Opportunities and Challenges of Kava in Lung Cancer Prevention
Abstract
:1. Introduction—Urgency of Lung Cancer Prevention
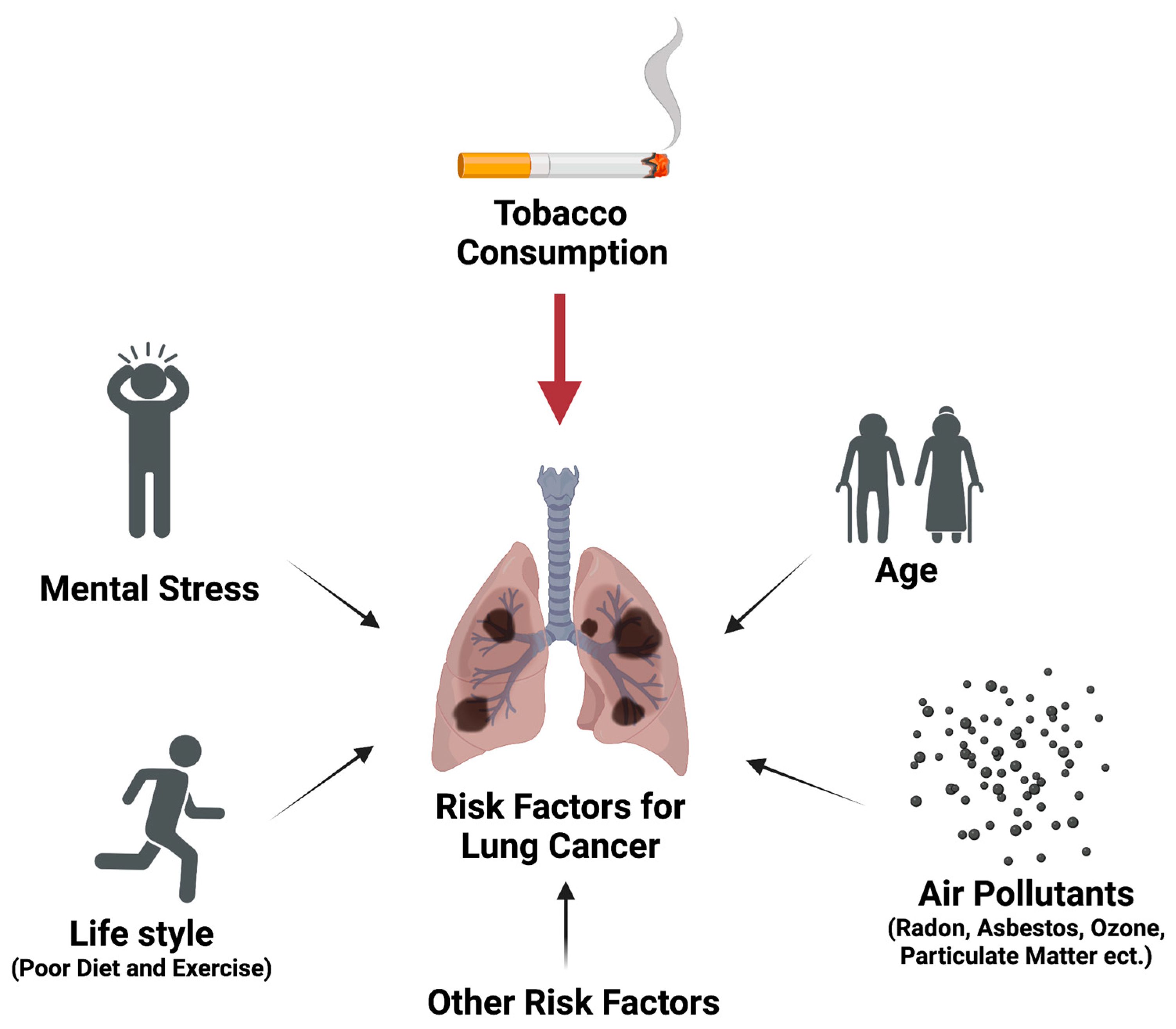
2. Different Risk Factors Contributing to Lung Carcinogenesis via Multiple Mechanisms
3. Lung Tumorigenesis Animal Models, Advantages, and Limitations, Particularly with Respect to Physiological Relevance for Clinical Translation
3.1. High-Dose Tobacco Carcinogen (NNK or BaP)-Induced Lung Tumorigenesis A/J Mouse Models
3.2. Chronic Low-Dose NNK in Drinking Water Induced Lung Tumorigenesis Model
3.3. A Chronic Tobacco Smoke-Exposure Induced Lung Tumorigenesis A/J Mouse Model
4. Kava’s Potential, Mechanisms, and Challenges in Cancer Risk Reduction, Particularly Lung Cancer
4.1. Knowledge about Kava, Its Traditional Use and Potential Benefits
4.2. Epidemiological Data Supporting Kava in Cancer Risk Reduction
4.3. Kava’s Potential in Cancer Risk Reduction in Animal Models, Responsible Ingredients, and Mechanisms
4.4. Potential Risks Associated with Kava Use, Particularly in the Chronic Use
5. Strategies and Opportunities for Future Kava Translational Development in Reducing Lung Cancer Risk
5.1. Rationale to Evaluate Kava Instead of Any Single-Chemical Entity in Kava
5.2. Evaluating Kava’s Preventive Potential in a Clinically More Relevant Lung Carcinogenesis Animal Models and Developing Mechanism-Based Non-Invasive Clinically Translatable Biomarkers
5.3. Identification and Intervention for Individuals with Higher Lung Cancer Risk and Timely Efficacy Monitoring for Precision Prevention and Interception
5.4. Rigorous Quality Control and Quality Assurance of Kava Product
5.5. Safety of Chronic Kava Use
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OH-Gua | 8-hydroxyguanine |
| ADC | Adenocarcinoma |
| AM | Alveolar macrophage |
| APAP | Potentiate acetaminophen |
| β-AR | β-Adrenergic receptor |
| cAMP | Cyclic adenosine monophosphate |
| COPD | Chronic obstructive pulmonary disease |
| COX-2 | Cyclooxygenase-2 |
| CTC | Circulating tumor cell |
| CtDNA | Cell free circulating tumor DNA |
| CYP | Cytochrome P450 enzyme |
| DHK | Dihydrokavain |
| DHM | Dihydromethysticin |
| DMY | Desmethoxyyangonin |
| EGCG | Epigallocatechin gallate |
| FKA | Flavokavain A |
| FKB | Flavokavain B |
| GCR | Glucocorticoid receptor resistance |
| GEMM | Genetically engineered mouse models |
| HPA | Hypothalamic-pituitary-adrenocortical axis |
| i.p. | Intraperitoneal |
| IPA | Ingenuity pathway analysis |
| LCC | Large cell carcinoma |
| LDCT | Low-dose CT |
| LPS | Lipopolysaccharide |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer cell |
| NLST | The National Lung Screening Trial |
| NNK | Nicotine-derived nitrosamine ketone |
| NSCLC | Non-small-cell lung cancer |
| OH-BBN | Hydroxy butyl(butyl) nitrosamine |
| PAH | Polycyclic aromatic hydrocarbons |
| PEITC | Phenethyl isothiocyanate |
| PGE2 | Prostaglandin-E2 |
| PKA | Protein kinase A |
| ROS | Reactive oxygen species |
| SCC | Squamous cell carcinoma |
| SCLC | Small cell lung cancer |
| SOX2 | SRY-box 2 |
| TEP | Tumor-educated platelet |
| UGT | UDP-Glucuronosyltransferases |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Saji, H. A worldwide trend of increasing primary adenocarcinoma of the lung. Surg. Today 2014, 44, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Devesa, S.S.; Bray, F.; Vizcaino, A.P.; Parkin, D.M. International lung cancer trends by histologic type: Male:female differences diminishing and adenocarcinoma rates rising. Int. J. Cancer 2005, 117, 294–299. [Google Scholar] [CrossRef]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef]
- Saltos, A.; Shafique, M.; Chiappori, A. Update on the Biology, Management, and Treatment of Small Cell Lung Cancer (SCLC). Front. Oncol. 2020, 10, 1074. [Google Scholar] [CrossRef]
- Zang, E.A.; Wynder, E.L. Differences in lung cancer risk between men and women: Examination of the evidence. J. Natl. Cancer Inst. 1996, 88, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.I.; McKinley, M.; Cheng, I.; Haile, R.; Wakelee, H.; Gomez, S.L. Lung cancer incidence trends in California by race/ethnicity, histology, sex, and neighborhood socioeconomic status: An analysis spanning 28 years. Lung Cancer 2017, 108, 140–149. [Google Scholar] [CrossRef]
- Youlden, D.R.; Cramb, S.M.; Baade, P.D. The International Epidemiology of Lung Cancer: Geographical distribution and secular trends. J. Thorac. Oncol. 2008, 3, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Shah, R.; Sabanathan, S.; Richardson, J.; Mearns, A.J.; Goulden, C. Results of surgical treatment of stage I and II lung cancer. J. Cardiovasc. Surg. 1996, 37, 169–172. [Google Scholar]
- Inage, T.; Nakajima, T.; Yoshino, I.; Yasufuku, K. Early Lung Cancer Detection. Clin. Chest Med. 2018, 39, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.R.; Abtin, F.; Brown, K. Computed tomography screening for lung cancer: Has it finally arrived? Implications of the national lung screening trial. J. Clin. Oncol. 2013, 31, 1002–1008. [Google Scholar] [CrossRef]
- Santarpia, M.; Karachaliou, N.; González-Cao, M.; Altavilla, G.; Giovannetti, E.; Rosell, R. Feasibility of cell-free circulating tumor DNA testing for lung cancer. Biomark. Med. 2016, 10, 417–430. [Google Scholar] [CrossRef]
