Advances in Rodent Experimental Models of Sepsis
Abstract
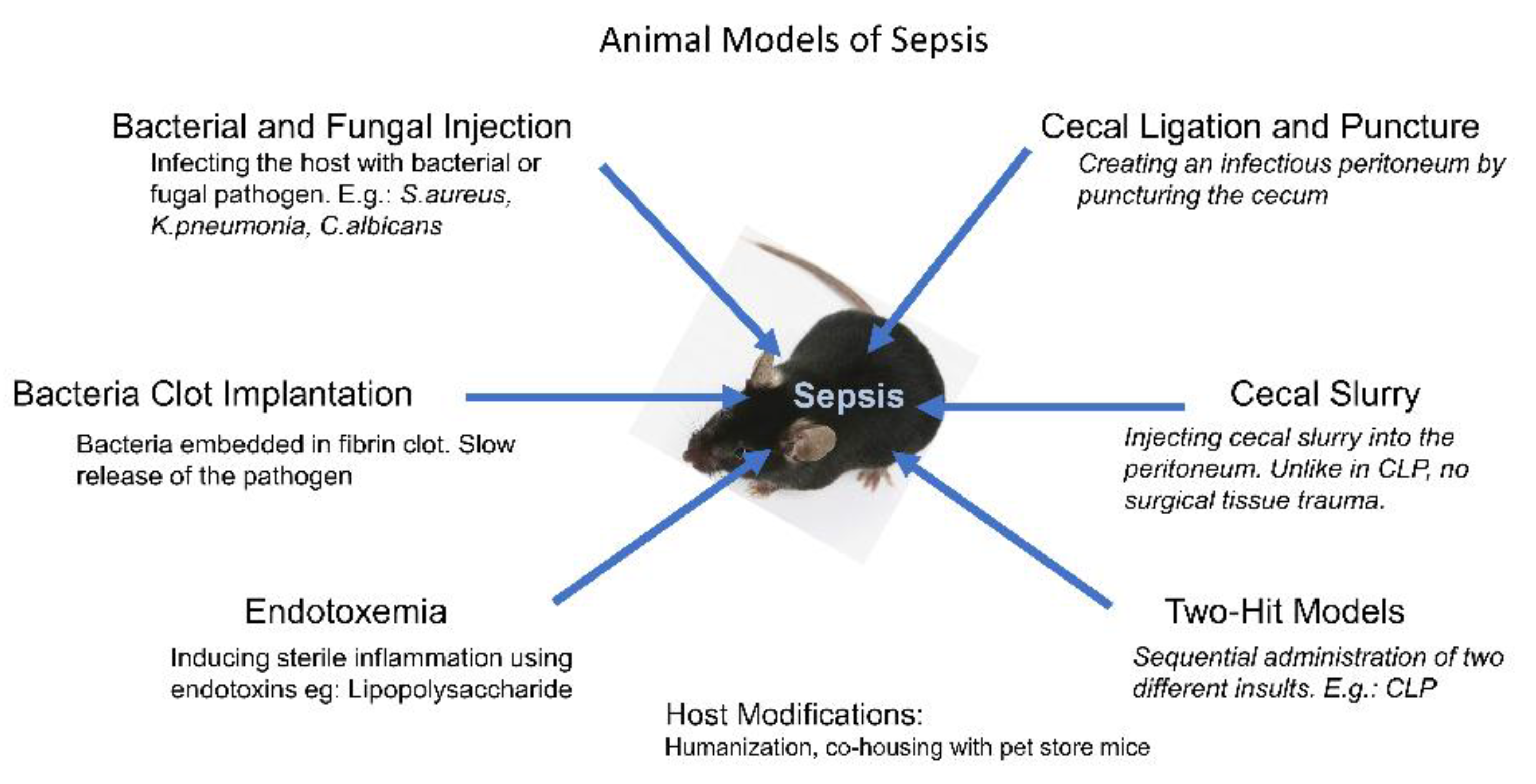
:1. Introduction
2. Bacterial and Fungal Infection Models
3. Bacteria Clot Implantation Models
4. Endotoxemia Models
5. Intraperitoneal Sepsis
6. The Two-Hit Models
7. Genetic Background and Phylogenetic Distance
8. Humanized Mice and “Dirty” Mice in Sepsis Studies
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA-J. Am. Med. Assoc. 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Coopersmith, C.M.; McDunn, J.E.; Ferguson, T.A. The sepsis seesaw: Tilting toward immunosuppression. Nat. Med. 2009, 15, 496–497. [Google Scholar] [CrossRef]
- Hilburger, M.E.; Adler, M.W.; Truant, A.L.; Meissler, J.J., Jr.; Satishchandran, V.; Rogers, T.J.; Eisenstein, T.K. Morphine induces sepsis in mice. J. Infect. Dis. 1997, 176, 183–188. [Google Scholar] [CrossRef]
- Martin, G.S. Sepsis, severe sepsis and septic shock: Changes in incidence, pathogens and outcomes. Expert Rev. Anti-Infect. Ther. 2012, 10, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Hu, X.G.; Liu, C.; Chen, M.; Wang, J.N.; Wang, M.Y.; Gao, F.; Han, J.Q.; Sun, D.; Zhang, C.X.; et al. Gelsolin Inhibits the Inflammatory Process Induced by LPS. Cell. Physiol. Biochem. 2017, 41, 205–212. [Google Scholar] [CrossRef]
- Wallet, F.; Loiez, C.; Herwegh, S.; Courcol, R.J. Usefulness of real-time PCR for the diagnosis of sepsis in ICU-acquired infections. Infect. Disord. Drug. Targets 2011, 11, 348–353. [Google Scholar] [CrossRef]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; on behalf of the International Forum of Acute Care Trialists. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, A.B.; Bronsveld, W.; Thijs, L.G. Hemodynamic determinants of mortality in human septic shock. Surgery 1986, 99, 140–153. [Google Scholar] [PubMed]
- Buras, J.A.; Holzmann, B.; Sitkovsky, M. Animal models of sepsis: Setting the stage. Nat. Rev. Drug. Discov. 2005, 4, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Bland, R.D.; Cobo, J.C.; Shoemaker, W.C. Sequential cardiorespiratory patterns associated with outcome in septic shock. Chest 1984, 85, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Raju, R. Immune and metabolic alterations following trauma and sepsis—An overview. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2523–2525. [Google Scholar] [CrossRef]
- Laudanski, K. Humanized Mice as a Tool to Study Sepsis-More Than Meets the Eye. Int. J. Mol. Sci. 2021, 22, 2403. [Google Scholar] [CrossRef] [PubMed]
- Schuurman, A.R.; Sloot, P.M.A.; Wiersinga, W.J.; van der Poll, T. Embracing complexity in sepsis. Crit. Care 2023, 27, 102. [Google Scholar] [CrossRef] [PubMed]
