Capacitive Electrode-Based Electric Field Treatments on Redox-Toxic Iron Deposits in Transgenic AD Mouse Models: The Electroceutical Targeting of Alzheimer’s Disease Feasibility Study
Abstract
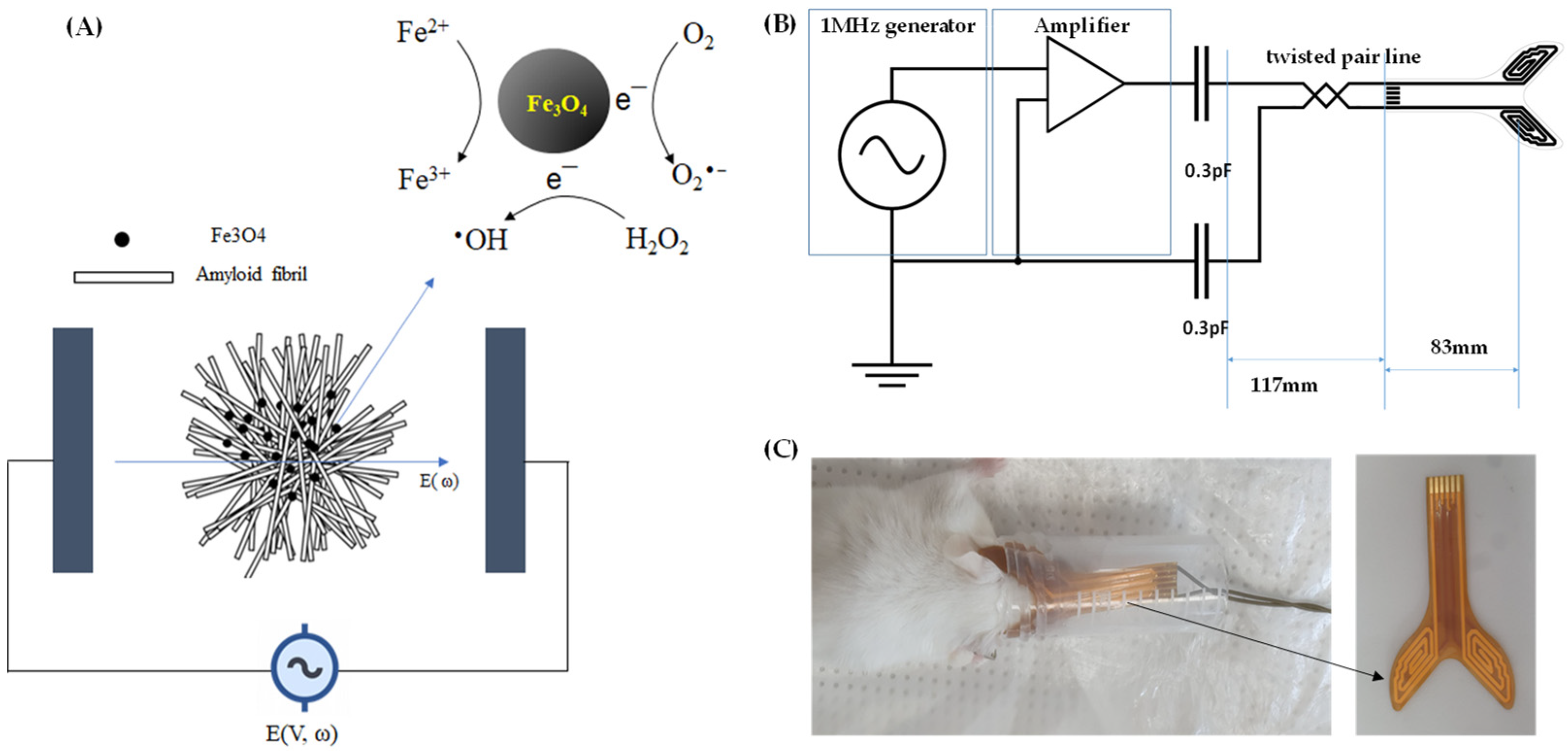
:1. Introduction
2. Results
2.1. ROS Measurement
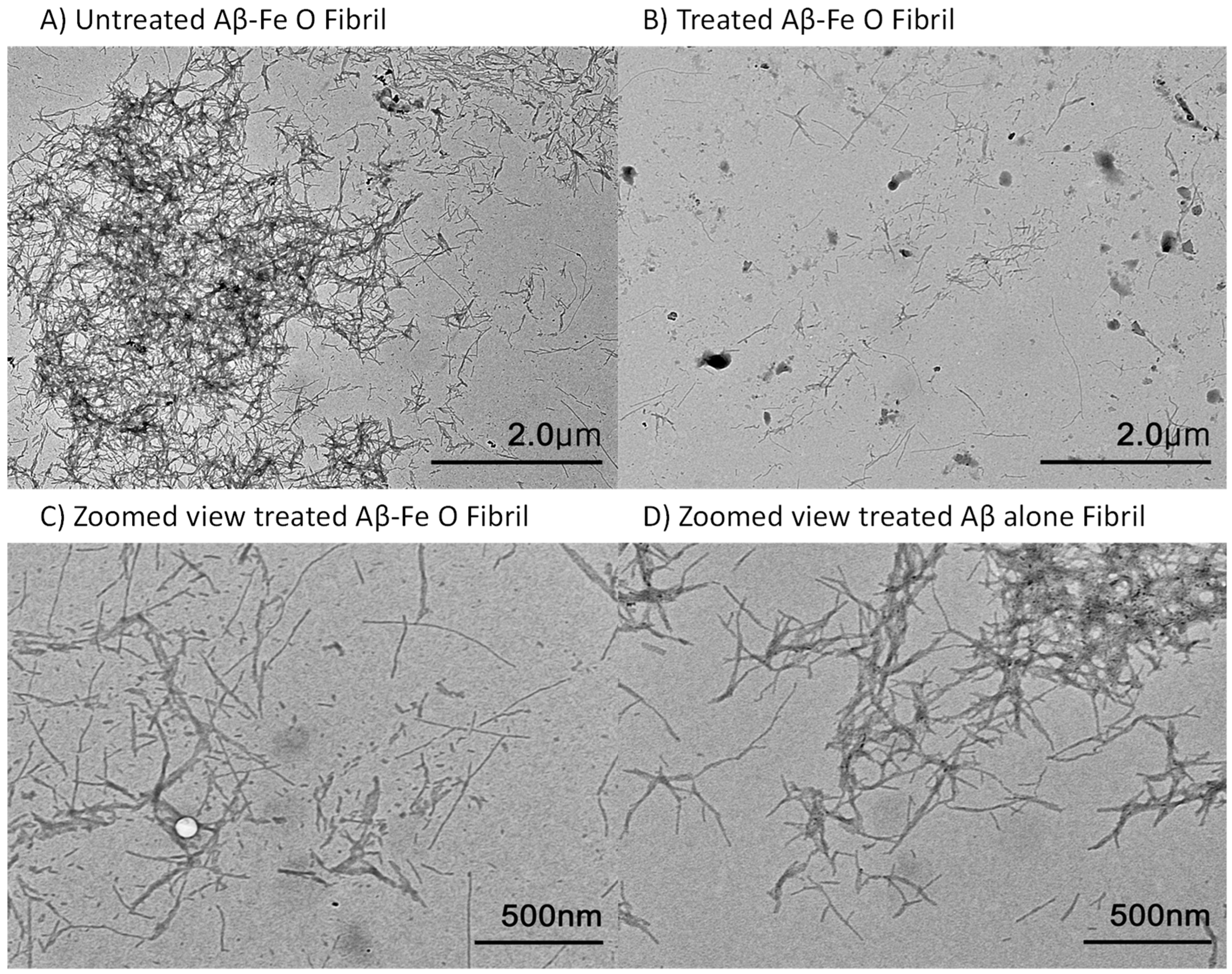
2.2. AEF Treatment on Aβ-Fe3O4 or Aβ Alone Fibril
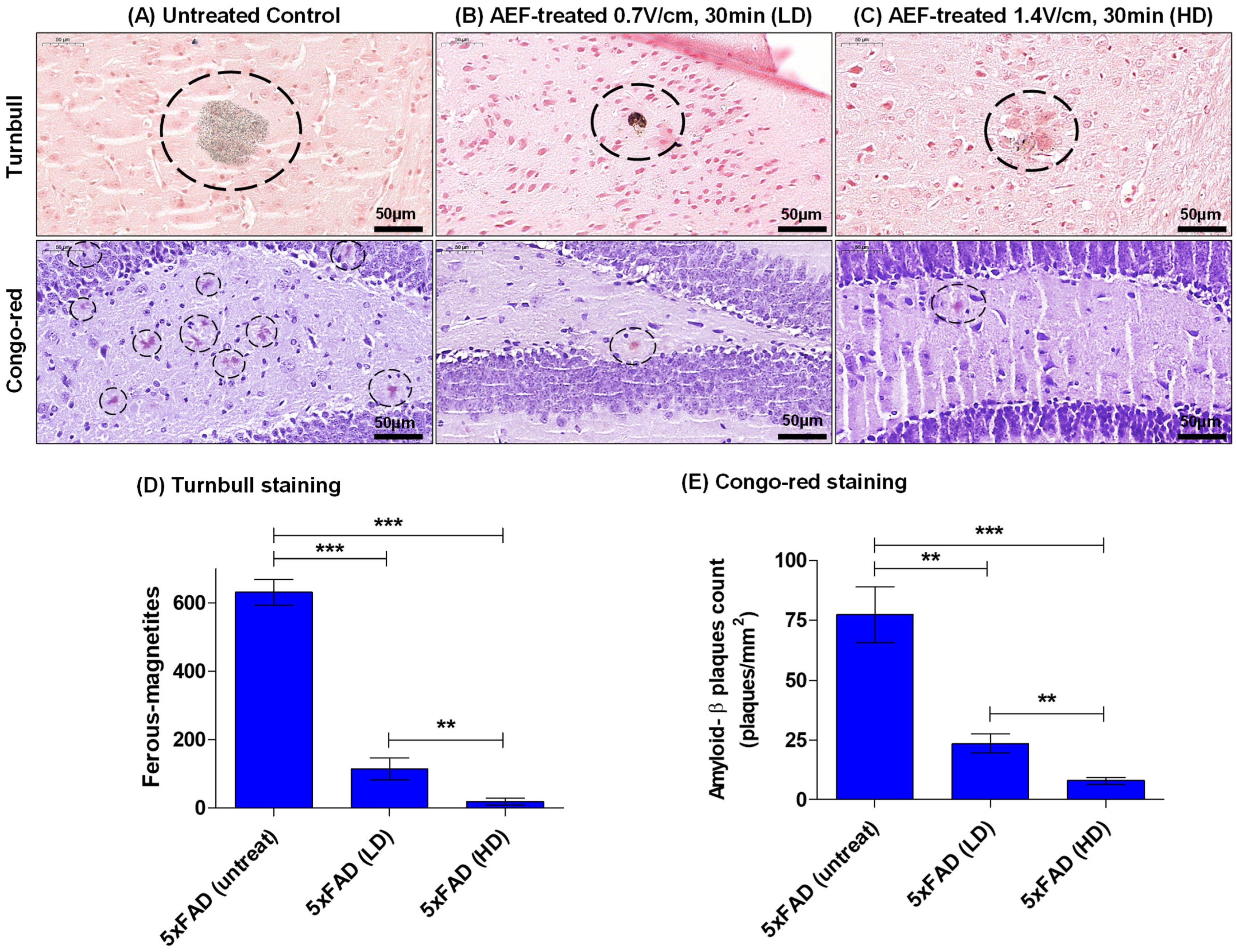
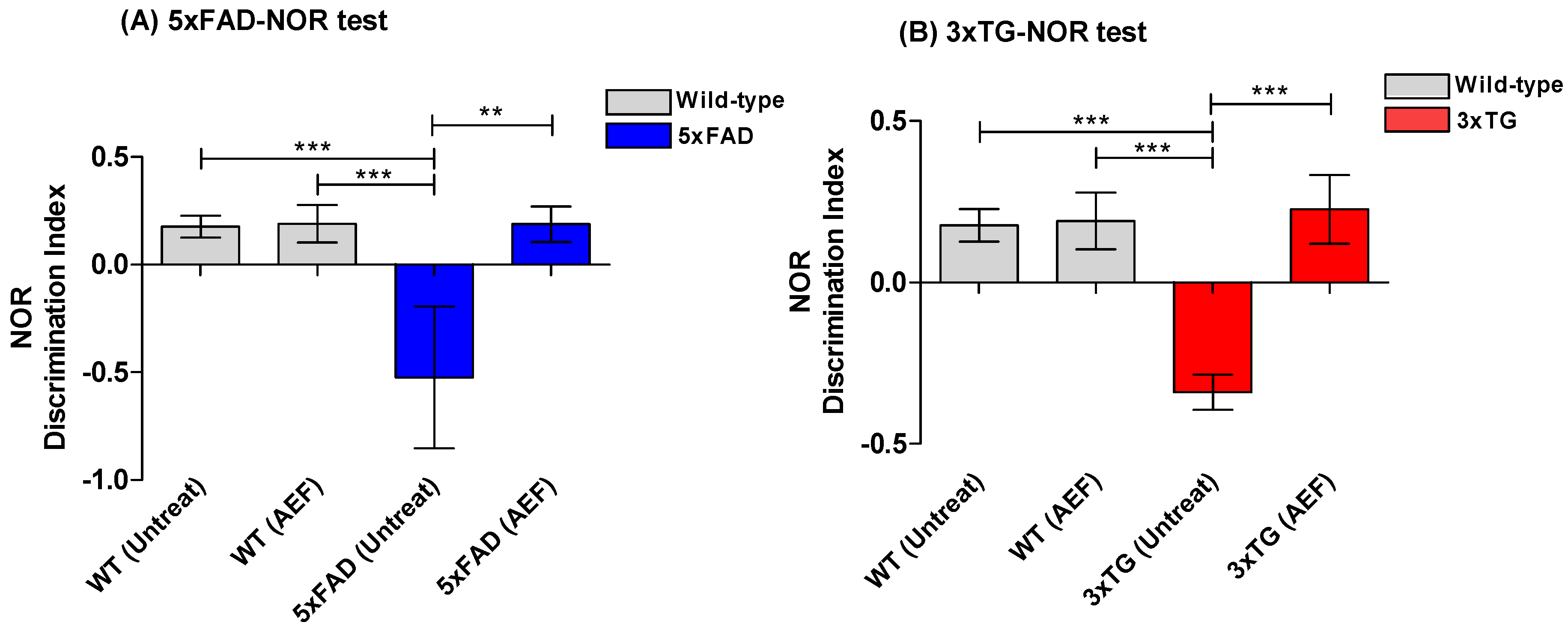
2.3. AEF Treatment on AD Mouse Model
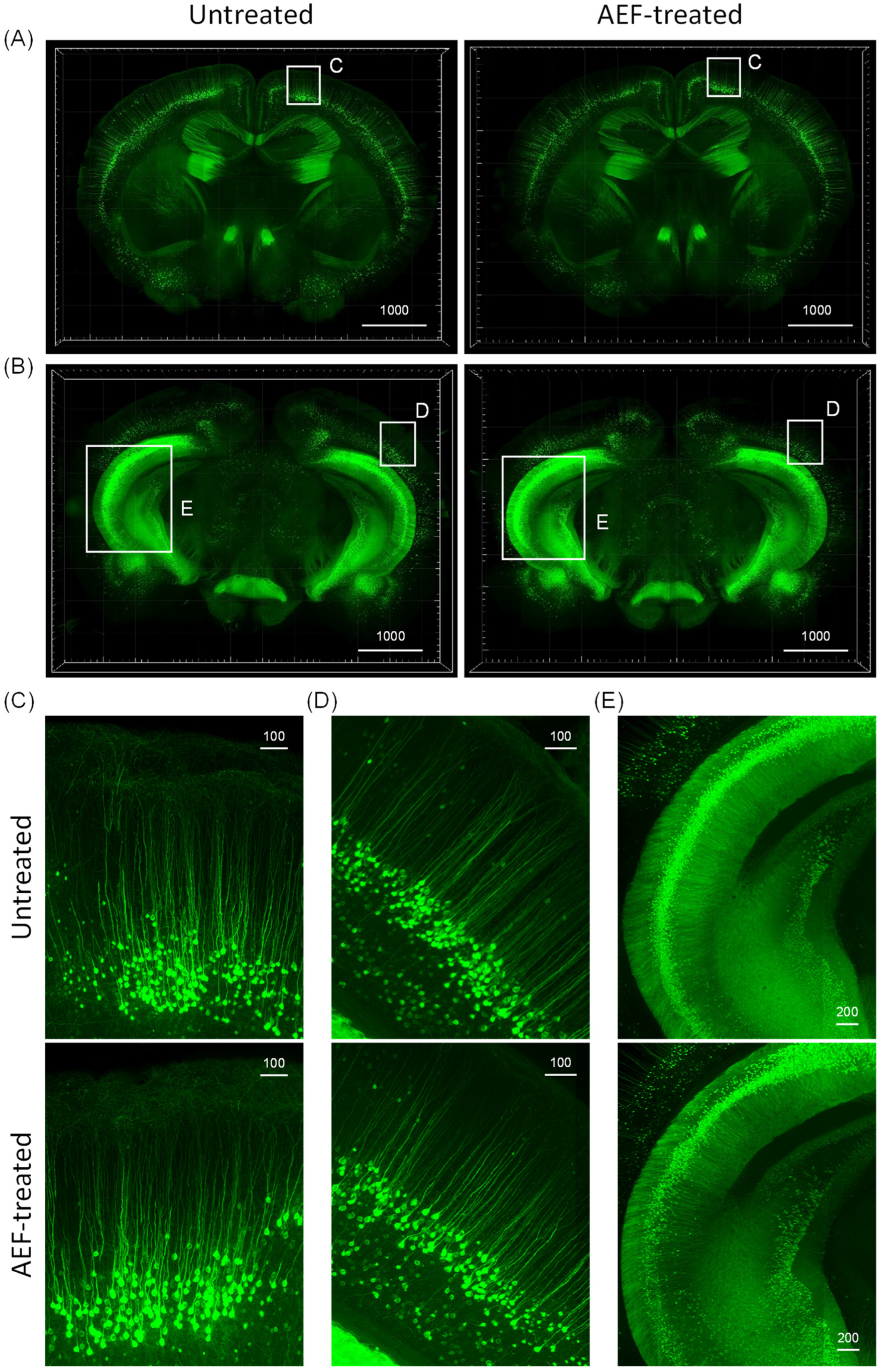
2.4. Assessment of Overall Neuronal Morphology in the AEF-Treated Brain
3. Discussion
4. Materials and Methods
4.1. Synthesis of Magnetite and Aβ/Magnetite or Aβ Fibril
4.2. Electric Field Stimulation
4.3. ROS Measurement
4.4. TEM Imaging
4.5. Animal Model and AEF Treatment
4.6. Sample Preparation and Histological Staining of Plaques
4.7. Behavioral Tests
4.7.1. Radial Arm Maze (RAM) Test
4.7.2. Novel Object Recognition (NOR) Test
4.8. Safety Evaluation of AEF Treatment with Large-Volume Imaging by Tissue Clearing
4.9. Statistics
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinshenker, D. Long Road to Ruin: Noradrenergic Dysfunction in Neurodegenerative Disease. Trends Neurosci. 2018, 42, 211–223. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Khan, U.A.; Liu, L.; Provenzano, F.A.; Berman, D.E.; Profaci, C.P.; Sloan, R.; Small, S.A. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat. Neurosci. 2014, 17, 304–311. [Google Scholar] [CrossRef]
- Schmitz, T.W.; Nathan Spreng, R. Alzheimer’s Disease Neuroimaging I. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. 2016, 7, 13249. [Google Scholar] [CrossRef]
- Schröder, N.; Figueiredo, L.S.; de Lima, M.N. Role of brain iron accumulation in cognitive dysfunction: Evidence from animal models and human studies. J. Alzheimers Dis. 2013, 34, 797–812. [Google Scholar] [CrossRef]
- Telling, N.D.; Everett, J.; Collingwood, J.F.; Dobson, J.; van der Laan, G.; Gallagher, J.J.; Wang, J.; Hitchcock, A.P. Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer’s disease. Cell Chem. Biol. 2017, 24, 1205–1215. [Google Scholar] [CrossRef]
- Spotorno, N.; Acosta-Cabronero, J.; Stomrud, E.; Lampinen, B.; Strandberg, O.T.; van Westen, D.; Hansson, O. Relationship between cortical iron and tau aggregation in Alzheimer’s disease. Brain 2020, 143, 1341–1349. [Google Scholar] [CrossRef]
- Rao, S.S.; Adlard, P.A. Untangling Tau and Iron: Exploring the Interaction Between Iron and Tau in Neurodegeneration. Front. Mol. Neurosci. 2018, 11, 276. [Google Scholar] [CrossRef]