- Krebs, M.G.; Metcalf, R.L.; Carter, L.; Brady, G.; Blackhall, F.H.; Dive, C. Molecular analysis of circulating tumour cells—Biology and biomarkers. Nat. Rev. Clin. Oncol. 2014, 11, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Birzele, F.; Kollmorgen, G.; Rüger, R. The Multiple Roles of Exosomes in Metastasis. Cancer Genom. Proteom. 2017, 14, 1–15. [Google Scholar] [CrossRef]
- Joosse, S.A.; Pantel, K. Tumor-Educated Platelets as Liquid Biopsy in Cancer Patients. Cancer Cell 2015, 28, 552–554. [Google Scholar] [CrossRef]
- Santarpia, M.; Liguori, A.; D’Aveni, A.; Karachaliou, N.; Gonzalez-Cao, M.; Daffinà, M.G.; Lazzari, C.; Altavilla, G.; Rosell, R. Liquid biopsy for lung cancer early detection. J. Thorac. Dis. 2018, 10, S882–S897. [Google Scholar] [CrossRef]
- Stone, E.; Vachani, A. Tobacco Control and Tobacco Cessation in Lung Cancer-Too Little, Too Late? Semin. Respir. Crit. Care Med. 2016, 37, 649–658. [Google Scholar] [CrossRef]
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Tobacco smoke and involuntary smoking. In IARC Monographs on the Evaluation of Carcinogenic Risks to Human; RARC; International Agency for Research on Cancer: Geneva, Switzerland, 2004; pp. 53–119. [Google Scholar]
- de Groot, P.; Munden, R.F. Lung cancer epidemiology, risk factors, and prevention. Radiol. Clin. N. Am. 2012, 50, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Gallaway, M.S.; Henley, S.J.; Steele, C.B.; Momin, B.; Thomas, C.C.; Jamal, A.; Trivers, K.F.; Singh, S.D.; Stewart, S.L. Surveillance for Cancers Associated with Tobacco Use—United States, 2010–2014. MMWR Surveill. Summ. 2018, 67, 1–42. [Google Scholar] [CrossRef]
- Jacob, L.; Freyn, M.; Kalder, M.; Dinas, K.; Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: A retrospective study of 422,010 patients followed for up to 30 years. Oncotarget 2018, 9, 17420–17429. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Cummings, K.M. Tobacco and lung cancer: Risks, trends, and outcomes in patients with cancer. Am. Soc. Clin. Oncol. Educ. Book 2013, 33, 359–364. [Google Scholar] [CrossRef]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Islami, F.; Torre, L.A.; Jemal, A. Global trends of lung cancer mortality and smoking prevalence. Transl. Lung Cancer Res. 2015, 4, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, L.; Odani, S.; Agaku, I.T. 20-Year Trends in Tobacco Sales and Self-Reported Tobacco Use in the United States, 2000–2020. Prev. Chronic Dis. 2022, 19, E45. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco smoke: Involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int. J. Environ. Res. Public Health 2009, 6, 445–462. [Google Scholar] [CrossRef]
- Eckel, S.P.; Cockburn, M.; Shu, Y.H.; Deng, H.; Lurmann, F.W.; Liu, L.; Gilliland, F.D. Air pollution affects lung cancer survival. Thorax 2016, 71, 891–898. [Google Scholar] [CrossRef]
- Riudavets, M.; Garcia de Herreros, M.; Besse, B.; Mezquita, L. Radon and Lung Cancer: Current Trends and Future Perspectives. Cancers 2022, 14, 3142. [Google Scholar] [CrossRef]
- Reddy, A.; Conde, C.; Peterson, C.; Nugent, K. Residential radon exposure and cancer. Oncol. Rev. 2022, 16, 558. [Google Scholar] [CrossRef]
- Lorenzo-Gonzalez, M.; Ruano-Ravina, A.; Torres-Duran, M.; Kelsey, K.T.; Provencio, M.; Parente-Lamelas, I.; Leiro-Fernandez, V.; Vidal-Garcia, I.; Castro-Anon, O.; Martinez, C.; et al. Lung cancer and residential radon in never-smokers: A pooling study in the Northwest of Spain. Environ. Res. 2019, 172, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Jafri, S.H.; Ali, F.; Mollaeian, A.; Mojiz Hasan, S.; Hussain, R.; Akkanti, B.; Williams, J.; Shoukier, M.; El-Osta, H. Major Stressful Life Events and Risk of Developing Lung Cancer: A Case-Control Study. Clin. Med. Insights Oncol. 2019, 13, 1179554919835798. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, E.S.; Sood, A.K.; Lutgendorf, S.K. Biobehavioral influences on cancer progression. Immunol. Allergy Clin. N. Am. 2011, 31, 109–132. [Google Scholar] [CrossRef] [PubMed]
- Chida, Y.; Hamer, M.; Wardle, J.; Steptoe, A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008, 5, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.; Fang, F.; Valdimarsdottir, U.; Udumyan, R.; Montgomery, S.; Fall, K. Stress resilience and cancer risk: A nationwide cohort study. J. Epidemiol. Community Health 2017, 71, 947–953. [Google Scholar] [CrossRef]
- Baird, W.M.; Hooven, L.A.; Mahadevan, B. Carcinogenic polycyclic aromatic hydrocarbon-DNA adducts and mechanism of action. Environ. Mol. Mutagen. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Shi, B.; Yang, Z.; Luo, Y.; Xu, T.; Liu, D.; Jiang, C.; Du, G.; Lu, N.; et al. Investigation of exposure biomarkers in human plasma following differing levels of tobacco-specific N-nitrosamines and nicotine in cigarette smoke. Environ. Res. 2022, 214, 113811. [Google Scholar] [CrossRef]
- Leanderson, P. Cigarette smoke-induced DNA damage in cultured human lung cells. Ann. N. Y. Acad. Sci. 1993, 686, 249–259, discussion 259–261. [Google Scholar] [CrossRef]
- Yalcin, E.; de la Monte, S. Tobacco nitrosamines as culprits in disease: Mechanisms reviewed. J. Physiol. Biochem. 2016, 72, 107–120. [Google Scholar] [CrossRef]
