Metastatic Dissemination: Role of Tumor-Derived Extracellular Vesicles and Their Use as Clinical Biomarkers
Abstract
:1. Introduction
2. Metastatic Processes
- -
- Platelet activation: increasing evidence supports the fact that tumor cells can interact with platelets and that tumor-activated platelets play several roles in cancer metastatic dissemination, including the formation of an early metastatic niche by preparing a fertile soil for cancer cell mestastisis [10,11,12,13,14]. In a mouse model of lung cancer, the platelets-derived chemokines CXCL-5 and CXCL-7 attract granulocytes, contributing to the creation of an early metastatic niche; indeed, the inhibition of these chemokine receptors prevented granulocyte recruitment, thus impairing metastasis formation [15]. The role of platelets as mediators of communication between tumor cells and bone, before metastasis formation, has also been suggested; prostate cancer and melanoma cells are able to stimulate bone formation in distant sites (the stimulation of the bone turnover could benefit the metastasis, as cancer cells have been demonstrated to be more prone to bone colonization during the bone remodeling process) and platelet depletion inhibited this process, suggesting a key role of platelets for contributing to the generation of a tumor-favorable microenvironment [16]. Similarly, platelet involvement in PMN formation was also described for bone metastasis colonization by breast cancer cells, although it was based on different molecular mechanisms: the secretion of autotaxin by activated platelets, and its subsequent binding to tumor cell integrin ανβ3, promoted the formation of lysophosphatidic acid, which induced the osteoclast-mediated bone destruction, thus controlling the early stages of bone colonization [17]. The role of tumor-derived EVs (tEVs) in stimulating platelet activation is starting to be understood; interestingly, once activated, platelets, in turn, can release platelet EVs (pEVs), whose role in tumor biology is becoming clear, including their involvement in metastasis formation [18].
- -
- Vascular leakiness: normal endothelial cells provide a physical barrier controlling the transfer of fluids, proteins, and cells from the blood to tissues and vice versa. Normally, endothelial cells are tightly connected by adherent and tight junctions, while tumor-associated vessels are featured by increased vessel hyperpermeability supported by endothelial fenestrae, transcellular holes, loosened inter-endothelial junctions, and an irregular basement membrane [19,20,21]. This impaired vascular barrier function and the associated vascular leakage are considered to be crucial for controlling the movement of cancer cells from primary sites to the blood (intravasation) and from blood to metastatic sites (extravasation) [7,22] (see below). Many soluble or EV-associated molecules and many signaling pathways are involved in the induction of a defective endothelium, such as miRNAs, VEGF, SDF-1, and angiopoietin-like molecules [23,24,25].
- -
- Anti-tumor immunity: NK and T cells can attack transformed cells acting as a natural shield against cancer; tumor cells, indeed, are “altered self” cells that express “non-self” antigens triggering immune cells, thus being threatened by immune defense [26]. To break through this protective barrier, tumor cells play out several mechanisms (some of them based on EVs) at metastatic sites; first, being able to evade the immune system and, second, educating immune cells to establish a pro-inflammatory microenvironment that supports tumor growth and metastasis [9,27,28,29].
- -
- Education of neighboring cells: it is known that once the tumor has formed, it starts to modify the surrounding stroma to become tumor supporting; even cells in distant organs are a common target of this education activity, including CAFs, endothelial cells, or TAMs; this “priming” process, triggering the formation of PMN, can be sustained by soluble mediators released by tumor cells, as well as by tumor-derived EVs, as they are able to travel through the blood and other biological fluids [9,28,30,31,32]. It has also been shown that EVs contribute to the organ-specific tropism of metastases by integrins [33,34,35].