- Everett, J.; Brooks, J.; Collingwood, J.F.; Telling, N.D. Nanoscale chemical speciation of β-amyloid/iron aggregates using soft X-ray spectromicroscopy. Inorg. Chem. Front. 2021, 8, 1439–1448. [Google Scholar] [CrossRef]
- James, S.A.; Churches, Q.I.; de Jonge, M.D.; Birchall, I.E.; Streltsov, V.; McColl, G.; Hare, D.J. Iron, Copper, and Zinc Concentration in Aβ Plaques in the APP/PS1 Mouse Model of Alzheimer’s Disease Correlates with Metal Levels in the Surrounding Neuropil. ACS Chem. Neurosci. 2017, 8, 629–637. [Google Scholar] [CrossRef]
- Yuan, P.; Grutzendler, J. Attenuation of β-Amyloid Deposition and Neurotoxicity by Chemogenetic Modulation of Neural Activity. J. Neurosci. 2016, 36, 632–641. [Google Scholar] [CrossRef]
- Querol-Vilaseca, M.; Colom-Cadena, M.; Pegueroles, J.; Nuñez-Llaves, R.; Luque-Cabecerans, J.; Muñoz-Llahuna, L.; Andilla, J.; Belbin, O.; Spires-Jones, T.L.; Gelpi, E.; et al. Nanoscale structure of amyloid-β plaques in Alzheimer’s disease. Sci. Rep. 2019, 9, 5181. [Google Scholar] [CrossRef]
- Esteras, N.; Kundel, F.; Amodeo, G.F.; Pavlov, E.V.; Klenerman, D.; Abramov, A.Y. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J. 2021, 288, 127–141. [Google Scholar] [CrossRef]