- Arora, J.; Mendelson, A.A.; Fox-Robichaud, A. Sepsis: Network pathophysiology and implications for early diagnosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R613–R624. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Singer, M.; Dal-Pizzol, F. Biomarkers for sepsis: More than just fever and leukocytosis-a narrative review. Crit. Care 2022, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Korneev, K.V. Mouse Models of Sepsis and Septic Shock. Mol. Biol. 2019, 53, 799–814. [Google Scholar] [CrossRef] [PubMed]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Komorowski, M.; Green, A.; Tatham, K.C.; Seymour, C.; Antcliffe, D. Sepsis biomarkers and diagnostic tools with a focus on machine learning. EBioMedicine 2022, 86, 104394. [Google Scholar] [CrossRef]
- Kim, M.H.; Choi, J.H. An Update on Sepsis Biomarkers. Infect. Chemother. 2020, 52, 1–18. [Google Scholar] [CrossRef]
- de Stoppelaar, S.F.; van ’t Veer, C.; Claushuis, T.A.; Albersen, B.J.; Roelofs, J.J.; van der Poll, T. Thrombocytopenia impairs host defense in gram-negative pneumonia-derived sepsis in mice. Blood 2014, 124, 3781–3790. [Google Scholar] [CrossRef] [PubMed]
- Kahn, F.; Hurley, S.; Shannon, O. Platelets promote bacterial dissemination in a mouse model of streptococcal sepsis. Microbes Infect. 2013, 15, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Powers, M.E.; Becker, R.E.N.; Sailer, A.; Turner, J.R.; Wardenburg, J.B. Synergistic Action of Staphylococcus aureus alpha-Toxin on Platelets and Myeloid Lineage Cells Contributes to Lethal Sepsis. Cell. Host Microbe 2015, 17, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.G.; Zhang, G.Y.; Guo, L.; Li, X.A.; Morris, A.J.; Daugherty, A.; Whiteheart, S.W.; Smyth, S.S.; Li, Z.Y. Platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the cyclooxygenase 1 signalling pathway. Nat. Commun. 2013, 4, 2657. [Google Scholar] [CrossRef] [PubMed]
- van den Boogaard, F.E.; Schouten, M.; de Stoppelaar, S.F.; Roelofs, J.J.T.H.; Brands, X.; Schultz, M.J.; van’t Veer, C.; van der Poll, T. Thrombocytopenia Impairs Host Defense During Murine Streptococcus pneumoniae Pneumonia. Crit. Care Med. 2015, 43, E75–E83. [Google Scholar] [CrossRef]
- Assinger, A.; Schrottmaier, W.C.; Salzmann, M.; Rayes, J. Platelets in Sepsis: An Update on Experimental Models and Clinical Data. Front. Immunol. 2019, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Deitch, E.A. Animal models of sepsis and shock: A review and lessons learned. Shock 1998, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Stanojcic, M.; Jeschke, M.G. Differences Between Murine and Human Sepsis. Surg. Clin. N. Am. 2014, 94, 1135–1149. [Google Scholar] [CrossRef]
- Poli-De-Figueiredo, L.F.; Garrido, A.G.; Nakagawa, N.K.; Sannomiya, P. Experimental models of sepsis and their clinical relevance. Shock 2008, 30, 53–59. [Google Scholar] [CrossRef]
- Parker, S.J.; Watkins, P.E. Experimental models of gram-negative sepsis. Br. J. Surg. 2001, 88, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Feldman, C.; Shaddock, E. Epidemiology of lower respiratory tract infections in adults. Expert Rev. Respir. Med. 2019, 13, 63–77. [Google Scholar] [CrossRef]