- Robertson, A.; Allen, J.; Laney, R.; Curnow, A. The cellular and molecular carcinogenic effects of radon exposure: A review. Int. J. Mol. Sci. 2013, 14, 14024–14063. [Google Scholar] [CrossRef] [PubMed]
- Burchiel, S.W.; Luster, M.I. Signaling by environmental polycyclic aromatic hydrocarbons in human lymphocytes. Clin. Immunol. 2001, 98, 2–10. [Google Scholar] [CrossRef]
- Bian, T.; Ding, H.; Wang, Y.; Hu, Q.; Chen, S.; Fujioka, N.; Aly, F.Z.; Lu, J.; Huo, Z.; Xing, C. Suppressing the activation of protein kinase A as a DNA damage-independent mechanistic lead for dihydromethysticin (DHM) prophylaxis of NNK-induced lung carcinogenesis. Carcinogenesis 2022, 43, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.N.; Ren, C.X.; Gong, Y.X.; Xie, D.P.; Kwon, T. Regulatory function of peroxiredoxin I on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung cancer development. Oncol. Lett. 2021, 21, 465. [Google Scholar] [CrossRef] [PubMed]
- Walser, T.; Cui, X.; Yanagawa, J.; Lee, J.M.; Heinrich, E.; Lee, G.; Sharma, S.; Dubinett, S.M. Smoking and lung cancer: The role of inflammation. Proc. Am. Thorac. Soc. 2008, 5, 811–815. [Google Scholar] [CrossRef]
- Cohen, S.; Janicki-Deverts, D.; Doyle, W.J.; Miller, G.E.; Frank, E.; Rabin, B.S.; Turner, R.B. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc. Natl. Acad. Sci. USA 2012, 109, 5995–5999. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.W. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain Behav. Immun. 2008, 22, 1049–1055. [Google Scholar] [CrossRef]
- Singh, A.P.; Adrianzen Herrera, D.; Zhang, Y.; Perez-Soler, R.; Cheng, H. Mouse models in squamous cell lung cancer: Impact for drug discovery. Expert Opin. Drug Discov. 2018, 13, 347–358. [Google Scholar] [CrossRef]
- Ge, G.Z.; Xu, T.R.; Chen, C. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim. Biophys. Sin. 2015, 47, 477–487. [Google Scholar] [CrossRef]
- Vikis, H.G.; Rymaszewski, A.L.; Tichelaar, J.W. Mouse models of chemically-induced lung carcinogenesis. Front. Biosci. 2013, 5, 939–946. [Google Scholar] [CrossRef]
- Wakamatsu, N.; Devereux, T.R.; Hong, H.H.; Sills, R.C. Overview of the molecular carcinogenesis of mouse lung tumor models of human lung cancer. Toxicol. Pathol. 2007, 35, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E.; Kassie, F.; O’Sullivan, M.G.; Negia, M.; Hanson, T.E.; Upadhyaya, P.; Ruvolo, P.P.; Hecht, S.S.; Xing, C. Chemopreventive effect of kava on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumorigenesis in A/J mice. Cancer Prev. Res. 2008, 1, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Leitzman, P.; Narayanapillai, S.C.; Balbo, S.; Zhou, B.; Upadhyaya, P.; Shaik, A.A.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Kava blocks 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in association with reducing O6-methylguanine DNA adduct in A/J mice. Cancer Prev. Res. 2014, 7, 86–96. [Google Scholar] [CrossRef]
- Castonguay, A.; Rioux, N. Inhibition of lung tumourigenesis by sulindac: Comparison of two experimental protocols. Carcinogenesis 1997, 18, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Rioux, N.; Castonguay, A. Prevention of NNK-induced lung tumorigenesis in A/J mice by acetylsalicylic acid and NS-398. Cancer Res. 1998, 58, 5354–5360. [Google Scholar] [PubMed]
- Rioux, N.; Castonguay, A. Recovery from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced immunosuppression in A/J mice by treatment with nonsteroidal anti-inflammatory drugs. J. Natl. Cancer Inst. 1997, 89, 874–880. [Google Scholar] [CrossRef]
- Balbo, S.; Johnson, C.S.; Kovi, R.C.; James-Yi, S.A.; O’Sullivan, M.G.; Wang, M.; Le, C.T.; Khariwala, S.S.; Upadhyaya, P.; Hecht, S.S. Carcinogenicity and DNA adduct formation of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and enantiomers of its metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F-344 rats. Carcinogenesis 2014, 35, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Kovi, R.C.; Johnson, C.S.; Balbo, S.; Hecht, S.S.; O’Sullivan, M.G. Metastasis to the F344 Rat Pancreas from Lung Cancer Induced by 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone and Enantiomers of Its Metabolite 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol, Constituents of Tobacco Products. Toxicol. Pathol. 2018, 46, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Witschi, H.P.; Nylen, E.; Joshi, P.A.; Correa, E.; Becker, K.L. Pathobiology of lung tumors induced in hamsters by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the modulating effect of hyperoxia. Cancer Res. 1990, 50, 1960–1965. [Google Scholar]
- Coggins, C.R. An updated review of inhalation studies with cigarette smoke in laboratory animals. Int. J. Toxicol. 2007, 26, 331–338. [Google Scholar] [CrossRef]
- Witschi, H.; Espiritu, I.; Dance, S.T.; Miller, M.S. A mouse lung tumor model of tobacco smoke carcinogenesis. Toxicol. Sci. 2002, 68, 322–330. [Google Scholar] [CrossRef]
- Laniado-Laborin, R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int. J. Environ. Res. Public Health 2009, 6, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Shah, A.M.; Blaha, M.J.; Chang, P.P.; Rosamond, W.D.; Matsushita, K. Cigarette Smoking, Cessation, and Risk of Heart Failure With Preserved and Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2022, 79, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Witschi, H. Successful and not so successful chemoprevention of tobacco smoke-induced lung tumors. Exp. Lung Res. 2000, 26, 743–755. [Google Scholar] [CrossRef]