- Limorenko, G.; Lashuel, H.A. Revisiting the grammar of Tau aggregation and pathology formation: How new insights from brain pathology are shaping how we study and target Tauopathies. Chem. Soc. Rev. 2022, 51, 513–565. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, J.K. Investigation of the redox state of magnetite upon Aβ-fibril formation or proton irradiation; implication of iron redox inactivation and β-amyloidolysis. MRS Commun. 2018, 8, 955–960. [Google Scholar] [CrossRef]
- Ward, R.J.; Dexter, D.T.; Crichton, R.R. Iron, neuroinflammation and neurodegeneration. Int. J. Mol. Sci. 2022, 23, 7267. [Google Scholar] [CrossRef]
- Wang, F.; Wang, J.; Shen, Y.; Li, H.; Rausch, W.-D.; Huang, X. Iron dyshomeostasis and ferroptosis: A new Alzheimer’s disease hypothesis? Front. Aging Neurosci. 2022, 14, 830569. [Google Scholar] [CrossRef]
- Singh, N.; Haldar, S.; Tripathi, A.K.; Horback, K.; Wong, J.; Sharma, D.; Singh, A. Brain iron homeostasis: From molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid. Redox Signal. 2014, 20, 1324–1363. [Google Scholar] [CrossRef]
- Gleason, A.; Bush, A.I. Iron and Ferroptosis as Therapeutic Targets in Alzheimer’s Disease. Neurotherapeutics 2021, 18, 252–264. [Google Scholar] [CrossRef]
- Slater, C.; Wang, Q. Alzheimer’s disease: An evolving understanding of noradrenergic involvement and the promising future of electroceutical therapies. Clin. Transl. Med. 2021, 11, e397. [Google Scholar] [CrossRef]