- van der Poll, T. Preclinical Sepsis Models. Surg. Infect. 2012, 13, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.L.; Pennington, J.E. Effects of aging on antibacterial mechanisms in experimental pneumonia. Am. Rev. Respir. Dis. 1983, 128, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Mares, C.A.; Sharma, J.; Ojeda, S.S.; Li, Q.; Campos, J.A.; Morris, E.G.; Coalson, J.J.; Teale, J.M. Attenuated response of aged mice to respiratory Francisella novicida is characterized by reduced cell death and absence of subsequent hypercytokinemia. PLoS ONE 2010, 5, e14088. [Google Scholar] [CrossRef] [PubMed]
- Shivshankar, P.; Boyd, A.R.; Le Saux, C.J.; Yeh, I.T.; Orihuela, C.J. Cellular senescence increases expression of bacterial ligands in the lungs and is positively correlated with increased susceptibility to pneumococcal pneumonia. Aging Cell 2011, 10, 798–806. [Google Scholar] [CrossRef]
- Antonini, J.M.; Roberts, J.R.; Clarke, R.W.; Yang, H.M.; Barger, M.W.; Ma, J.Y.; Weissman, D.N. Effect of age on respiratory defense mechanisms: Pulmonary bacterial clearance in Fischer 344 rats after intratracheal instillation of Listeria monocytogenes. Chest 2001, 120, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.E.; Saito, H. Sepsis in old age: Review of human and animal studies. Aging Dis. 2014, 5, 126–136. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Yadav, B. Microbe Profile: Candida albicans: A shape-changing, opportunistic pathogenic fungus of humans. Microbiology (Reading) 2017, 163, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Fajardo, P.; Cuenda, A.; Sanz-Ezquerro, J.J. A Mouse Model of Candidiasis. Methods Mol. Biol. 2021, 2321, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Holder, I.A.; Nathan, P. Effect in mice of injection of viable Candida albicans and a cell-free sonic extract on circulating platelets. Infect. Immun. 1973, 7, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Wurster, S.; Albert, N.D.; Kontoyiannis, D.P. Candida auris Bloodstream Infection Induces Upregulation of the PD-1/PD-L1 Immune Checkpoint Pathway in an Immunocompetent Mouse Model. mSphere 2022, 7, e0081721. [Google Scholar] [CrossRef]
- Ghanta, S.; Kwon, M.Y.; Perrella, M.A. Induction of Sepsis Via Fibrin Clot Implantation. Methods Mol. Biol. 2021, 2321, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Mathiak, G.; Szewczyk, D.; Abdullah, F.; Ovadia, P.; Feuerstein, G.; Rabinovici, R. An improved clinically relevant sepsis model in the conscious rat. Crit. Care Med. 2000, 28, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Toky, V.; Sharma, S.; Arora, B.B.; Chhibber, S. Establishment of a sepsis model following implantation of Klebsiella pneumoniae-infected fibrin clot into the peritoneal cavity of mice. Folia Microbiol. 2003, 48, 665–669. [Google Scholar] [CrossRef]
- Ahrenholz, D.H.; Simmons, R.L. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery 1980, 88, 41–47. [Google Scholar]
- Natanson, C.; Fink, M.P.; Ballantyne, H.K.; MacVittie, T.J.; Conklin, J.J.; Parrillo, J.E. Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. J. Clin. Investig. 1986, 78, 259–270. [Google Scholar] [CrossRef]
- Deitch, E.A. Rodent models of intra-abdominal infection. Shock 2005, 24 (Suppl. 1), 19–23. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, ESP-0001-2018. [Google Scholar] [CrossRef]