- Witschi, H. A/J mouse as a model for lung tumorigenesis caused by tobacco smoke: Strengths and weaknesses. Exp. Lung Res. 2005, 31, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Witschi, H.; Espiritu, I.; Uyeminami, D. Chemoprevention of tobacco smoke-induced lung tumors in A/J strain mice with dietary myo-inositol and dexamethasone. Carcinogenesis 1999, 20, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Witschi, H.; Espiritu, I.; Yu, M.; Willits, N.H. The effects of phenethyl isothiocyanate, N-acetylcysteine and green tea on tobacco smoke-induced lung tumors in strain A/J mice. Carcinogenesis 1998, 19, 1789–1794. [Google Scholar] [CrossRef]
- Hecht, S.S.; Kassie, F.; Hatsukami, D.K. Chemoprevention of lung carcinogenesis in addicted smokers and ex-smokers. Nat. Rev. Cancer 2009, 9, 476–488. [Google Scholar] [CrossRef]
- Wang, Y.; Rouggly, L.; You, M.; Lubet, R. Animal models of lung cancer characterization and use for chemoprevention research. Prog. Mol. Biol. Transl. Sci. 2012, 105, 211–226. [Google Scholar] [CrossRef]
- Sanchez-Cespedes, M. A role for LKB1 gene in human cancer beyond the Peutz-Jeghers syndrome. Oncogene 2007, 26, 7825–7832. [Google Scholar] [CrossRef]
- Gill, R.K.; Yang, S.H.; Meerzaman, D.; Mechanic, L.E.; Bowman, E.D.; Jeon, H.S.; Roy Chowdhuri, S.; Shakoori, A.; Dracheva, T.; Hong, K.M.; et al. Frequent homozygous deletion of the LKB1/STK11 gene in non-small cell lung cancer. Oncogene 2011, 30, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Iwakawa, R.; Takahashi, K.; Kohno, T.; Nakanishi, Y.; Matsuno, Y.; Suzuki, K.; Nakamoto, M.; Shimizu, E.; Minna, J.D.; et al. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene 2007, 26, 5911–5918. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Hiroshima, K.; Iyoda, A.; Hoshi, K.; Honma, K.; Kuroki, M.; Kokubo, T.; Fujisawa, T.; Miyagi, Y.; Nakatani, Y. LKB1 protein expression in neuroendocrine tumors of the lung. Pathol. Int. 2008, 58, 84–88. [Google Scholar] [CrossRef]
- Ji, H.; Ramsey, M.R.; Hayes, D.N.; Fan, C.; McNamara, K.; Kozlowski, P.; Torrice, C.; Wu, M.C.; Shimamura, T.; Perera, S.A.; et al. LKB1 modulates lung cancer differentiation and metastasis. Nature 2007, 448, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fillmore, C.M.; Koyama, S.; Wu, H.; Zhao, Y.; Chen, Z.; Herter-Sprie, G.S.; Akbay, E.A.; Tchaicha, J.H.; Altabef, A.; et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 2014, 25, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Berrett, K.C.; Kc, U.; Clair, P.M.; Pop, S.M.; Carr, S.R.; Witt, B.L.; Oliver, T.G. Sox2 cooperates with Lkb1 loss in a mouse model of squamous cell lung cancer. Cell Rep. 2014, 8, 40–49. [Google Scholar] [CrossRef]
- Ghaffar, H.; Sahin, F.; Sanchez-Cepedes, M.; Su, G.H.; Zahurak, M.; Sidransky, D.; Westra, W.H. LKB1 protein expression in the evolution of glandular neoplasia of the lung. Clin. Cancer Res. 2003, 9, 2998–3003. [Google Scholar]
- Gurumurthy, S.; Hezel, A.F.; Sahin, E.; Berger, J.H.; Bosenberg, M.W.; Bardeesy, N. LKB1 deficiency sensitizes mice to carcinogen-induced tumorigenesis. Cancer Res. 2008, 68, 55–63. [Google Scholar] [CrossRef]
- Bian, T.; Wang, Y.; Botello, J.F.; Hu, Q.; Jiang, Y.; Zingone, A.; Ding, H.; Wu, Y.; Zahra Aly, F.; Salloum, R.G.; et al. LKB1 phosphorylation and deactivation in lung cancer by NNAL, a metabolite of tobacco-specific carcinogen, in an isomer-dependent manner. Oncogene 2022, 41, 4042–4054. [Google Scholar] [CrossRef]
- Volgin, A.; Yang, L.; Amstislavskaya, T.; Demin, K.; Wang, D.; Yan, D.; Wang, J.; Wang, M.; Alpyshov, E.; Hu, G.; et al. DARK Classics in Chemical Neuroscience: Kava. ACS Chem. Neurosci. 2020, 11, 3893–3904. [Google Scholar] [CrossRef]
- Finau, S.A.; Stanhope, J.M.; Prior, I.A. Kava, alcohol and tobacco consumption among Tongans with urbanization. Soc. Sci. Med. 1982, 16, 35–41. [Google Scholar] [CrossRef]
- Wheatley, D. Kava and valerian in the treatment of stress-induced insomnia. Phytother. Res. 2001, 15, 549–551. [Google Scholar] [CrossRef] [PubMed]
- Munte, T.F.; Heinze, H.J.; Matzke, M.; Steitz, J. Effects of oxazepam and an extract of kava roots (Piper methysticum) on event-related potentials in a word recognition task. Neuropsychobiology 1993, 27, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, M.P.; Whitty, A.; Merrill, J.C.; Tang, X.; Ashton, T.D.; Amar, S. Identification and characterization of kava-derived compounds mediating TNF-alpha suppression. Chem. Biol. Drug Des. 2009, 74, 121–128. [Google Scholar] [CrossRef]
- He, X.G.; Lin, L.Z.; Lian, L.Z. Electrospray high performance liquid chromatography-mass spectrometry in phytochemical analysis of kava (Piper methysticum) extract. Planta Med. 1997, 63, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Kanumuri, S.R.R.; Mamallapalli, J.; Nelson, R.; McCurdy, C.R.; Mathews, C.A.; Xing, C.; Sharma, A. Clinical pharmacokinetics of kavalactones after oral dosing of standardized kava extract in healthy volunteers. J. Ethnopharmacol. 2022, 297, 115514. [Google Scholar] [CrossRef] [PubMed]