- Seo, S.-J.; Chang, W.-S.; Jeon, J.-G.; Choi, Y.; Kim, E.H.; Kim, J.-K. Proton Stimulation Targeting Plaque Magnetite Reduces Amyloid-β Plaque and Iron Redox Toxicity and Improves Memory in an Alzheimer’s Disease Mouse Model. J. Alzheimers Dis. 2021, 84, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Ben Haim, L.; Carrillo-de Sauvage, M.A.; Ceyzériat, K.; Escartin, C. Elusive roles for reactive astrocytes in neurodegenerative diseases. Front. Cell. Neurosci. 2015, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef] [PubMed]
- Sheltraw, D.J.; Inglis, B.; Labruna, L.; Ivry, R. Comparing the electric fields of transcranial electric and magnetic perturbation. J. Neural. Eng. 2021, 18, 046067. [Google Scholar] [CrossRef]
- Liu, H.; Di Valentin, C. Band Gap in Magnetite above Verwey Temperature Induced by Symmetry Breaking. J. Phys. Chem. C 2017, 121, 25736–25742. [Google Scholar] [CrossRef]
- Guo, Q.; Lei, C.; Chen, W.; Zhang, J.; Huang, B. Electric-Field-Driven Nanoparticles Produce Dual-Functional Bipolar Electrodes and Nanoelectrolytic Cells for Water Remediation. Cell Rep. Phys. Sci. 2021, 2, 100299. [Google Scholar] [CrossRef]