- Fink, M.P. Animal models of sepsis. Virulence 2014, 5, 143–153. [Google Scholar] [CrossRef]
- Traber, D.L.; Flynn, J.T.; Herndon, D.N.; Redl, H.; Schlag, G.; Traber, L.D. Comparison of the Cardiopulmonary Responses to Single Bolus and Continuous Infusion of Endotoxin in an Ovine Model. Circ. Shock 1989, 27, 123–138. [Google Scholar] [PubMed]
- Boomer, J.S.; Green, J.M.; Hotchkiss, R.S. The changing immune system in sepsis: Is individualized immuno-modulatory therapy the answer? Virulence 2014, 5, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Stortz, J.A.; Raymond, S.L.; Mira, J.C.; Moldawer, L.L.; Mohr, A.M.; Efron, P.A. Murine Models of Sepsis and Trauma: Can We Bridge the Gap? Ilar J. 2017, 58, 90–105. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, S.E.; Rahman, M.; Herwald, H.; Thorlacius, H. Streptococcal M1 Protein-Induced Lung Injury Is Independent of Platelets in Mice. Shock 2011, 35, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Bjorck, V.; Pahlman, L.I.; Tornebrant, J.; Bodelsson, M. Streptococcal M1 protein induces hyporesponsiveness and cytokine release from human arteries in a fibrinogen-dependent manner: A translational study. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 61. [Google Scholar] [CrossRef]
- Rao, T.S.; Currie, J.L.; Shaffer, A.F.; Isakson, P.C. In-Vivo Characterization of Zymosan-Induced Mouse Peritoneal Inflammation. J. Pharmacol. Exp. Ther. 1994, 269, 917–925. [Google Scholar]
- Volman, T.J.H.; Hendriks, T.; Goris, R.J.A. Zymosan-induced generalized inflammation: Experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock 2005, 23, 291–297. [Google Scholar] [CrossRef]
- Vonasmuth, E.J.U.; Maessen, J.G.; Vanderlinden, C.J.; Buurman, W.A. Tumor Necrosis Factor-Alpha (Tnf-Alpha) and Interleukin-6 in a Zymosan-Induced Shock Model. Scand. J. Immunol. 1990, 32, 313–319. [Google Scholar] [CrossRef]
- Dejager, L.; Pinheiro, I.; Dejonckheere, E.; Libert, C. Cecal ligation and puncture: The gold standard model for polymicrobial sepsis? Trends Microbiol. 2011, 19, 198–208. [Google Scholar] [CrossRef]
- Li, J.L.; Li, G.; Jing, X.Z.; Li, Y.F.; Ye, Q.Y.; Jia, H.H.; Liu, S.H.; Li, X.J.; Li, H.; Huang, R.; et al. Assessment of clinical sepsis-associated biomarkers in a septic mouse model. J. Int. Med. Res. 2018, 46, 2410–2422. [Google Scholar] [CrossRef]
- Bounes, F.V.; Memier, V.; Marcaud, M.; Jacquemin, A.; Hamzeh-Cognasse, H.; Garcia, C.; Series, J.; Sie, P.; Minville, V.; Gratacap, M.P.; et al. Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis. Sci. Rep. 2018, 8, 13536. [Google Scholar] [CrossRef]
- Wichterman, K.A.; Baue, A.E.; Chaudry, I.H. Sepsis and Septic Shock—A Review of Laboratory Models and a Proposal. J. Surg. Res. 1980, 29, 189–201. [Google Scholar] [CrossRef]
- Cai, L.; Arbab, A.S.; Lee, T.J.; Sharma, A.; Thomas, B.; Igarashi, K.; Raju, R.P. BACH1-Hemoxygenase-1 axis regulates cellular energetics and survival following sepsis. Free Radic. Biol. Med. 2022, 188, 134–145. [Google Scholar] [CrossRef]
