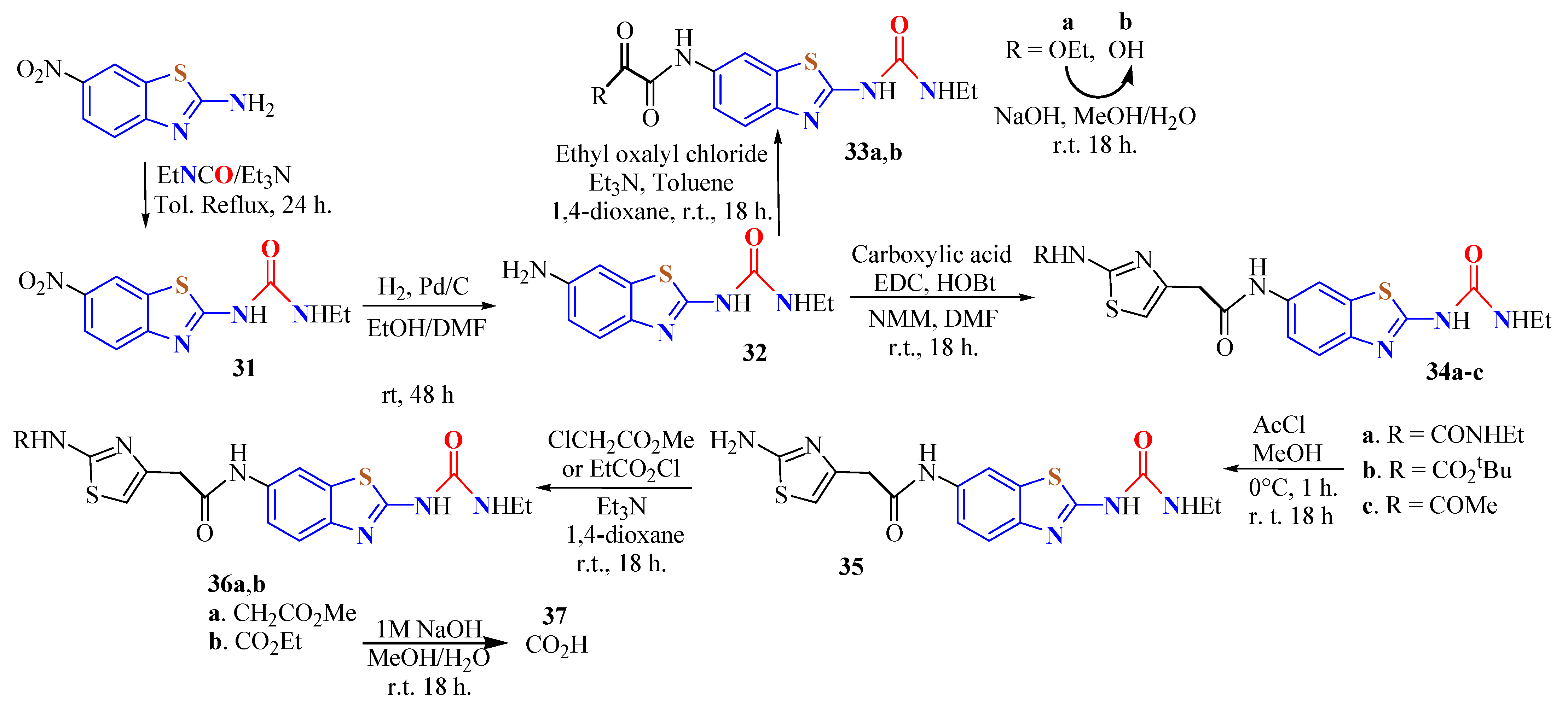
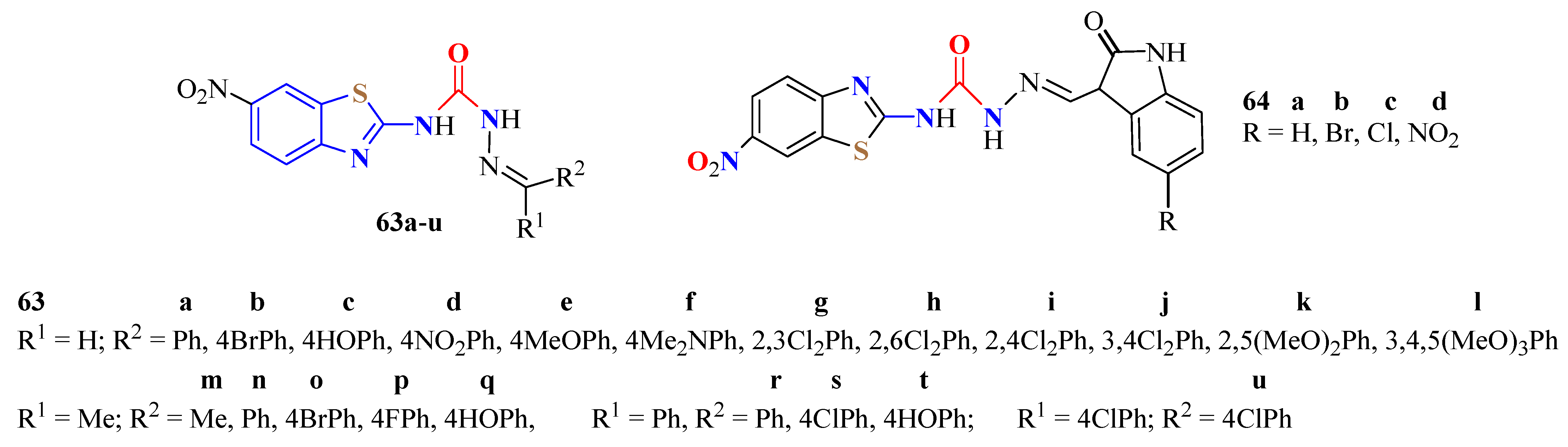
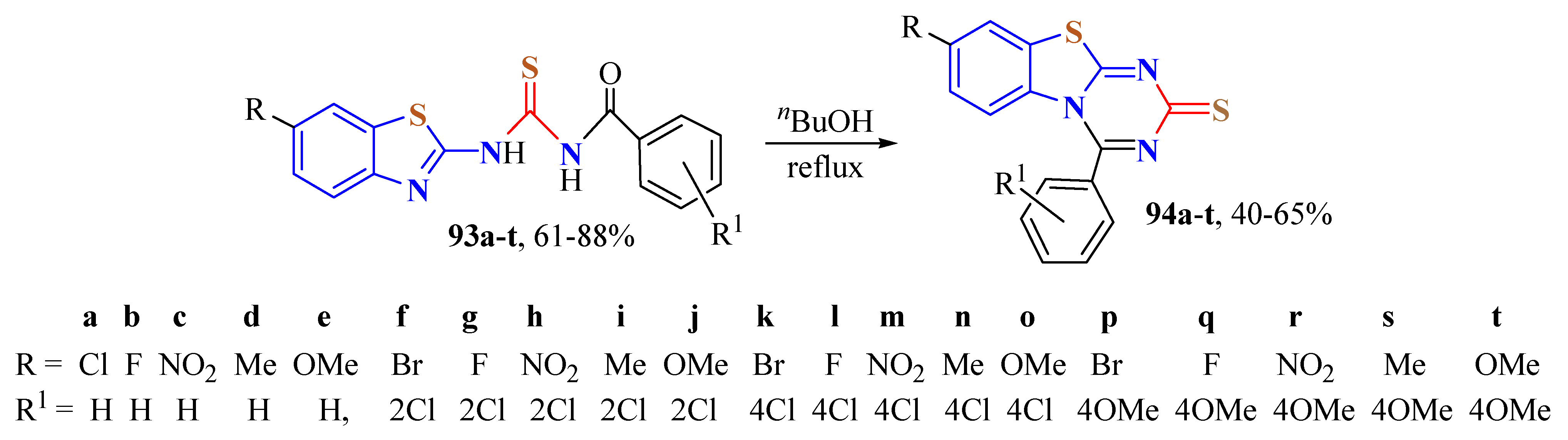
4. N-acyltUBT Derivatives
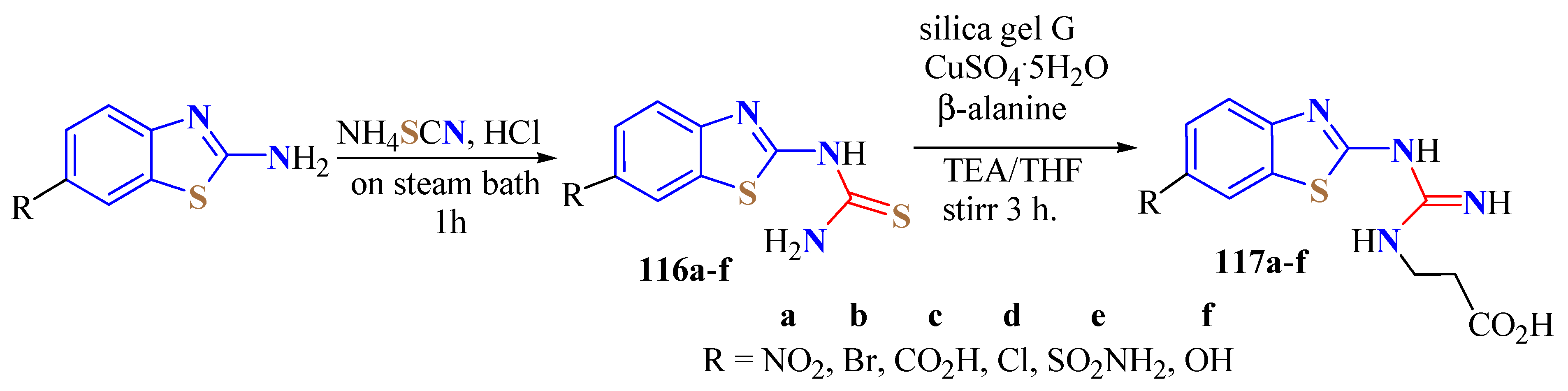
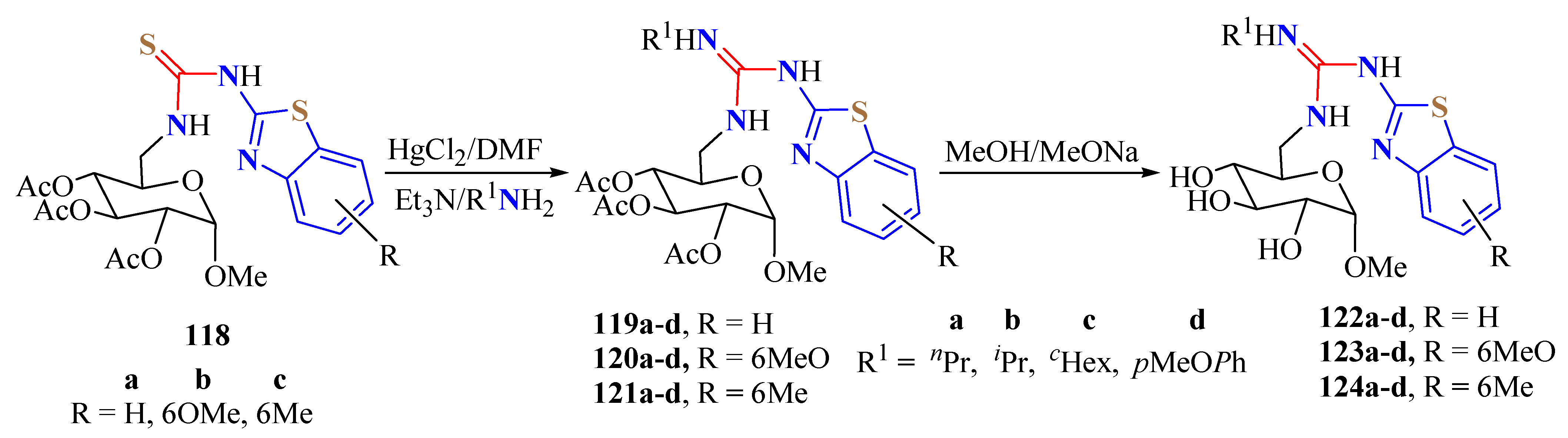
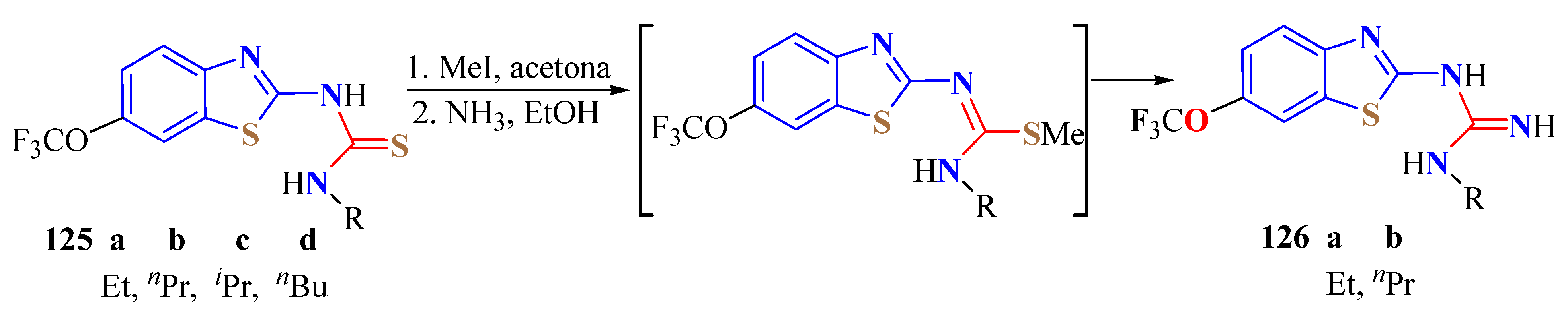
5. Guanidine Derivatives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- Venkatachalam, T.K.; Mao, C.; Uckun, F.M. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 2004, 12, 4275–4284. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, S.-U.; Choi, H.-K.; Lee, J.; Lim, J.-O.; Kil, M.-J.; Jin, M.-K.; Kim, K.-P.; Sung, J.-H.; Chung, S.-J.; et al. Analysis of structure–activity relationships for the B-region of N-(3-acyloxy-2-benzylpropyl)-N0-[4-(methylsulfonylamino)-benzyl]thiourea analogues as vanilloid receptor antagonists: Discovery of an N-hydroxythiourea analogue with potent analgesic activity. Bioorg. Med. Chem. Lett. 2004, 14, 2291–2297. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Cho, J.H.; Oh, C.H. Synthesis and biological evaluation of 1β-methylcarbapenems having cyclic thiourea moieties and their related compounds. Eur. J. Med. Chem. 2006, 41, 825–832. [Google Scholar] [CrossRef]
- Khan, S.A.; Singh, N.; Saleem, K. Synthesis, characterization and in vitro antibacterial activity of thiourea and urea derivatives of steroids. Eur. J. Med. Chem. 2008, 43, 2272–2277. [Google Scholar] [CrossRef]
- Saeed, A.; Saeed, N.; Hummera, R.; Sadaf, R.; Hameed, A. Synthesis, characterization and antibacterial activity of some 1-aroyl-3-aryl tioureas. Chemistry 2009, 18, 152–158. [Google Scholar]
- Keche, A.P.; Hatnapure, G.D.; Tale, R.H.; Rodge, A.H.; Birajdar, S.S.; Kamble, V.M. A novel pyrimidine derivatives with aryl urea, thiourea and sulfonamide moieties: Synthesis, anti-inflammatory and antimicrobial evaluation. Bioorg. Med. Chem. Lett. 2012, 22, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef]
- Pavić, K.; Rajić, Z.; Michnová, H.; Jampílek, J.; Perković, I.; Zorc, B. Second generation of primaquine ureas and bis-ureas as potential antimycobacterial agents. Mol. Divers. 2019, 23, 657–667. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Turan-Zitouni, G.; Ozdemir, A.; Revial, G. New triazole and triazolothiadiazine derivatives as possible antimicrobial agents. Eur. J. Med. Chem. 2008, 43, 155–159. [Google Scholar] [CrossRef]
- Koçyiğit-Kaymakçıoğlu, B.; Çalışır, M.M.; Özbek, B.; Ötük, G. Synthesis and antimicrobial activities of schiff bases derived from 4-amino-5-(1phenylethyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione. Eur. J. Chem. 2010, 7, 458–464. [Google Scholar]
- Vega-Perez, J.M.; Perinan, I.; Argandona, M.; Vega-Holm, M.; Palo-Nieto, C.; Burgos-Morón, E.; López-Lázaro, M.; Vargas, C.; Nieto, J.J.; Iglesias-Guerra, F. Isoprenylthiourea and urea derivatives as new farnesyl diphos phate analogues: Synthesis and in vitro antimicrobial and cytotoxic activities. Eur. J. Med. Chem. 2012, 58, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Karipcin, F.; Atis, M.; Sariboga, B.; Celik, H.; Tas, M. Structural, spectral, optical and antimicrobial properties of synthesized 1-benzoyl-3-furan2-ylmethyl-thiourea. J. Mol. Struct. 2013, 1048, 69–77. [Google Scholar] [CrossRef]
- Zhong, Z.; Xing, R.; Liu, S.; Wang, L.; Cai, S.; Li, P. Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro. Carbohydr. Res. 2008, 343, 566–570. [Google Scholar] [CrossRef]
- Pingaew, R.; Sinthupoom, N.; Mandi, P.; Prachayasittikul, V.; Cherdtrakulkiat, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, biological evaluation and in silico study of bis-thiourea derivatives as anticancer, antimalarial and antimicrobial agents. Med. Chem. Res. 2017, 26, 3136–3148. [Google Scholar] [CrossRef]
- Lee, J.; La, S.; Ahn, B.R.; Jeong, T.C.; Kim, D.H. Metabolism of 1-{3-[3-(4-cyanobenzyl)-3H-imidazol-4-yl]-propyl}-3-(6-methoxypyridin-3-yl)-1-(2-trifluoro-methyl-benzyl)tiourea (YH3945), a novel anticancer drug, in rats using the14C-labeled compound. Rapid Commun. Mass Spectrom. 2004, 18, 1901–1910. [Google Scholar] [CrossRef]
- Manjula, S.N.; Noolvi, N.M.; Parihar, K.V.; Reddy, S.A.M.; Ramani, V.; Gadad, A.K.; Singh, G.; Kutty, N.G.; Rao, C.M. Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzo thiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar] [CrossRef]
- Azimian, F.; Hamzeh-Mivehroud, M.; Mojarrad, J.S.; Hemmati, S.; Dastmalchi, S. Synthesis and biological evaluation of diaryl urea derivatives designed as potential anticarcinoma agents using de novo structure-based lead optimization approach. Eur. J. Med. Chem. 2020, 201, 112461. [Google Scholar] [CrossRef]