- He, Z.; Gao, C.; Qian, M.; Shi, Y.; Chen, J.; Song, S. Electro-Fenton Process Catalyzed by Fe3O4 Magnetic Nanoparticles for Degradation of C.I. Reactive Blue 19 in Aqueous Solution: Operating Conditions, Influence, and Mechanism. Ind. Eng. Chem. Res. 2014, 53, 3435–3447. [Google Scholar] [CrossRef]
- Wydra, R.J.; Oliver, C.E.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. Accelerated generation of free radicals by iron oxide nanoparticles in the presence of an alternating magnetic field. RSC Adv. 2015, 5, 18888–18893. [Google Scholar] [CrossRef]
- Everett, J.; Brooks, J.; Lermyte, F.; O’Connor, P.B.; Peter JSadler, P.J.; Dobson, J.; Collingwood, J.F.; Telling, N.D. Iron stored in ferritin is chemically reduced in the presence of aggregating Aβ(1-42). Sci. Rep. 2020, 10, 10332. [Google Scholar] [CrossRef]
- Balejcikova, L.; Siposova, K.; Kopcansky, P.; Safarik, I. Fe(II) formation after interaction of the amyloid β-peptide with iron-storage protein ferritin. J. Biol. Phys. 2018, 44, 237–243. [Google Scholar] [CrossRef]
- Falangola, M.F.; Lee, S.P.; Nixon, R.A.; Duff, K.; Helpern, J.A. Histological co-localization of iron in Abeta plaques of PS/APP transgenic mice. Neurochem. Res. 2005, 30, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Chinnu, B.; Prasad, K.R.S.; Rao, G.N.; Babu, K.S. Dielectric response of magnetite nanoparticles. Eur. J. Mol. Clin. Med. 2020, 7, 573–581. [Google Scholar]