- Subramani, K.; Raju, S.P.; Chu, X.; Warren, M.; Pandya, C.D.; Hoda, N.; Fulzele, S.; Raju, R. Effect of plasma-derived extracellular vesicles on erythrocyte deformability in polymicrobial sepsis. Int. Immunopharmacol. 2018, 65, 244–247. [Google Scholar] [CrossRef]
- Singer, G.; Houghton, J.; Rivera, C.A.; Anthoni, C.; Granger, D.N. Role of LPS in the hepatic microvascular dysfunction elicited by cecal ligation and puncture in mice. J. Hepatol. 2007, 47, 799–806. [Google Scholar] [CrossRef]
- Remick, D.G.; Newcomb, D.E.; Bolgos, G.L.; Call, D.R. Comparison of the mortality and inflammatory response of two models of sepsis: Lipopolysaccharide vs. cecal ligation find puncture. Shock 2000, 13, 110–116. [Google Scholar] [CrossRef]
- Gentile, L.F.; Nacionales, D.C.; Lopez, M.C.; Vanzant, E.; Cuenca, A.; Szpila, B.E.; Cuenca, A.G.; Joseph, A.; Moore, F.A.; Leeuwenburgh, C.; et al. Host responses to sepsis vary in different low-lethality murine models. PLoS ONE 2014, 9, e94404. [Google Scholar] [CrossRef]
- Mourelatos, M.G.; Enzer, N.; Ferguson, J.L.; Rypins, E.B.; Burhop, K.E.; Law, W.R. The effects of diaspirin cross-linked hemoglobin in sepsis. Shock 1996, 5, 141–148. [Google Scholar] [CrossRef]
- Starr, M.E.; Steele, A.M.; Saito, M.; Hacker, B.J.; Evers, B.M.; Saito, H. A new cecal slurry preparation protocol with improved long-term reproducibility for animal models of sepsis. PLoS ONE 2014, 9, e115705. [Google Scholar] [CrossRef]
- Dyson, A.; Rudiger, A.; Singer, M. Temporal changes in tissue cardiorespiratory function during faecal peritonitis. Intensive Care Med. 2011, 37, 1192–1200. [Google Scholar] [CrossRef]
- Wynn, J.L.; Scumpia, P.O.; Delano, M.J.; O’Malley, K.A.; Ungaro, R.; Abouhamze, A.; Moldawer, L.L. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 2007, 28, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Zantl, N.; Uebe, A.; Neumann, B.; Wagner, H.; Siewert, J.R.; Holzmann, B.; Heidecke, C.D.; Pfeffer, K. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect. Immun. 1998, 66, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.; Traeger, T.; Entleutner, M.; Westerholt, A.; Kleist, B.; Huser, N.; Holzmann, B.; Stier, A.; Pfeffer, K.; Heidecke, C.D. Cecal ligation and puncture versus colon ascendens stent peritonitis: Two distinct animal models for polymicrobial sepsis. Shock 2004, 21, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Hoesel, L.M.; Ward, P.A. The disconnect between animal models of sepsis and human sepsis. J. Leukocyte Biol. 2007, 81, 137–143. [Google Scholar] [CrossRef]
- Barthlen, W.; Zantl, N.; Pfeffer, K.; Heidecke, C.D.; Holzmann, B.; Stadler, J. Impact of experimental peritonitis on bone marrow cell function. Surgery 1999, 126, 41–47. [Google Scholar] [CrossRef]
- Maier, S.; Emmanuilidis, K.; Entleutner, M.; Zantl, N.; Werner, M.; Pfeffer, K.; Heidecke, C.D. Massive chemokine transcription in acute renal failure due to polymicrobial sepsis. Shock 2000, 14, 187–192. [Google Scholar] [CrossRef]