- Holla, B.S.; Veerenda, B.; Shivananda, K.; Boja, P. Synthesis characterization and anticancer activity studies on some Mannich bases derived from 1,2,4-triazoles. Eur. J. Med. Chem. 2003, 38, 759–767. [Google Scholar] [CrossRef]
- Ramadas, K.; Suresh, G.; Janarthanan, N.; Masilamani, S. Antifungal Activityof 1,3-DisubstitutedSymmetrical and Unsymmetrical Thioureas. Pestic. Sci. 1998, 52, 145–151. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, E.; Manzano, J.L.; Benito, J.J.; Hermosa, R.; Monte, E.; Criado, J.J. Thiourea, triazole and thiadiazine compounds and their metal complexes as antifungal agents. J. Inorg. Biochem. 2005, 99, 1558–1572. [Google Scholar] [CrossRef]
- Esteva-Font, C.; Phuan, P.-W.; Lee, S.; Su, T.; Anderson, M.O.; Verkman, A.S. Structure-activity analysis of thiourea analogs as inhibitors of UT-A and UT-B urea transporters. Biochim. Biophys. Acta 2015, 1848, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Shalini, S.; Kumar, K.S.; Rajak, H.; Agrawal, R.K. Design, Synthesis and Evaluation of Thiourea Derivatives as Antimicrobial and Antiviral Agents. Lett. Drug Des. Discov. 2019, 16, 618–624. [Google Scholar] [CrossRef]
- Chen, M.-H.; Chen, Z.; Song, B.-A.; Bhadury, P.S.; Yang, S.; Cai, X.-J.; Hu, D.-Y.; Xue, W.; Zeng, S. Synthesis and Antiviral Activities of Chiral Thiourea Derivatives Containing an α-Aminophosphonate Moiety. J. Agric. Food Chem. 2009, 57, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Karakus, S.; Koçyıgıt-Kaymakcioglu, B.; Toklu, H.Z.; Aricioglu, F.; Rollas, S. Synthesis and Anticonvulsant Activity of NewN-(Alkyl/Sub-stituted aryl)-N′-[4-(5-cyclohexylamino)-1,3,4-thiadiazole-2-yl)phenyl]tioureas. Arch. Pharm. Chem. Life Sci. 2009, 342, 48–53. [Google Scholar] [CrossRef]
- Mehrotra, S.; Roychowdhury, P.; Pandey, K.K.; Srivastava, P.K. Synthesis of 1-aryl-2-mercapto-4-aryl-1,6-dihydro-1,3,5-triazine-6-thione and their latentiation products as antithyroidal agent. Arch. Pharmacal Res. 1995, 18, 356. [Google Scholar] [CrossRef]
- Li, J.-Z.; Xue, S.-J.; Guo, Y.-L. Synthesis and Biological Activity of Oxidized Nicotinicacyl Thiourea Derivatives. Chin. J. Org. Chem. 2007, 27, 1278–1281. [Google Scholar]
- Moneer, A.A.; Mohammed, K.O.; El-Nassan, H.B. Synthesis of Novel Substituted Thiourea andBenzimidazole Derivatives Containing a PyrazoloneRing as Anti-Inflammatory Agents. Chem. Biol. Drug Des. 2016, 87, 784–793. [Google Scholar] [CrossRef]
- Mumtaz, A.; Majeed, A.; Zaib, S.; Rahman, S.U.; Hameed, S.; Saeed, A.; Rafique, H.; Mughal, E.; Maalik, A.; Hussain, I.; et al. Investigation of potent inhibitors of cholinesterase based on thiourea and pyrazoline derivatives: Synthesis, inhibition assay and molecular modeling studies. Bioorg. Chem. 2019, 90, 103036. [Google Scholar] [CrossRef]
- Begum, S.; Choudhary, M.I.; And Khan, K.M. Synthesis, phytotoxic, cytotoxic, acetylcholinesterase and butrylcholinesterase activities of N, N′-diaryl unsymmetrically substituted tioureas. Nat. Prod. Res. 2009, 23, 1719–1730. [Google Scholar] [CrossRef]
- Dixit, P.P.; Patil, V.J.; Nair, P.S.; Jain, S.; Sinha, N.; Arora, S.K. Synthesis of 1-[3-(4-benzotriazol-1/2-yl-3-fluoro-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-3-substituted-thiourea derivatives as antituberculosis agents. Eur. J. Med. Chem. 2006, 41, 423–428. [Google Scholar] [CrossRef]
- Liav, A.; Angala, S.K.; Brennan, P.J.; Jackson, M. N-α-AldopentofuranosylN′-[p-(isoamyloxy)phenyl]-thiourea derivatives: Potential anti-TB therapeutic agents. Bioorg. Med. Chem. Lett. 2008, 18, 2649–2651. [Google Scholar] [CrossRef] [PubMed]
- Ekoue-Kovi, K.; Yearick, K.; Iwaniuk, D.P.; Natarajan, J.K.; Alumasa, J.; de Dios, A.C.; Roepe, P.D.; Wolf, C. Synthesis and antimalarial activity of new 4-amino-7-chloroquinolyl amides, sulfonamides, ureas and tioureas. Bioorg. Med. Chem. 2009, 17, 270–283. [Google Scholar] [CrossRef]
- Sunduru, N.; Srivastava, K.; Rajakumar, S.; Puri, S.K.; Saxena, J.K.; Chauhan, P.M.S. Synthesis of novel thiourea, thiazolidinedione, and thioparabanic acid derivatives of 4-aminoquinoline as potent antimalarials. Bioorg. Med. Chem. Lett. 2009, 19, 2570–2573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Wu, G.; Zhou, J.; Huang, W.; Hu, X. Synthesis and biological evaluation of sulfonylurea and thiourea derivatives substituted with benzenesulfonamide groups as potential hypoglycemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 1740–1744. [Google Scholar] [CrossRef]