- Botello, J.F.; Corral, P.; Bian, T.; Xing, C. Kava and its Kavalactones Inhibit Norepinephrine-induced Intracellular Calcium Influx in Lung Cancer Cells. Planta Med. 2020, 86, 26–31. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; Balbo, S.; Leitzman, P.; Grill, A.E.; Upadhyaya, P.; Shaik, A.A.; Zhou, B.; O’Sullivan, M.G.; Peterson, L.A.; Lu, J.; et al. Dihydromethysticin from kava blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis and differentially reduces DNA damage in A/J mice. Carcinogenesis 2014, 35, 2365–2372. [Google Scholar] [CrossRef]
- Lebot, V.; Do, T.K.T.; Legendre, L. Detection of flavokavins (A, B, C) in cultivars of kava (Piper methysticum) using high performance thin layer chromatography (HPTLC). Food Chem. 2014, 151, 554–560. [Google Scholar] [CrossRef]
- Bian, T.; Corral, P.; Wang, Y.; Botello, J.; Kingston, R.; Daniels, T.; Salloum, R.G.; Johnston, E.; Huo, Z.; Lu, J.; et al. Kava as a Clinical Nutrient: Promises and Challenges. Nutrients 2020, 12, 3044. [Google Scholar] [CrossRef]
- Simeoni, P.; Lebot, V. Identification of factors determining kavalactone content and chemotype in Kava (Piper methysticum Forst. f.). Biochem. Syst. Ecol. 2002, 30, 413–424. [Google Scholar] [CrossRef]
- Lebot, V.; Michalet, S.; Legendre, L. Kavalactones and Flavokavins Profiles Contribute to Quality Assessment of Kava (Piper methysticum G. Forst.), the Traditional Beverage of the Pacific. Beverages 2019, 5, 34. [Google Scholar] [CrossRef]
- Lasme, P.; Davrieux, F.; Montet, D.; Lebot, V. Quantification of kavalactones and determination of kava (Piper methysticum) chemotypes using near-infrared reflectance spectroscopy for quality control in Vanuatu. J. Agric. Food Chem. 2008, 56, 4976–4981. [Google Scholar] [CrossRef]
- Mamallapalli, J.; Kanumuri, S.R.R.; Corral, P.; Johnston, E.; Zhuang, C.; McCurdy, C.R.; Mathews, C.A.; Sharma, A.; Xing, C. Characterization of Different Forms of Kava (Piper methysticum) Products by UPLC-MS/MS. Planta Med. 2022, 88, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qu, W.Y.; Bittenbender, H.C.; Li, Q.X. Kavalactone content and chemotype of kava beverages prepared from roots and rhizomes of Isa and Mahakea varieties and extraction efficiency of kavalactones using different solvents. J. Food Sci. Technol. 2015, 52, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Steiner, G.G. The correlation between cancer incidence and kava consumption. Hawaii Med. J. 2000, 59, 420–422. [Google Scholar]
- Tuomilehto, J.; Zimmet, P.; Taylor, R.; Bennet, P.; Wolf, E.; Kankaanpaa, J. Smoking rates in Pacific islands. Bull. World Health Organ. 1986, 64, 447–456. [Google Scholar]
- Henderson, B.E.; Kolonel, L.N.; Dworsky, R.; Kerford, D.; Mori, E.; Singh, K.; Thevenot, H. Cancer incidence in the islands of the Pacific. Natl. Cancer Inst. Monogr. 1985, 69, 73–81. [Google Scholar]
- Foliaki, S.; Best, D.; Akau’ola, S.; Cheng, S.; Borman, B.; Pearce, N. Cancer incidence in four pacific countries: Tonga, Fiji Islands, Cook Islands and Niue. Pac. Health Dialog 2011, 17, 21–32. [Google Scholar]
- Haiti, S.; Hu, Q.; Huo, Z.; Lu, J.; Xing, C. Structure-activity relationship of dihydromethysticin on NNK-induced lung DNA damage in A/J mice. ChemMedChem 2021, 17, e202100727. [Google Scholar] [CrossRef]
- Hu, Q.; Corral, P.; Narayanapillai, S.C.; Leitzman, P.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Oral dosing of dihydromethysticin ahead of tobacco carcinogen NNK effectively prevents lung tumorigenesis in A/J mice. Chem. Res. Toxicol. 2020, 33, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Ding, H.; Fujioka, N.; Salloum, R.; Huo, Z.; Lu, J.; Xing, C. Chemoprevention effect of dihydromethysticin on lung cancer via transcriptional activation of UGTs to enhance NNK detoxification. Carcinogenesis, 2022; under review. [Google Scholar]
- Johnson, T.E.; Hermanson, D.; Wang, L.; Kassie, F.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am. J. Chin. Med. 2011, 39, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, L.; Xie, J.; Wu, X.R.; Gin, G.E.; Wang, B.; Uchio, E.; Zi, X. Kavalactone Kawain Impedes Urothelial Tumorigenesis in UPII-Mutant Ha-Ras Mice via Inhibition of mTOR Signaling and Alteration of Cancer Metabolism. Molecules 2023, 28, 1666. [Google Scholar] [CrossRef] [PubMed]
- Narayanapillai, S.C.; Lin, S.H.; Leitzman, P.; Upadhyaya, P.; Baglole, C.J.; Xing, C. Dihydromethysticin (DHM) Blocks Tobacco Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-Induced O6-Methylguanine in a Manner Independent of the Aryl Hydrocarbon Receptor (AhR) Pathway in C57BL/6 Female Mice. Chem. Res. Toxicol. 2016, 29, 1828–1834. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; von Weymarn, L.B.; Carmella, S.G.; Leitzman, P.; Paladino, J.; Upadhyaya, P.; Hecht, S.S.; Murphy, S.E.; Xing, C. Dietary Dihydromethysticin Increases Glucuronidation of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol in A/J Mice, Potentially Enhancing Its Detoxification. Drug Metab. Dispos. 2016, 44, 422–427. [Google Scholar] [CrossRef]