- Neumann, B.; Zantl, N.; Veihelmann, A.; Emmanuilidis, K.; Pfeffer, K.; Heidecke, C.D.; Holzmann, B. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int. Immunol. 1999, 11, 217–227. [Google Scholar] [CrossRef]
- Feterowski, C.; Mack, M.; Weighardt, H.; Bartsch, B.; Kaiser-Moore, S.; Holzmann, B. CC chemokine receptor 2 regulates leukocyte recruitment and IL-10 production during acute polymicrobial sepsis. Eur. J. Immunol. 2004, 34, 3664–3673. [Google Scholar] [CrossRef]
- Traeger, T.; Koerner, P.; Kessler, W.; Cziupka, K.; Diedrich, S.; Busemann, A.; Heidecke, C.D.; Maier, S. Colon ascendens stent peritonitis (CASP)—A standardized model for polymicrobial abdominal sepsis. J. Vis. Exp. 2010, 46, e2299. [Google Scholar] [CrossRef]
- Lustig, M.K.; Bac, V.H.; Pavlovic, D.; Maier, S.; Grundling, M.; Grisk, O.; Wendt, M.; Heidecke, C.D.; Lehmann, C. Colon ascendens stent peritonitis—A model of sepsis adopted to the rat: Physiological, microcirculatory and laboratory changes. Shock 2007, 28, 59–64. [Google Scholar] [CrossRef]
- Ai, H.; Li, B.; Meng, F.; Ai, Y. CASP-Model Sepsis Triggers Systemic Innate Immune Responses Revealed by the Systems-Level Signaling Pathways. Front. Immunol. 2022, 13, 907646. [Google Scholar] [CrossRef]
- van der Linde, J.; Diedrich, S.; Klee, T.; Heidecke, C.D.; Kersting, S.; Kessler, W. Disseminated Intravascular Coagulation (DIC): Old player creates new perspectives on the polymicrobial sepsis model of CASP. PLoS ONE 2022, 17, e0277492. [Google Scholar] [CrossRef]
- Saadia, R.; Schein, M. Multiple organ failure. How valid is the “two hit” model? J. Accid. Emerg. Med. 1999, 16, 163–166, discussion 166–167. [Google Scholar] [CrossRef]
- Sundarasivarao, P.Y.K.; Walker, J.M.; Rodriguez, A.; Spur, B.W.; Yin, K. Resolvin D2 induces anti-microbial mechanisms in a model of infectious peritonitis and secondary lung infection. Front. Immunol. 2022, 13, 1011944. [Google Scholar] [CrossRef]
- Bastarache, J.A.; Smith, K.; Jesse, J.J.; Putz, N.D.; Meegan, J.E.; Bogart, A.M.; Schaaf, K.; Ghosh, S.; Shaver, C.M.; Ware, L.B. A two-hit model of sepsis plus hyperoxia causes lung permeability and inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L273–L282. [Google Scholar] [CrossRef]
- Restagno, D.; Venet, F.; Paquet, C.; Freyburger, L.; Allaouchiche, B.; Monneret, G.; Bonnet, J.M.; Louzier, V. Mice Survival and Plasmatic Cytokine Secretion in a “Two Hit” Model of Sepsis Depend on Intratracheal Pseudomonas Aeruginosa Bacterial Load. PLoS ONE 2016, 11, e0162109. [Google Scholar] [CrossRef]
- Mileski, W.J.; Winn, R.K.; Harlan, J.M.; Rice, C.L. Sensitivity to endotoxin in rabbits is increased after hemorrhagic shock. J. Appl. Physiol. 1992, 73, 1146–1149. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, F.A.; Franciose, R.J.; Kim, F.J.; Biffl, W.L.; Banerjee, A. The postischemic gut serves as a priming bed for circulating neutrophils that provoke multiple organ failure. J. Trauma 1994, 37, 881–887. [Google Scholar] [CrossRef]