- Zhao, F.; Xiao, J.H.; Wang, Y.; Li, S. Synthesis of thiourea derivatives as CCR4 antagonists. Chin. Chem. Lett. 2009, 20, 296–299. [Google Scholar] [CrossRef]
- Esteves-Souza, A.; Pissinate, K.; Nascimento, M.G.; Grynberg, N.F.; Echevarria, A. Synthesis, cytotoxicity, and DNA-topoisomerase inhibitory activity of new asymmetric ureas and tioureas. Bioorg. Med. Chem. 2006, 14, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Harriet, M.B.; Bret, F.; Paul, B. Riluzole. A review of its pharmacodynamics and Pharmacokinetic Properties and Therapeutic Potential in Amyotrophic Lateral Sclerosis. Drugs 1996, 52, 549–563. [Google Scholar]
- Pittenger, C.; Coric, V.; Banasr, M.; Bloch, M.; Krystal, J.H.; Sanacora, G. Rluzole in the Treatment of Mood and Anxiety Disorders. CNS Drugs 2008, 22, 761–786. [Google Scholar] [CrossRef]
- Gupta, A.; Rawat, S. Synthesis and Cyclization of Benzothiazole: Review. J. Curr. Pharm. Res. 2010, 3, 13–23. [Google Scholar]
- Rana, A.; Siddiqui, N.S.; Khan, S.A. Benzothiazoles: A new profile of biological activities. Indian J. Pharm. Sci. 2007, 69, 10–17. [Google Scholar]
- Chaudhar, P.; Sharma, P.K.; Sharma, A.; Varshney, J. Recent Advances in Pharmacological Activity of Benzothiazole Derivatives. Int. J. Curr. Pharm. Res. 2010, 2, 5–11. [Google Scholar]
- Akhilesh, G.; Swati, R. Therapeutic Importance of Benzothiazole: Review. Asian J. Res. Chem. 2010, 3, 821–836. [Google Scholar]
- Yadav, P.S.; Devprakash, D.; Senthilkumar, G.P. Benzothiazole: Different Methods of Synthesis and Diverse Biological Activities. Int. J. Pharm. Sci. Drug Res. 2011, 3, 01–07. [Google Scholar]
- Victor, F.; Raisa, R.d.R.; Claudia, R.B.G.; Thatyana, R.A.V. Chemistry and Biological Activities of 1,3-Benzothiazoles. Mini-Rev. Org. Chem. 2012, 9, 44–53. [Google Scholar]
- Jaiswal, S.; Mishra, A.P.; Srivastava, A. The Different Kinds of Reaction Involved in Synthesis of 2-Substituted Benzothiazole and its Derivatives: A Review. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 631–641. [Google Scholar]
- Ali, R.; Siddiqui, N. Biological Aspects of Emerging Benzothiazoles: A Short Review. J. Chem. 2013, 2013, 345198. [Google Scholar] [CrossRef]
- Henary, M.; Paranjpe, S.; Owens, E.A. Substituted benzothiazoles: Synthesis and medicinal characteristics. Heterocycl. Commun. 2013, 19, 89–99. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sinhmar, A.; Sharma, A.; Rajak, H.; Pathak, D.P. Medicinal significance of benzothiazole scaffold: An insight view. J. Enzyme Inhib. Med. Chem. 2013, 28, 240–266. [Google Scholar] [CrossRef]
- Sompalle, R.; Roopan, S.M. Review on Benzothiazoles: Synthesis and Diverse Biological Activities. Chem. Sci. Rev. Lett. 2014, 2, 408–414. [Google Scholar]
- Xie, X.; Yan, Y.; Zhu, N.; Liu, G. Benzothiazoles exhibit broad-spectrum antitumor activity: Their potency, structure-activity and structure-metabolism relationship. Eur. J. Med. Chem. 2014, 76, 67–78. [Google Scholar] [CrossRef]
- Kamal, A.; Syed, M.-A.H.; Mohammed, S.M. Therapeutic potential of benzothiazoles: A patent review (2010–2014). Expert Opin. Ther. Pat. 2015, 25, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.N.; Karumudi, B.S.; Rao, N.R. Benzothiazole-Versatile heterocyclic nucleus in medicinal chemistry: A Review. Int. J. Pharm. Chem. 2015, 5, 104–114. [Google Scholar]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Seth, S. A Comprehensive Review on Recent advances in Synthesis & Pharmacotherapeutic potential of Benzothiazoles. Antiinflamm. Antiallergy Agents Med. Chem. 2015, 14, 98–112. [Google Scholar]
- Gill, R.K.; Rawal, R.K.; Bariwal, J. Recent advances in the chemistry and biology of benzothiazoles. Arch. Pharm. 2015, 348, 155–178. [Google Scholar] [CrossRef]
- Mene, D.; Kale, M. Exploration of Different Methodologies for Synthesizing Biologically Important Benzothiazoles: An Overview. Curr. Org. Synth. 2016, 13, 41–57. [Google Scholar] [CrossRef]
- Achaiah, G.; Sridhar Goud, S.N.; Praveen Kumar, K.; Mayuri, P. Review on 2-substituted benzothiazoles: Diversity of synthetic methods and biological activities. Int. J. Pharm. Sci. Res. 2016, 7, 1375–1382. [Google Scholar]
- Gulati, S.; Wakode, S.; Kaur, A.; Anand, K. Synthesis, biological activity and recent advancement of benzothiazoles: A classical Review. World J. Pharm. Pharm. Sci. 2017, 6, 1842–1869. [Google Scholar]