- Radoń, A.; Kądziołka-Gaweł, M.; Łukowiec, D.; Gębara, P.; Cesarz-Andraczke, K.; Kolano-Burian, A.; Włodarczyk, P.; Polak, M.; Babilas, R. Influence of Magnetite Nanoparticles Shape and Spontaneous Surface Oxidation on the Electron Transport Mechanism. Materials 2021, 14, 5241. [Google Scholar] [CrossRef] [PubMed]
- Radoń, A.; Łukowiec, D.; Kremzer, M.; Mikuła, J.; Włodarczyk, P. Electrical Conduction Mechanism and Dielectric Properties of Spherical Shaped Fe3O4 Nanoparticles Synthesized by Co-Precipitation Method. Materials 2018, 11, 735. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Im, H.; Kang, Y.J.; Kim, Y.; Shin, J.H.; Won, W.; Lim, J.; Ju, Y.; Park, Y.M.; Kim, S.; et al. Severe reactive astrocytes precipitate pathological hallmarks of Alzheimer’s disease via H2O2− production. Nat. Neurosci. 2020, 23, 1555–1566. [Google Scholar] [CrossRef]
- Navarro, A.; Tolivia, J.; Del Valle, E. Congo red method for demonstrating amyloid in paraffin sections. J. Histotechnol. 1999, 22, 305–308. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Lee, W.-S.; Lee, J.; Park, S.-H.; Kim, S.; Kim, K.-H.; Park, S.; Kim, E.H.; Kim, J.-K. Capacitive Electrode-Based Electric Field Treatments on Redox-Toxic Iron Deposits in Transgenic AD Mouse Models: The Electroceutical Targeting of Alzheimer’s Disease Feasibility Study. Int. J. Mol. Sci. 2023, 24, 9552. https://doi.org/10.3390/ijms24119552