- Perl, M.; Hohmann, C.; Denk, S.; Kellermann, P.; Lu, D.; Braumuller, S.; Bachem, M.G.; Thomas, J.; Knoferl, M.W.; Ayala, A.; et al. Role of activated neutrophils in chest trauma-induced septic acute lung injury. Shock 2012, 38, 98–106. [Google Scholar] [CrossRef]
- Efron, P.A.; Mohr, A.M.; Moore, F.A.; Moldawer, L.L. The future of murine sepsis and trauma research models. J. Leukoc. Biol. 2015, 98, 945–952. [Google Scholar] [CrossRef]
- Sellers, R.S.; Clifford, C.B.; Treuting, P.M.; Brayton, C. Immunological Variation Between Inbred Laboratory Mouse Strains: Points to Consider in Phenotyping Genetically Immunomodified Mice. Vet. Pathol. 2012, 49, 32–43. [Google Scholar] [CrossRef]
- Carreras, E.; Velasco de Andres, M.; Orta-Mascaro, M.; Simoes, I.T.; Catala, C.; Zaragoza, O.; Lozano, F. Discordant susceptibility of inbred C57BL/6 versus outbred CD1 mice to experimental fungal sepsis. Cell. Microbiol. 2019, 21, e12995. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chan, J.F.; Yuen, K.Y. Animal models in SARS-CoV-2 research. Nat. Methods 2022, 19, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Shuai, H.; Chan, J.F.; Yuen, T.T.; Yoon, C.; Hu, J.C.; Wen, L.; Hu, B.; Yang, D.; Wang, Y.; Hou, Y.; et al. Emerging SARS-CoV-2 variants expand species tropism to murines. EBioMedicine 2021, 73, 103643. [Google Scholar] [CrossRef]
- Engoren, M.; Jewell, E.S.; Douville, N.; Moser, S.; Maile, M.D.; Bauer, M.E. Genetic variants associated with sepsis. PLoS ONE 2022, 17, e0265052. [Google Scholar] [CrossRef]
- Rosier, F.; Nunez, N.F.; Torres, M.; Loriod, B.; Rihet, P.; Pradel, L.C. Transcriptional Response in a Sepsis Mouse Model Reflects Transcriptional Response in Sepsis Patients. Int. J. Mol. Sci. 2022, 23, 821. [Google Scholar] [CrossRef]
- Mural, R.J.; Adams, M.D.; Myers, E.W.; Smith, H.O.; Miklos, G.L.; Wides, R.; Halpern, A.; Li, P.W.; Sutton, G.G.; Nadeau, J.; et al. A comparison of whole-genome shotgun-derived mouse chromosome 16 and the human genome. Science 2002, 296, 1661–1671. [Google Scholar] [CrossRef]
- Ernst, W.; Zimara, N.; Hanses, F.; Mannel, D.N.; Seelbach-Gobel, B.; Wege, A.K. Humanized mice, a new model to study the influence of drug treatment on neonatal sepsis. Infect. Immun. 2013, 81, 1520–1531. [Google Scholar] [CrossRef]
- Ito, R.; Takahashi, T.; Katano, I.; Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012, 9, 208–214. [Google Scholar] [CrossRef]
- Unsinger, J.; McDonough, J.S.; Shultz, L.D.; Ferguson, T.A.; Hotchkiss, R.S. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J. Leukocyte Biol. 2009, 86, 219–227. [Google Scholar] [CrossRef]
- Mullen, Y. Development of the Nonobese Diabetic Mouse and Contribution of Animal Models for Understanding Type 1 Diabetes. Pancreas 2017, 46, 455–466. [Google Scholar] [CrossRef]
- Letson, H.L.; Morris, J.; Biros, E.; Dobson, G.P. Conventional and Specific-Pathogen Free Rats Respond Differently to Anesthesia and Surgical Trauma. Sci. Rep. 2019, 9, 9399. [Google Scholar] [CrossRef]