- Agarwal, S.; Gandhi, D.; Kalal, P. Benzothiazole: A Versatile and Multitarged Pharmacofore in the field of Medicinal Cehemistry. Lett. Org. Chem. 2017, 14, 724–729. [Google Scholar] [CrossRef]
- Banerjee, S.; Payra, S.; Saha, A. A Review on Synthesis of Benzothiazole Derivatives. Curr. Organocatal. 2017, 4, 164–181. [Google Scholar] [CrossRef]
- Shaista, A.; Amrita, P. Benzothiazole—A Magic Molecule. IJPSR 2017, 8, 4909–4929. [Google Scholar]
- Srivastava, A.; Mishra, A.P.; Chandra, S.; Bajpai, A. Benzothiazole derivative: A review on its pharmacological importance towards synthesis of lead. Int. J. Pharm. Sci. Res. 2019, 10, 1553–1566. [Google Scholar]
- Tariq, S.; Kanboj, P.; Amir, M. Therapeutic advancement of benzothiazole derivatives in the last decennial period. Arch. Pharm. 2019, 352, 1800170. [Google Scholar] [CrossRef]
- Abrol, S.; Bodla, R.B.; Goswami, C. A Comprehensive Review on Benzothiazole Derivatives for Their Biological Activities. Int. J. Pharm. Sci. Res. 2019, 10, 3196–3209. [Google Scholar]
- Liu, X.; Dong, Z.-B. A Review on Domino Condensation/Cyclization Reactions for the Synthesis of 2-Substituted 1,3-Benzothiazole Derivatives. Eur. J. Org. Chem. 2020, 2020, 408–419. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Zuo, X.; Feng, X.; Gao, Y. Recent Advances in Synthesis of Benzothiazole Compounds Related to Green Chemistry. Molecules 2020, 25, 1675. [Google Scholar] [CrossRef]
- Pathak, N.; Rathi, E.; Kumar, N.; Kini, S.G.; Rao, C.M. A Review on Anticancer Potentials of Benzothiazole Derivatives. Mini-Rev. Med. Chem. 2020, 20, 12–23. [Google Scholar] [CrossRef]
- Mahesh, B.; Shiddappa, B.L. Structural Activity Relationship and Importance of Benzothiazole Derivatives in Medicinal Chemistry: A Comprehensive Review. Mini-Rev. Org. Chem. 2020, 17, 323–350. [Google Scholar]
- Elgemeire, G.H.; Azzam, R.A.; Osman, R.R. Recent advances in synthesis, metal complexes and biological evaluation of 2-aryl, 2-pyridyl and 2-pyrimidylbenzothiazoles as potential chemotherapeutics. Inorg. Chim. Acta 2020, 502, 119302. [Google Scholar] [CrossRef]
- Irfana, A.; Batoola, F.; Naqvia, S.A.Z.; Islamb, A.; Osmanc, S.M.; Nocentinid, A.; Alissae, S.A.; Supuran, C.T. Benzothiazole derivatives as anticancer agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 265–279. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Bansal, K.K.; Rajak, H.; Sharma, S.; Thakur, V.K. New horizons in benzothiazole scaffold for cancer therapy: Advances in bioactivity, functionality, and chemistry. Appl. Mater. Today 2020, 20, 100783. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Elamin, M.B.; Elaziz, A.A.E.S.A.; Abdallah, E.M. Benzothiazole moieties and their derivatives as antimicrobial and antiviral agents: A mini-review. Int. J. Res. Pharm. Sci. 2020, 3, 3309–3315. [Google Scholar] [CrossRef]
- Law, C.S.W.; Yeong, K.Y. Current trends of benzothiazoles in drug discovery: A patent review (2015–2020). Expert Opin. Ther. Pat. 2022, 32, 299–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Y.; Zhang, Y.; Hu, L.; Han, S. Progress in Synthesis of 2-Substituted Benzothiazole Compounds. Chin. J. Org. Chem. 2021, 41, 1053–1071. [Google Scholar] [CrossRef]
- Dhadda, S.; Raigar, A.K.; Saini, K.; Manju, G.A. Benzothiazoles: From recent advances in green synthesis to anti-cancer potential. Sustain. Chem. Pharm. 2021, 24, 100521. [Google Scholar] [CrossRef]
- Sumit; Kumar, A.; Mishra, A.K. Advancement in Pharmacological Activities of Benzothiazole and its Derivatives: An Up to Date Review. Mini-Rev. Med. Chem. 2021, 21, 314–335. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Sahiba, N.; Sethiya, A.; Soni, J.; Teli, P.; Agarwal, S.; Goyal, P.K. Insight View on Synthetic Strategies and Biological Applications of Pyrimidobenzothiazoles. Mini-Rev. Org. Chem. 2021, 18, 1012–1025. [Google Scholar] [CrossRef]
- Mohammad, A.; Mohd, I. A Mini-Review on Pharmacological Importance of Benzothiazole Scaffold. Mini-Rev. Org. Chem. 2021, 18, 1086–1097. [Google Scholar]
- Thube, U.S.; Pawar, P.Y.; Sawant, R.L. Synthesis and Antidiabetic Evaluation of Some 2-substituted Benzothiazole Derivatives. Eur. J. Mol. Clin. Med. 2021, 8, 2343–2350. [Google Scholar]
- Bhagdev, K.; Sarkar, S. Benzothiazole: As an Antidiabetic Agent. Ann. Rom. Soc. Cell Biol. 2021, 25, 20269–20285. [Google Scholar]
- Larisa, V.; Zhilitskaya, L.V.; Yarosh, N.O. Synthesis of biologically active derivatives of 2-aminobenzothiazole. Chem. Heterocycl. Comp. 2021, 57, 369–373. [Google Scholar]