- Puppala, M.; Narayanapillai, S.C.; Leitzman, P.; Sun, H.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Xing, C. Pilot in Vivo Structure-Activity Relationship of Dihydromethysticin in Blocking 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone-Induced O(6)-Methylguanine and Lung Tumor in A/J Mice. J. Med. Chem. 2017, 60, 7935–7940. [Google Scholar] [CrossRef]
- Tang, S.N.; Zhang, J.; Jiang, P.; Datta, P.; Leitzman, P.; O’Sullivan, M.G.; Jiang, C.; Xing, C.; Lu, J. Gene expression signatures associated with suppression of TRAMP prostate carcinogenesis by a kavalactone-rich Kava fraction. Mol. Carcinog. 2016, 55, 2291–2303. [Google Scholar] [CrossRef]
- Triolet, J.; Shaik, A.A.; Gallaher, D.D.; O’Sullivan, M.G.; Xing, C. Reduction in colon cancer risk by consumption of kava or kava fractions in carcinogen-treated rats. Nutr. Cancer 2012, 64, 838–846. [Google Scholar] [CrossRef]
- Xu, X.; Tian, X.; Song, L.; Xie, J.; Liao, J.C.; Meeks, J.J.; Wu, X.R.; Gin, G.E.; Wang, B.; Uchio, E.; et al. Kawain Inhibits Urinary Bladder Carcinogenesis through Epigenetic Inhibition of LSD1 and Upregulation of H3K4 Methylation. Biomolecules 2023, 13, 521. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Chou, T.W.; Feng, J.H.; Huang, C.C.; Cheng, Y.W.; Chien, S.C.; Wang, S.Y.; Shyur, L.F. A Plant Kavalactone Desmethoxyyangonin Prevents Inflammation and Fulminant Hepatitis in Mice. PLoS ONE 2013, 8, e77626. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fu, T.; Wang, C.; Ning, C.; Liu, K.; Liu, Z.; Sun, H.; Ma, X.; Huo, X.; Yang, X.; et al. Yangonin protects against cholestasis and hepatotoxity via activation of farnesoid X receptor in vivo and in vitro. Toxicol. Appl. Pharmacol. 2018, 348, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Gao, X.; Wang, C.; Ning, C.; Liu, K.; Liu, Z.; Sun, H.; Ma, X.; Sun, P.; Meng, Q. Protective effects of yangonin from an edible botanical Kava against lithocholic acid-induced cholestasis and hepatotoxicity. Eur. J. Pharmacol. 2018, 824, 64–71. [Google Scholar] [CrossRef]
- Alshammari, A.; Patel, J.; Al-Hashemi, J.; Cai, B.; Panek, J.; Huck, O.; Amar, S. Kava-241 reduced periodontal destruction in a collagen antibody primed Porphyromonas gingivalis model of periodontitis. J. Clin. Periodontol. 2017, 44, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Panek, J.S.; Amar, S. Kava analogues as agents for treatment of periodontal diseases: Synthesis and initial biological evaluation. Bioorganic Med. Chem. Lett. 2018, 28, 2667–2669. [Google Scholar] [CrossRef]
- Huck, O.; You, J.; Han, X.; Cai, B.; Panek, J.; Amar, S. Reduction of Articular and Systemic Inflammation by Kava-241 in a Porphyromonas gingivalis-Induced Arthritis Murine Model. Infect. Immun. 2018, 86, e00356-18. [Google Scholar] [CrossRef]
- Singh, S.P.; Huck, O.; Abraham, N.G.; Amar, S. Kavain Reduces Porphyromonas gingivalis-Induced Adipocyte Inflammation: Role of PGC-1alpha Signaling. J. Immunol. 2018, 201, 1491–1499. [Google Scholar] [CrossRef]
- Tang, X.R.; Amar, S. Kavain inhibition of LPS-induced TNF-alpha via ERK/LITAF. Toxicol. Res. 2016, 5, 188–196. [Google Scholar] [CrossRef]
- Tang, X.R.; Amar, S. Kavain Involvement in LPS-Induced Signaling Pathways. J. Cell. Biochem. 2016, 117, 2272–2280. [Google Scholar] [CrossRef]
- Hashimoto, T.; Suganuma, M.; Fujiki, H.; Yamada, M.; Kohno, T.; Asakawa, Y. Isolation and synthesis of TNF-alpha release inhibitors from Fijian kawa (Piper methysticum). Phytomedicine 2003, 10, 309–317. [Google Scholar] [CrossRef]
- Wang, Y.; Narayanapillai, S.C.; Tessier, K.; Strayer, L.; Upadhyaya, P.; Hu, Q.; Kingston, R.; Salloum, R.G.; Lu, J.; Hecht, S.S.; et al. The Impact of One-week Dietary Supplementation with Kava on Biomarkers of Tobacco Use and Nitrosamine-based Carcinogenesis Risk among Active Smokers. Cancer Prev. Res. 2020, 13, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Schuller, H.M.; Tithof, P.K.; Williams, M.; Plummer, H., 3rd. The tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acid. Cancer Res. 1999, 59, 4510–4515. [Google Scholar] [PubMed]
- Pavel, E.; Nadella, K.; Towns, W.H., 2nd; Kirschner, L.S. Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A. Mol. Endocrinol. 2008, 22, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Codina, A.; Renauer, P.A.; Wang, G.; Chow, R.D.; Park, J.J.; Ye, H.; Zhang, K.; Dong, M.B.; Gassaway, B.; Ye, L.; et al. Convergent Identification and Interrogation of Tumor-Intrinsic Factors that Modulate Cancer Immunity In Vivo. Cell Syst. 2019, 8, 136–151.e7. [Google Scholar] [CrossRef] [PubMed]
- Coles, G.L.; Cristea, S.; Webber, J.T.; Levin, R.S.; Moss, S.M.; He, A.; Sangodkar, J.; Hwang, Y.C.; Arand, J.; Drainas, A.P.; et al. Unbiased Proteomic Profiling Uncovers a Targetable GNAS/PKA/PP2A Axis in Small Cell Lung Cancer Stem Cells. Cancer Cell 2020, 38, 129–143.e7. [Google Scholar] [CrossRef]
- Cvijic, M.E.; Kita, T.; Shih, W.; DiPaola, R.S.; Chin, K.V. Extracellular catalytic subunit activity of the cAMP-dependent protein kinase in prostate cancer. Clin. Cancer Res. 2000, 6, 2309–2317. [Google Scholar]
- Cho, Y.S.; Park, Y.G.; Lee, Y.N.; Kim, M.K.; Bates, S.; Tan, L.; Cho-Chung, Y.S. Extracellular protein kinase A as a cancer biomarker: Its expression by tumor cells and reversal by a myristate-lacking Calpha and RIIbeta subunit overexpression. Proc. Natl. Acad. Sci. USA 2000, 97, 835–840. [Google Scholar] [CrossRef]