Choi Y, Lee W-S, Lee J, Park S-H, Kim S, Kim K-H, Park S, Kim EH, Kim J-K. Capacitive Electrode-Based Electric Field Treatments on Redox-Toxic Iron Deposits in Transgenic AD Mouse Models: The Electroceutical Targeting of Alzheimer’s Disease Feasibility Study. International Journal of Molecular Sciences. 2023; 24(11):9552. https://doi.org/10.3390/ijms24119552
Chicago/Turabian StyleChoi, Younshick, Won-Seok Lee, Jaemeun Lee, Sun-Hyun Park, Sunwoung Kim, Ki-Hong Kim, Sua Park, Eun Ho Kim, and Jong-Ki Kim. 2023. "Capacitive Electrode-Based Electric Field Treatments on Redox-Toxic Iron Deposits in Transgenic AD Mouse Models: The Electroceutical Targeting of Alzheimer’s Disease Feasibility Study" International Journal of Molecular Sciences 24, no. 11: 9552. https://doi.org/10.3390/ijms24119552
APA StyleChoi, Y., Lee, W. -S., Lee, J., Park, S. -H., Kim, S., Kim, K. -H., Park, S., Kim, E. H., & Kim, J. -K. (2023). Capacitive Electrode-Based Electric Field Treatments on Redox-Toxic Iron Deposits in Transgenic AD Mouse Models: The Electroceutical Targeting of Alzheimer’s Disease Feasibility Study. International Journal of Molecular Sciences, 24(11), 9552. https://doi.org/10.3390/ijms24119552