- Bhoge, N.D.; Mohite, P.B.; Deshmukh, V.K.; Magare, B.K. A Comprehensive Review on Synthetic Strategy of Benzothiazole Lead and Pharmacological Importance. Indian J. Pharm. Drug Stud. 2021, 2, 15–19. [Google Scholar]
- García-Báez, E.V.; Padilla-Martínez, I.I.; Tamay-Cach, F.; Cruz, A. Benzothiazoles from Condensation of o-Aminothiophenoles with Carboxylic Acids and Their Derivatives: A Review. Molecules 2021, 26, 6518. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Shrivstava, N.; Pathak, A.; Dewangan, R.P.; Yahya, S.; Yar, S. Recent advances and SAR study of 2-substituted benzothiazole scaffold based potent chemotherapeutic agents. Results Chem. 2022, 4, 100258. [Google Scholar] [CrossRef]
- Chanchal, S.; Rajnish, K.; Avijit, M.; Salahuddin, A.K.; Rakesh, S.; Shivali, M.; Mohd, A.M. Benzothiazole: Synthetic Strategies, Biological Potential, and Interactions with Targets. Mini-Rev. Org. Chem. 2022, 19, 242–256. [Google Scholar]
- Asiri, Y.I.; Alsayari, A.; Muhsinah, A.B.; Mabkhotc, Y.N.; Hassan, M.Z. Benzothiazoles as potential antiviral agents. J. Pharm. Pharmacol. 2020, 72, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Bhagdev, K.; Sarkar, S. Benzothiazole: As an Antiviral Agent. Med. Sci. Forum 2021, 7, 9. [Google Scholar] [CrossRef]
- Abdel-Rahman, H.M.; Morsy, M.A. Novel benzothiazolyl urea and thiourea derivatives with potential cytotoxic and antimicrobial activities. J. Enzyme Inhib. Med. Chem. 2007, 22, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lind, P.T.; Morin, J.M., Jr.; Noreen, R.; Ternansky, R.J. Thiourea Derivatives and Methods for Inhibition of HIV and Related Viruses. WO Patent 9303022, 18 February 1993. [Google Scholar]
- Kumbhare, R.M.; Dadmal, T.; Kosurkara, U.; Sridhar, V.; Rao, J.V. Synthesis and cytotoxic evaluation of thiourea and N-bis-benzothiazolederivatives: A novel class of cytotoxic agents. Bioorg. Med. Chem. Lett. 2012, 22, 453–455. [Google Scholar] [CrossRef]
- Hartley, D.; Kidd, H. The Agrochemical Handbook; The Royal Society of Chemistry: Nottingham, UK, 1987. [Google Scholar]
- Wegler, R.; Eue, L. Chemie der Pflanzenschutz- und Schädlingsbekämp- fungsmittel. In Herbizide; Springer: Berlin, Germany, 1977; Volume 5. [Google Scholar]
- Ondrej, B.; Lukas, H.; Laura, A.; Rafael, D.; Patrick, G.; Marketa, B.; Ondrej, S.; Karel, M.; Kamil, K.; Terry, S.; et al. 6-Benzothiazolyl Ureas, Thioureas and Guanidines are Potent Inhibitors of ABAD/17&:β-HSD10 and Potential Drugs for Alzheimer’s Disease Treatment: Design, Synthesis and in vitro Evaluation. J. Med. Chem. 2017, 13, 345–358. [Google Scholar]
- Hroch, L.; Benek, O.; Guest, P.; Aitken, L.; Soukup, O.; Janockova, J.; Musil, K.; Dohnal, V.; Dolezal, R.; Kuca, K.; et al. Design, synthesis and in vitro evaluation of benzothiazole-based ureas as potential ABAD/17β-HSD10 modulators for Alzheimer’s disease treatment. Bioorg. Med. Chem. Lett. 2016, 26, 3675–3678. [Google Scholar] [CrossRef]
- Aitken, L.; Benek, O.; McKelvie, B.E.; Hughes, R.E.; Hroch, L.; Schmidt, M.; Major, L.L.; Vinklarova, L.; Kamil Kuca, K.; Smith, T.K.; et al. Novel Benzothiazole-Based Ureas as 17β-HSD10 Inhibitors, A Potential Alzheimer’s Disease Treatment. Molecules 2019, 24, 2757. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Benek, O.; Vinklarova, L.; Hrabinova, M.; Zemanova, L.; Chribek, M.; Kralova, V.; Hroch, L.; Dolezal, R.; Lycka, A.; et al. Benzothiazolyl Ureas are Low Micromolar and Uncompetitive Inhibitors of 17β-HSD10 with Implications to Alzheimer’s Disease Treatment. Int. J. Mol. Sci. 2020, 21, 2059. [Google Scholar] [CrossRef] [PubMed]
- Lukas, H.; Patrick, G.; Ondrej, B.; Ondrej, S.; Jana, J.; Rafael, D.; Kamil, K.; Laura, A.; Terry, K.S.; Frank, G.-M.; et al. Synthesis and evaluation of frentizole-based indolyl thiourea analogues as MAO/ABAD inhibitors for Alzheimer’s disease treatment. Biorg. Med. Chem. 2017, 25, 1143–1152. [Google Scholar]
- Rosales-Hernández, M.C.; Mendieta-Wejebe, J.E.; Padilla-Martínez, I.I.; García-Báez, E.V.; Cruz, A. Synthesis and Biological Importance of 2-(thio)ureabenzothiazoles. Molecules 2022, 27, 6104. [Google Scholar] [CrossRef]
- Sutoris, V.; Algas, J. Benzothiazole compounds. III. Synthesis and biological activity of substituted N-(2-benzothiazolyl)ureas and benzothiazolyldihydrouracils. Chem. Zvesti (Chem. Pap.) 1973, 27, 829–833. [Google Scholar]
- Youssef, K.M.; Al-Abbdullah, E.; El-Khamees, H. Synthesis of sulofenur analoges as antitumour agents: Part II. Med. Chem. Res. 2002, 11, 481–503. [Google Scholar]