- Huggins, M.A.; Jameson, S.C.; Hamilton, S.E. Embracing microbial exposure in mouse research. J. Leukoc. Biol. 2019, 105, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Beura, L.K.; Hamilton, S.E.; Bi, K.; Schenkel, J.M.; Odumade, O.A.; Casey, K.A.; Thompson, E.A.; Fraser, K.A.; Rosato, P.C.; Filali-Mouhim, A.; et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016, 532, 512–516. [Google Scholar] [CrossRef]
- Hamilton, S.E.; Badovinac, V.P.; Beura, L.K.; Pierson, M.; Jameson, S.C.; Masopust, D.; Griffith, T.S. New Insights into the Immune System Using Dirty Mice. J. Immunol. 2020, 205, 3–11. [Google Scholar] [CrossRef]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef]
- Franco, N.H. Animal Experiments in Biomedical Research: A Historical Perspective. Animals 2013, 3, 238–273. [Google Scholar] [CrossRef]
- Berton, R.R.; Jensen, I.J.; Harty, J.T.; Griffith, T.S.; Badovinac, V.P. Inflammation Controls Susceptibility of Immune-Experienced Mice to Sepsis. Immunohorizons 2022, 6, 528–542. [Google Scholar] [CrossRef]
| Pros | Cons | |
|---|---|---|
| Bacterial and Fungal Infection | Selected pathogens of choice can be tested, lack of surgical insult, and ability to study progression of infection in relation to severity. | Strain, dose, and route dependence on severity; single pathogen may not reflect human sepsis. |
| Bacteria Clot Implantation | Allows slow pathogen release, produces progressive sepsis, and prolonged immunometabolic dysregulation. | Reproducibility depends on clot standardization. |
| Endotoxemia | Simple procedure, reproducibility, and acute response. | Dependence on toxin, dose, and route. Differs from clinical sepsis. |
| Intraperitoneal | ||
| Cecal Ligation and Puncture | Polymicrobial sepsis. Cardio-metabolic and immune response similar to clinical sepsis. Organ dysfunction. Simple surgical procedure. | Variability of the model (needle size, number of punctures, ligated cecum length). Surgical insult. |
| Cecal Slurry | Reproducibility, ease of use, lack of surgical trauma, and organ dysfunction. | Batch-to-batch variation in the slurry. |
| Colon Ascendens Stent Peritonitis | Polymicrobial infection, organ dysfunction, and inflammatory response. | Surgical insult, variability due to stent size, and challenging surgical model. |
| Two-Hit (e.g., CLP followed by lung infection) | Mimics biphasic multiorgan failure. | Variability depending on duration between hits, nature of each hit, and sequence of hits. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Rodgers, E.; Schoenmann, N.; Raju, R.P. Advances in Rodent Experimental Models of Sepsis. Int. J. Mol. Sci. 2023, 24, 9578. https://doi.org/10.3390/ijms24119578
Cai L, Rodgers E, Schoenmann N, Raju RP. Advances in Rodent Experimental Models of Sepsis. International Journal of Molecular Sciences. 2023; 24(11):9578. https://doi.org/10.3390/ijms24119578
Chicago/Turabian StyleCai, Lun, Elizabeth Rodgers, Nick Schoenmann, and Raghavan Pillai Raju. 2023. "Advances in Rodent Experimental Models of Sepsis" International Journal of Molecular Sciences 24, no. 11: 9578. https://doi.org/10.3390/ijms24119578