- Kita, T.; Goydos, J.; Reitman, E.; Ravatn, R.; Lin, Y.; Shih, W.C.; Kikuchi, Y.; Chin, K.V. Extracellular cAMP-dependent protein kinase (ECPKA) in melanoma. Cancer Lett. 2004, 208, 187–191. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Lin, W.; Wang, W.; Zhang, Z.; Rayburn, E.R.; Lu, J.; Chen, D.; Yue, X.; Shen, F.; et al. Extracellular activity of cyclic AMP-dependent protein kinase as a biomarker for human cancer detection: Distribution characteristics in a normal population and cancer patients. Cancer Epidemiol. Biomark. Prev. 2007, 16, 789–795. [Google Scholar] [CrossRef]
- Kong, D.H.; Jung, S.H.; Jeon, H.Y.; Kim, W.J.; Kim, Y.M.; Ha, K.S. A peptide array-based serological protein kinase A activity assay and its application in cancer diagnosis. Analyst 2015, 140, 6588–6594. [Google Scholar] [CrossRef]
- Keil, M.F.; Briassoulis, G.; Stratakis, C.A.; Wu, T.J. Protein Kinase A and Anxiety-Related Behaviors: A Mini-Review. Front. Endocrinol. 2016, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Keil, M.F.; Briassoulis, G.; Stratakis, C.A. The Role of Protein Kinase A in Anxiety Behaviors. Neuroendocrinology 2016, 103, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Keil, M.F.; Briassoulis, G.; Gokarn, N.; Nesterova, M.; Wu, T.J.; Stratakis, C.A. Anxiety phenotype in mice that overexpress protein kinase A. Psychoneuroendocrinology 2012, 37, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Briassoulis, G.; Keil, M.F.; Naved, B.; Liu, S.; Starost, M.F.; Nesterova, M.; Gokarn, N.; Batistatos, A.; Wu, T.J.; Stratakis, C.A. Studies of mice with cyclic AMP-dependent protein kinase (PKA) defects reveal the critical role of PKA’s catalytic subunits in anxiety. Behav. Brain Res. 2016, 307, 1–10. [Google Scholar] [CrossRef]
- Westbom, C.M.; Shukla, A.; MacPherson, M.B.; Yasewicz, E.C.; Miller, J.M.; Beuschel, S.L.; Steele, C.; Pass, H.I.; Vacek, P.M.; Shukla, A. CREB-induced inflammation is important for malignant mesothelioma growth. Am. J. Pathol. 2014, 184, 2816–2827. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Gao, R.; Zhang, M.; Amelio, A.L.; Fallahi, M.; Chen, Z.; Gu, Y.; Hu, C.; Welsh, E.A.; Engel, B.E.; et al. Role of LKB1-CRTC1 on glycosylated COX-2 and response to COX-2 inhibition in lung cancer. J. Natl. Cancer Inst. 2015, 107, 358. [Google Scholar] [CrossRef]
- Ratovitski, E.A. LKB1/PEA3/DeltaNp63 pathway regulates PTGS-2 (COX-2) transcription in lung cancer cells upon cigarette smoke exposure. Oxidative Med. Cell. Longev. 2010, 3, 317–324. [Google Scholar] [CrossRef]
- Sarris, J.; Kavanagh, D.J.; Byrne, G.; Bone, K.M.; Adams, J.; Deed, G. The Kava Anxiety Depression Spectrum Study (KADSS): A randomized, placebo-controlled crossover trial using an aqueous extract of Piper methysticum. Psychopharmacology 2009, 205, 399–407. [Google Scholar] [CrossRef]
- Tang, Y.; Simoneau, A.R.; Xie, J.; Shahandeh, B.; Zi, X. Effects of the kava chalcone flavokawain A differ in bladder cancer cells with wild-type versus mutant p53. Cancer Prev. Res. 2008, 1, 439–451. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, W.; Wang, Z.; Gao, M.; Wang, X.; Han, W.; Zhang, N.; Xu, X. Flavokawain A inhibits prostate cancer cells by inducing cell cycle arrest and cell apoptosis and regulating the glutamine metabolism pathway. J. Pharm. Biomed. Anal. 2020, 186, 113288. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Xu, X.; Blair, C.A.; Sun, Z.; Xie, J.; Lilly, M.B.; Zi, X. Kava components down-regulate expression of AR and AR splice variants and reduce growth in patient-derived prostate cancer xenografts in mice. PLoS ONE 2012, 7, e31213. [Google Scholar] [CrossRef] [PubMed]
- Rossette, M.C.; Moraes, D.C.; Sacramento, E.K.; Romano-Silva, M.A.; Carvalho, J.L.; Gomes, D.A.; Caldas, H.; Friedman, E.; Bastos-Rodrigues, L.; De Marco, L. The In Vitro and In Vivo Antiangiogenic Effects of Flavokawain B. Phytother. Res. 2017, 31, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Akhtar, M.N.; Yeap, S.K.; Lim, K.L.; Ho, W.Y.; Abdullah, M.P.; Ho, C.L.; Omar, A.R.; Ismail, J.; Alitheen, N.B. Flavokawain B induced cytotoxicity in two breast cancer cell lines, MCF-7 and MDA-MB231 and inhibited the metastatic potential of MDA-MB231 via the regulation of several tyrosine kinases In vitro. BMC Complement. Altern. Med. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In Vivo Anti-Tumor Effects of Flavokawain A in 4T1 Breast Cancer Cell-Challenged Mice. Anti-Cancer Agent Med. Chem. 2015, 15, 905–915. [Google Scholar] [CrossRef]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In vivo antitumor and antimetastatic effects of flavokawain B in 4T1 breast cancer cell-challenged mice. Drug Des. Dev. Ther. 2015, 9, 1401–1417. [Google Scholar] [CrossRef]
- Teschke, R.; Sarris, J.; Schweitzer, I. Kava hepatotoxicity in traditional and modern use: The presumed Pacific kava paradox hypothesis revisited. Br. J. Clin. Pharmacol. 2012, 73, 170–174. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Hepatic toxicity possibly associated with kava-containing products—United States, Germany, and Switzerland, 1999–2002. MMWR Morb. Mortal. Wkly. Rep. 2002, 51, 1065–1067. [Google Scholar]
- Mathews, J.M.; Etheridge, A.S.; Black, S.R. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab. Dispos. 2002, 30, 1153–1157. [Google Scholar] [CrossRef]