- Pareek, D.; Chaudhary, M.; Pareek, P.K.; Kantc, R.; Ojha, K.G.; Iraqi, S.M.U.; Pareek, A. Synthesis of some biologically important 2-thiobarbituric acid derivatives incorporating benzothiazole moiety. Der Pharm. Lett. 2010, 2, 274–283. [Google Scholar]
- LoMonte, F.; Kramer, T.; Boländer, A.; Plotkin, B.; Eldar-Finkelman, H.; Fuertes, A.; Dominguez, J.; Schmidt, B. Synthesis and biological evaluation of glycogen synthase kinase 3 (GSK-3) inhibitors: An fast and atom efficient access to 1-aryl-3-benzylureas. Bioorg. Med. Chem. Lett. 2011, 21, 5610–5615. [Google Scholar]
- Spadaro, A.; Negri, M.; Marchais-Oberwinkler, S.; Bey, N.; Frotscher, M. Hydroxybenzothiazoles as New Nonsteroidal Inhibitors of 17β-Hydroxysteroid Dehydrogenase Type 1 (17β-HSD1). PLoS ONE 2012, 7, e29252. [Google Scholar] [CrossRef] [PubMed]
- Axford, L.C.; Anderson, K.H.; Andrau, L.N.; Atherall, J.; Barker, S.; Bennett, J.M.; Blair, M.; Collins, I.; Czaplewski, L.G.; Davies, D.T.; et al. Design, synthesis and biological evaluation of α-substituted isonipecotic acid benzothiazole analogues as potent bacterial type II topoisomerase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 6598–6603. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.T.; Axford, L.C.; Barker, S.; Bennett, J.M.; Blair, M.; Collins, I.; Davies, D.T.; Ford, L.; Gannon, C.T.; Lancett, P.; et al. Discovery and in vivo evaluation of alcohol-containing benzothiazoles as potent dual-targeting bacterial DNA supercoiling inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 4215–4222. [Google Scholar] [CrossRef] [PubMed]
- Wagh, Y.B.; Kuwar, A.; Sahoo, S.K.; Galluccic, J.; Dalal, D.S. Highly selective fluorimetric sensor for Cu2+ and Hg2+ using a benzothiazole-based receptor in semi-aqueous media and molecular docking studies. RSC Adv. 2015, 5, 45528–45534. [Google Scholar] [CrossRef]
- Xie, X.-X.; Li, H.; Wang, J.; Mao, S.; Xin, M.-H.; Lu, S.-M.; Zhang, S.-Q. Synthesis and anticancer effects evaluation of 1-alkyl-3-(6-(2-methoxy-3-sulfonylaminopyridin-5-yl)benzo[d]thiazol-2-yl)urea as anticancer agents with low toxicity. Bioorg. Med. Chem. 2015, 23, 6477–6485. [Google Scholar] [CrossRef]
- Bielenica, A.; Sanna, G.; Madeddu, S.; Struga, M.; Jóźwiak, M.; Anna, E.; Kozioł, A.E.; Sawczenko, A.; Materek, I.B.; Serra, A.; et al. New Thiourea and 1,3-thiazolidin-4-one Derivatives Effective on the HIV-1Virus. Chem. Biol. Drug Des. 2017, 90, 883–891. [Google Scholar] [CrossRef]
- Tomašič, T.; Barančoková, M.; Zidar, N.; Ilaš, J.; Tammela, P.; Kikelj, D. Design, synthesis, and biological evaluation of 1-ethyl-3-(thiazol-2-yl)urea derivatives as Escherichia coliDNA gyrase inhibitors. Arch. Pharm. Chem. Life Sci. 2018, 351, e1700333. [Google Scholar] [CrossRef]
- Eshkil, F.; Eshghi, H.; Saljooghi, A.S.; Bakavoli, M. Synthesis of New Benzothiazole Derivatives and Evaluation of Cytotoxicity of N-(6-Substitued-1,3-benzothiazol-2-yl)-4-phenyl-1,3-thiazol-2(3H)-imine Compounds. Org. Chem. Res. 2019, 5, 87–94. [Google Scholar]
- Eshkil, F.; Eshghi, H.; Saljooghi, A.S.; Bakavoli, M.; Rahimizadeh, M. Benzothiazole Thiourea Derivatives as Anticancer Agents: Design, Synthesis, and Biological Screening. Russ. J. Bioorg. Chem. 2017, 43, 576–582. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D.; Jit, L.R.J.S.; Sathiyanesan Sathish Kumar, S.S.; James, P.; Stables, J.P. Anticonvulsant and neurotoxicity evaluation of some 6-chlorobenzothiazolyl-2-thiosemicarbazones. Eur. J. Med. Chem. 2002, 37, 231–236. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D.; Mehta, S.; Nigam, D.; Kumar, M.M.; Murugesan, S.; Stables, J.P. Anticonvulsant and neurotoxicity evaluation of some 6-substituted benzothiazolyl-2-thiosemicarbazones. Il. Farm. 2005, 60, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Bhat, M.A.; Haque, S.E. Synthesis of benzothiazole semicarbazones as novel anticonvulsants–the role of hydrophobic domain. Bioorg. Med. Chem. Lett. 2007, 17, 4178–4182. [Google Scholar] [CrossRef]
- Mahran, M.A.; William, S.; Ramzy, F.; Sembel, A.M. Synthesis and in vitro Evaluation of New Benzothiazole Derivatives as Schistosomicidal Agents. Molecules 2007, 12, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Haque, S.E.; Alam, M.S.; Ahsan, W.; Arshad, M.F. Anticonvulsant and toxicity evaluation of newly synthesized 1- [2-(3,4-disubstituted phenyl)-3-chloro-4-oxoazetidin-1-yl]-3-(6-substituted-1,3-benzothiazol-2-yl)ureas. Acta Chim. Slov. 2009, 56, 462–469. [Google Scholar]
- Pandurangan, A.; Sharma, A.; Sharma, N.; Sharma, P.K.; Visht, S. Synthesis and structural studies of novel benzothiazole derivative and evaluation of their antimicrobial activity. Der Pharma Chem. 2010, 2, 316–324. [Google Scholar]
- Sekar, V.; Perumal, P.; Gandhimathi, S. Screening of anticancer activity in newly synthesized benzothiazole derivatives. J. Pharm. Sci. Res. 2011, 3, 1520–1524. [Google Scholar]
- Gilani, S.J.; Khan, S.A.; Siddiqui, N.; Verma, S.P.; Mullick, P.; Alam, O. Synthesis and in vitro antimicrobial activity of novel N-(6-chlorobenzo[d]thiazol-2-yl) hydrazine carboxamide derivatives of benzothiazole class. J. Enzyme Inhib. Med. Chem. 2011, 26, 332–340. [Google Scholar] [CrossRef]
- Amir, M.; Asif, S.; Ali, I.; Hassan, M.Z. Synthesis of benzothiazole derivatives having acetamido and carbothioamido pharmacophore as anticonvulsant agents. Med. Chem. Res. 2012, 21, 2661–2670. [Google Scholar] [CrossRef]
- Tripathi, R.K.P.; Goshain, O.; Ayyannan, S.R. Design, Synthesis, in vitro MAO-B Inhibitory Evaluation and Computational Studies of Some 6-Nitrobenzothiazole-Derived Semicarbazones. Chem. Med. Chem. 2013, 8, 462–474. [Google Scholar] [CrossRef]
- Tripathi, R.K.P.; Ayyannan, S.R. Anticonvulsant activity, organotypic hippocampal neuroprotection assay and in-silico sodium channel blocking potential of 2-amino-6-nitrobenzothiazole derived semicarbazones. Biomed. Pharmacother. 2017, 95, 1451–1460. [Google Scholar] [CrossRef]
- Sarkar, S.; Dwivedi, J.; Chauhan, R. Synthesis of 1-[2 (substituted phenyl)-4-oxothiazolidin-3-yl]-3-(6-fluro-7-chloro-1,3-benzothiazol-2-yl)-ureas as anthelmintic agent. J. Pharm. Res. 2013, 7, 439–442. [Google Scholar] [CrossRef]
- Chen, J.; Ma, D.; Lu, K.; Wang, L.; Han, X.; Zhao, Y.; Gong, P. Design, synthesis and structure activity relationships of novel benzothiazole derivatives bearing the ortho-hydroxy N-carbamoylhydrazone moiety as potent antitumor agents. Eur. J. Med. Chem. 2014, 86, 257–269. [Google Scholar]
- Zhang, J.; Ma, G.; Han, X.; Bao, G.; Wang, L.; Zhai, X.; Gong, P. Synthesis and biological evaluation of benzothiazole derivatives bearing the ortho-hydroxy-N-acylhydrazone moiety as potent antitumor agents. Arch. Pharm. Chem. Life Sci. 2014, 347, 936–949. [Google Scholar]
- Ma, J.; Bao, G.; Wang, L.; Li, W.; Xu, B.; Du, B.; Lv, J.; Zhai, X.; Gong, P. Design, synthesis, biological evaluation and preliminary mechanism study of novel benzothiazole derivatives bearing indole-based moiety as potent antitumor agents. Eur. J. Med. Chem. 2015, 96, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Hu, G.; Xie, L.; Chen, L.; Xu, B.; Gong, P. Design, synthesis and biological evaluation of novel benzothiazole derivatives bearing semicarbazone moiety as antitumor agents. Chem. Res. Chin. Univ. 2015, 31, 958–963. [Google Scholar] [CrossRef]
- Gilani, S.J.; Nagarajan, K.; Dixit, S.P.; Taleuzzaman, M.; Khan, S.A. Benzothiazole incorporated thiazolidin-4-ones and azetidin-2-ones derivatives: Synthesis and in vitro antimicrobial evaluation. Arabian J. Chem. 2016, 9, S1523–S1531. [Google Scholar] [CrossRef]
- Gilani, S.J.; Hassan, M.Z.; Imam, S.S.; Kala, C.; Dixit, S.P. Novel benzothiazole hydrazine carboxamide hybrid scaffolds as potential in vitro GABA AT enzyme inhibitors: Synthesis, molecular docking and antiepileptic evaluation. Bioorg. Med. Chem. Lett. 2019, 29, 1825–1830. [Google Scholar] [CrossRef]
- Ma, J.; Ni, X.; Gao, Y.; Huang, K.; Liu, J.; Wang, Y.; Chen, R.; Wang, C. Identification and biological evaluation of novel benzothiazole derivatives bearing a pyridine-semicarbazone moiety as apoptosis inducers via activation of procaspase-3 to caspase-3. Med. Chem. Commun. 2019, 10, 465–477. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, C.; Li, S.; Hu, D.; Song, B. Discovery of novel bis-sulfoxide derivatives bearing acylhydrazone and benzothiazole moieties as potential antibacterial agents. Pestic. Biochem. Physiol. 2020, 167, 104605. [Google Scholar] [CrossRef]
- Saeed, A.; Rafique, H.; Hameed, A.; Rasheed, S. Synthesis and Antibacterial Activity of Some New 1-aroyl-3-(substituted-2-benzothiazolyl)tioureas. Pharm. Chem. J. 2008, 42, 191–195. [Google Scholar] [CrossRef]
- Rana, A.; Siddiqui, N.; Khan, S.A.; Ehtaishamul, H.S.; Bhat, M.A. N-{[(6-substituted-1,3-benzothiazole-2-yl)amino]carbonothioyl}-2/4-substituted benzamides: Synthesis and pharmacological evaluation. Eur. J. Med. Chem. 2008, 43, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- Yunus, U.; Tahir, M.K.; Moazzam Bhatti, M.H.; Ali, S.; Wong, W.-Y. 1-(1,3-Benzothiazol-2-yl)-3-benzoylthiourea. Acta Cryst. 2008, E64, o20. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Rashid, N.; Hussain, R.; Jones, P.G. 1-(Benzothiazol-2-yl)-3-(4-nitrobenzoyl)- tiourea. Acta Cryst. 2009, E65, o2106. [Google Scholar]