- Unger, M.; Holzgrabe, U.; Jacobsen, W.; Cummins, C.; Benet, L.Z. Inhibition of cytochrome P450 3A4 by extracts and kavalactones of Piper methysticum (Kava-Kava). Planta Med. 2002, 68, 1055–1058. [Google Scholar] [CrossRef]
- Smith, K.K.; Dharmaratne, H.R.; Feltenstein, M.W.; Broom, S.L.; Roach, J.T.; Nanayakkara, N.P.; Khan, I.A.; Sufka, K.J. Anxiolytic effects of kava extract and kavalactones in the chick social separation-stress paradigm. Psychopharmacology 2001, 155, 86–90. [Google Scholar] [CrossRef]
- Mamallapalli, J.; Freeman, B.; Botello, J.; Mathews, C.A.; Fujioka, N.; Xing, C. To be determined. Antagonism of the β-adrenergic receptor as a potential anti-stress mechanism of kava and kavalactones. Neurobiol. Stress, 2023; under review. [Google Scholar]
- Lindenberg, D.; Pitule-Schödel, H. D,L-kavain in comparison with oxazepam in anxiety disorders. A double-blind study of clinical effectiveness. Fortschr. Med. 1990, 108, 49–50, 53–54. [Google Scholar]
- Feltenstein, M.W.; Lambdin, L.C.; Ganzera, M.; Ranjith, H.; Dharmaratne, W.; Nanayakkara, N.P.; Khan, I.A.; Sufka, K.J. Anxiolytic properties of Piper methysticum extract samples and fractions in the chick social-separation-stress procedure. Phytother. Res. 2003, 17, 210–216. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; Leitzman, P.; O’Sullivan, M.G.; Xing, C. Flavokawains a and B in kava, not dihydromethysticin, potentiate acetaminophen-induced hepatotoxicity in C57BL/6 mice. Chem. Res. Toxicol. 2014, 27, 1871–1876. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Gross, S.; Liu, J.H.; Yu, B.Y.; Feng, L.L.; Nolta, J.; Sharma, V.; Piwnica-Worms, D.; Qiu, S.X. Flavokawain B, the hepatotoxic constituent from kava root, induces GSH-sensitive oxidative stress through modulation of IKK/NF-kappaB and MAPK signaling pathways. FASEB J. 2010, 24, 4722–4732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Narayanapillai, S.C.; Hu, Q.; Fujioka, N.; Xing, C. Detection and quantification of 4-hydroxy-1-(3-pyridyl)-1-butanone (HPB) from smoker albumin and its potential as a surrogate biomarker of tobacco-specific nitrosamines exposure and bioactivation. Toxicol. Lett. 2019, 311, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Narayanapillai, S.; Hu, Q.; Fujioka, N.; Xing, C. Contribution of Tobacco Use and 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone to Three Methyl DNA Adducts in Urine. Chem. Res. Toxicol. 2018, 31, 836–838. [Google Scholar] [CrossRef]
- Wang, Y.; Fujioka, N.; Xing, C. Quantitative profiling of cortisol metabolites in human urine by high-resolution accurate-mass MS. Bioanalysis 2018, 10, 2015–2026. [Google Scholar] [CrossRef]



| Age-Standardized Cancer Incidence Rates for All Sites per 100,000 Population in 1960s–1970s | |||
|---|---|---|---|
| Country | Male | Female | Kava Consumed/Person/Year (Kilograms) |
| Vanuatu | 70.9 | 83.7 | 6.7 |
| Fiji | 75 | 112.2 | 2.8 |
| Western Samoa | 90.2 | 93.7 | 2.2 |
| Micronesia | 132.9 | 97 | 1.4 |
| New Caledonia | 182 | 154 | 0.6 |
| Hawaii | 311.9 | 297.6 | 0 |
| New Zealand | 322.9 | 297.6 | 0 |
| USA, Los Angeles | 307.2 | 276.2 | 0 |
| Activity of Compounds in Kava and Related References | ||||||||
|---|---|---|---|---|---|---|---|---|
| DHM | M | Y | DHK | K | DMY | FKA | FKB | |
| Carcinogen detoxification and DNA damage reduction in mice [89] | ++ | + | - | - | - | - | - | - |
| PK in human [87] and mice [152] | ++ | + | - | ++ | + | - | N/A | N/A |
| NE-induced cAMP in cells [153] | + | N/A | ++ | - | - | + | N/A | N/A |
| Anxiolytic activity in human [154] # | N/A | N/A | N/A | N/A | + | N/A | N/A | N/A |
| Anxiolytic activity in chicken [152,155] # | N/A | N/A | N/A | + | N/A | N/A | N/A | N/A |
| Anti-inflammatory activity in mice [85,113,114,115,116,117,118,119,120,121,122] # | + | + | + | N/A | + | + | N/A | N/A |
| Hepatotoxic risk in mice [156,157] | - | - | - | - | - | - | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeman, B.; Mamallapalli, J.; Bian, T.; Ballas, K.; Lynch, A.; Scala, A.; Huo, Z.; Fredenburg, K.M.; Bruijnzeel, A.W.; Baglole, C.J.; et al. Opportunities and Challenges of Kava in Lung Cancer Prevention. Int. J. Mol. Sci. 2023, 24, 9539. https://doi.org/10.3390/ijms24119539
Freeman B, Mamallapalli J, Bian T, Ballas K, Lynch A, Scala A, Huo Z, Fredenburg KM, Bruijnzeel AW, Baglole CJ, et al. Opportunities and Challenges of Kava in Lung Cancer Prevention. International Journal of Molecular Sciences. 2023; 24(11):9539. https://doi.org/10.3390/ijms24119539
Chicago/Turabian StyleFreeman, Breanne, Jessica Mamallapalli, Tengfei Bian, Kayleigh Ballas, Allison Lynch, Alexander Scala, Zhiguang Huo, Kristianna M. Fredenburg, Adriaan W. Bruijnzeel, Carolyn J. Baglole, and et al. 2023. "Opportunities and Challenges of Kava in Lung Cancer Prevention" International Journal of Molecular Sciences 24, no. 11: 9539. https://doi.org/10.3390/ijms24119539
APA StyleFreeman, B., Mamallapalli, J., Bian, T., Ballas, K., Lynch, A., Scala, A., Huo, Z., Fredenburg, K. M., Bruijnzeel, A. W., Baglole, C. J., Lu, J., Salloum, R. G., Malaty, J., & Xing, C. (2023). Opportunities and Challenges of Kava in Lung Cancer Prevention. International Journal of Molecular Sciences, 24(11), 9539. https://doi.org/10.3390/ijms24119539