- Siddiqui, N.; Rana, A.; Khan, S.A.; Syed Haque, S.E.; Alam, M.S.; Ahsan, W.; Ahmed, S. Synthesis of 8-substituted-4-(2/4-substituted phenyl)- 2H-[1,3,5]triazino[2,1-b][1,3]benzothiazole-2-thiones and their anticonvulsant, anti-nociceptive, and toxicity evaluation in mice. J. Enzyme Inhib. Med. Chem. 2009, 24, 1344–1350. [Google Scholar] [CrossRef]
- Modi, J.A.; Patel, K.C. Design, synthesis of some 2,6,9-trisubstituted purinyl thioureido derivatives and evaluation of antimicrobial activity. Med. Chem. Res. 2012, 21, 1660–1664. [Google Scholar] [CrossRef]
- Malik, S.; Bahare, R.D.; Khan, S.A. Design, synthesis and anticonvulsant evaluation of N-(benzo[d]thiazol-2-ylcarbamoyl)-2-methyl-4-oxoquinazoline-3(4H)-carbothioamide deri-vatives: A hybrid pharmacophore approach. Eur. J. Med. Chem. 2013, 67, 1–13. [Google Scholar] [CrossRef]
- Odame, F.; Woodcock, G.; Hosten, E.C.; Lobb, K.; Tshentu, Z.N. A novel gold(I)-mediated intramolecular transamidation of benzoyl thiourea derivatives to form benzamides via dethiocyanation. J. Organomet. Chem. 2020, 922, 121359. [Google Scholar] [CrossRef]
- Bhargava, P.N.; Lakhan, R. Synthesis of Benzothiazolylguanidines as Antituberculars and Antibacterials. Agric. Biol. Chem. 1968, 32, 1392–1394. [Google Scholar] [CrossRef]
- Bhargava, P.N.; Singh, H. New Compounds. Synthesis of Some New N-o-Tolyl-N’-2-(substituted) Benzothiazolylguanidines. J. Med. Chem. 1969, 12, 558–559. [Google Scholar] [CrossRef]
- Bhargava, P.N.; Choubey, V.N. Benzothiazolyl Guanidines as Antibacterials. Agric. Biol. Chem. 1970, 34, 644–647. [Google Scholar] [CrossRef]
- Bhargava, P.N.; Singh, S.N. Substituted Benzothiazolylguanidines. Curr. Sci. 1971, 40, 430–432. [Google Scholar]
- Lakhan, R.; Sharma, B.P.; Shukla, B.N. Synthesis and antimicrobial activity of 1-aryl-2-amino-3-(4-arylthiazol-2-yl)/(benzothiazol-2-yl)-guanidines. Il. Farm. 2000, 55, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, P.; Tiwari, V.S. Design and synthesis of Quinazolinone, Benzothiazole derivatives bearing guanidinopropanoic acid moiety and their Schiff bases as cytotoxic and antimicrobial agents. Arabian J. Chem. 2016, 9, S914–S925. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Cao, L.-H. Synthesis and bioactivity of novel methyl 6-deoxy-6-(N’-alkyl/aryl-N’’-benzothiazol-2-yl)-guanidino-α-D-glucopyranosides. Carbohydr. Res. 2008, 343, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Anzini, M.; Chelini, A.; Mancini, A.; Cappelli, A.; Frosini, M.; Ricci, L.; Valoti, M.; Magistretti, J.; Castelli, L.; Giordani, A.; et al. Synthesis and biological evaluation of amidine, guanidine, and thiourea derivatives of 2-amino(6-trifluoromethoxy)-benzothiazole as neuroprotective agents potentially useful in brain diseases. J. Med. Chem. 2010, 53, 734–744. [Google Scholar] [CrossRef]


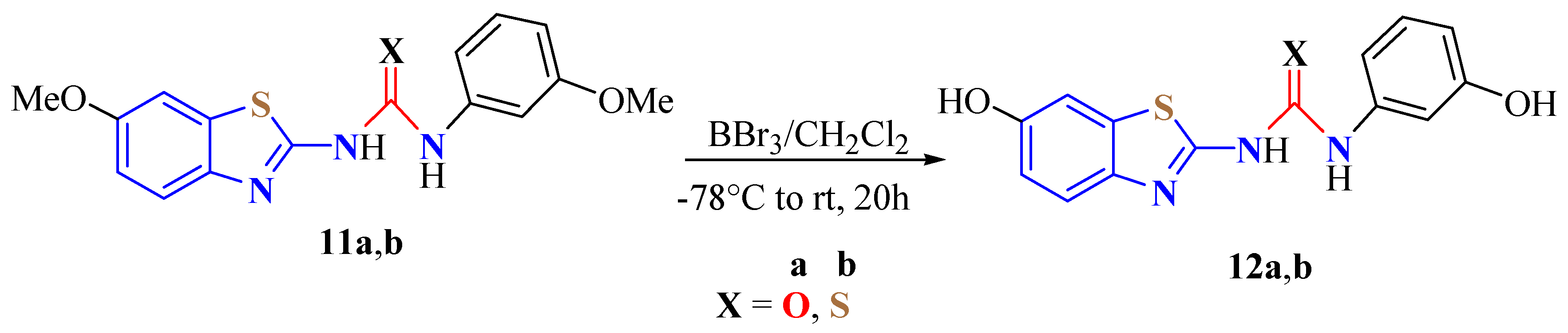













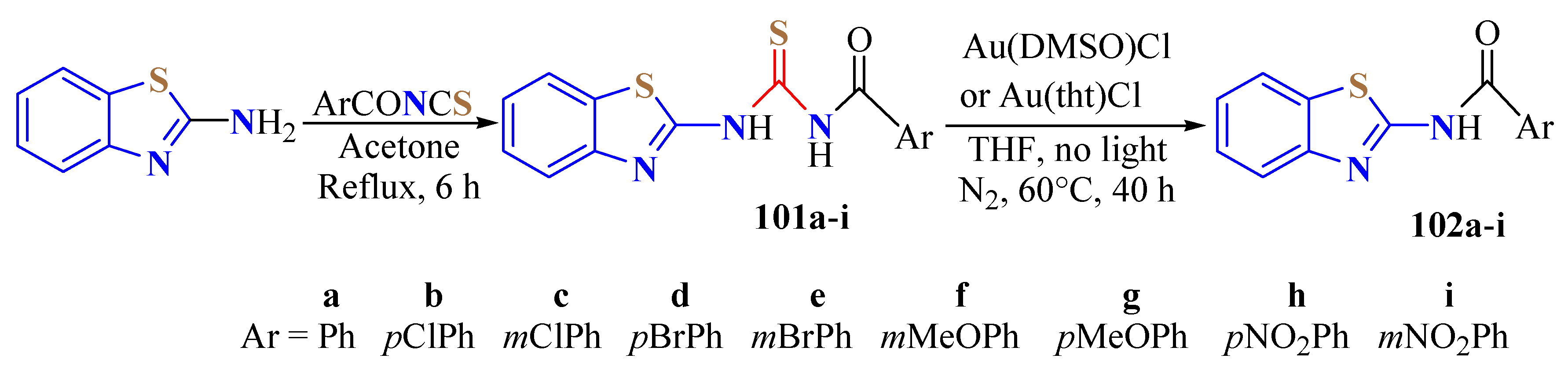
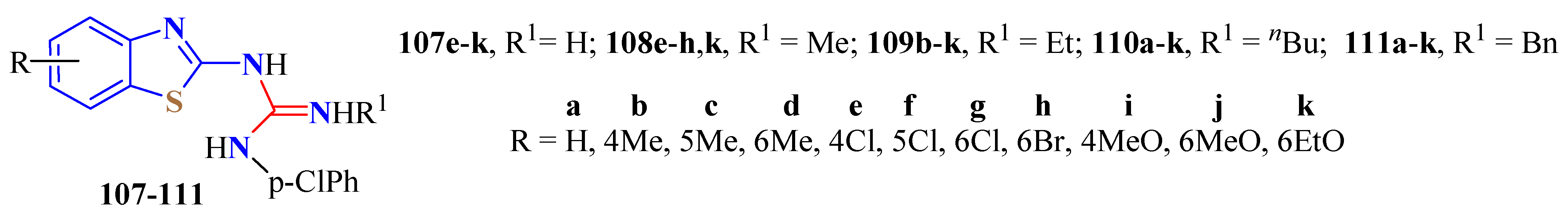
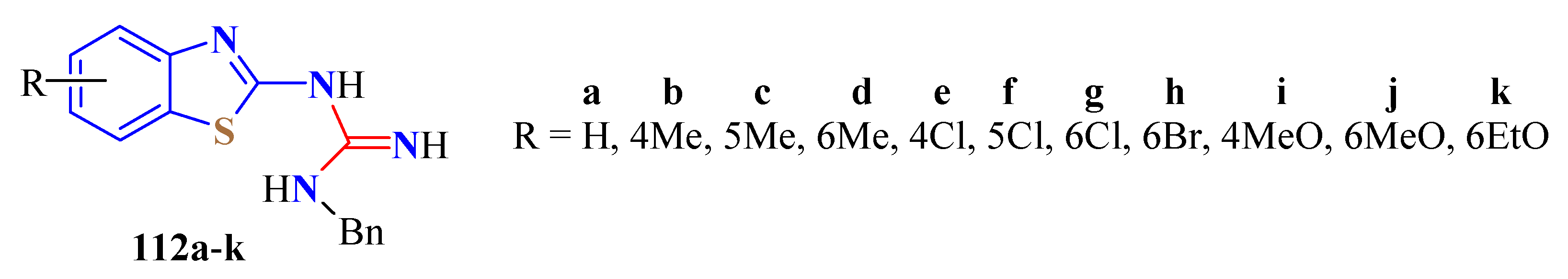
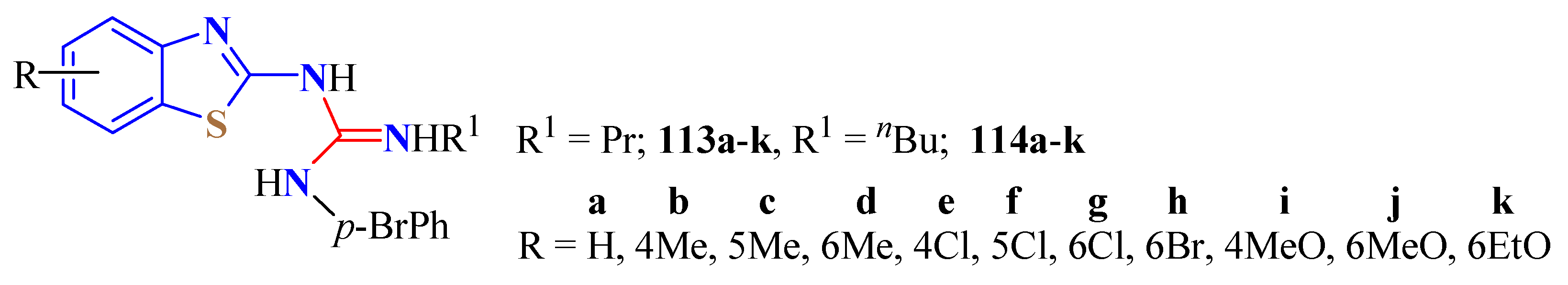

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendieta-Wejebe, J.E.; Rosales-Hernández, M.C.; Padilla-Martínez, I.I.; García-Báez, E.V.; Cruz, A. Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives. Int. J. Mol. Sci. 2023, 24, 9488. https://doi.org/10.3390/ijms24119488
Mendieta-Wejebe JE, Rosales-Hernández MC, Padilla-Martínez II, García-Báez EV, Cruz A. Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives. International Journal of Molecular Sciences. 2023; 24(11):9488. https://doi.org/10.3390/ijms24119488
Chicago/Turabian StyleMendieta-Wejebe, Jessica E., Martha C. Rosales-Hernández, Itzia I. Padilla-Martínez, Efrén V. García-Báez, and Alejandro Cruz. 2023. "Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives" International Journal of Molecular Sciences 24, no. 11: 9488. https://doi.org/10.3390/ijms24119488
APA StyleMendieta-Wejebe, J. E., Rosales-Hernández, M. C., Padilla-Martínez, I. I., García-Báez, E. V., & Cruz, A. (2023). Design, Synthesis and Biological Activities of (Thio)Urea Benzothiazole Derivatives. International Journal of Molecular Sciences, 24(11), 9488. https://doi.org/10.3390/ijms24119488