- -
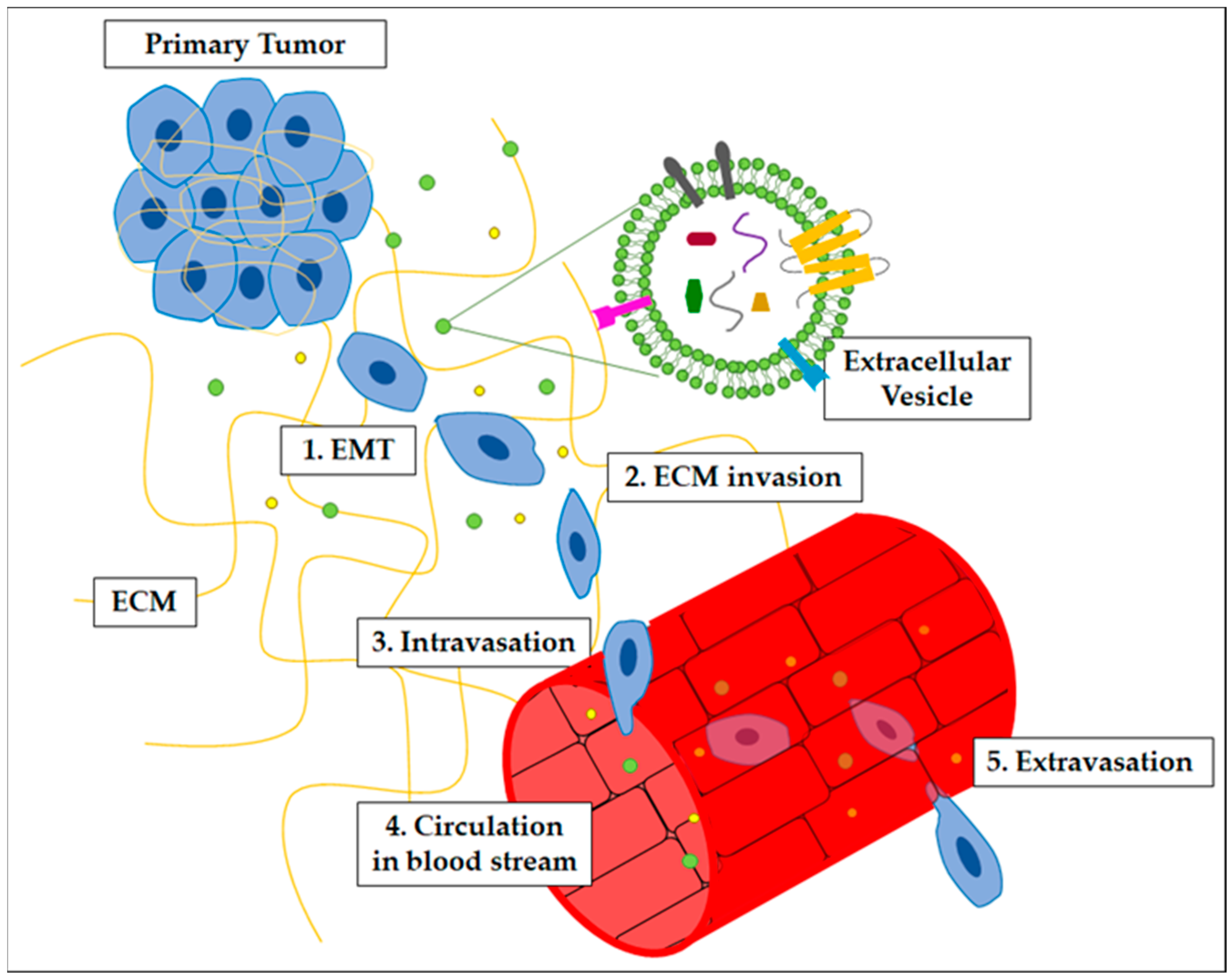
- Epithelial-to-Mesenchymal Transitions (EMT): a variety of transcription factors lead cancer cells to lose their epithelial phenotype and consequently their polarity, as well as the expression of adhesion molecules; this allows cells to move individually and acquire a fibroblast-like phenotype. The main characteristic of this transition is the increased level of invasiveness, motility, and ECM-degradation ability that the cells acquire [38].
- -
- Invasion and intravasation: to establish a successful metastatic process, cancer cells need to invade neighboring tissues by breaking the basement membrane and infiltrating themselves. A variety of ECM components such as collagen, fibronectin, and glycoprotein are involved in the invasion of tumor cells, which can migrate and invade as groups of cells, or singularly, towards blood vessels, through the primary site tissue [3]. After invasion, tumor cells undergo an intravasation process, which is driven by the establishment of an intra-tumor hypoxic environment and involves the enrolment of cells, such as CAFs and TAMs, that assist ECM modification to foster the intravasation of tumor cells in blood circulation. Intravasation is supported by invadopodia development in cancer cells. Once entered into blood circulation, cancer cells are also known as circulating tumor cells (CTCs) [39,40,41].
- -
- Resistance to death: once CTCs enter the bloodstream, they may be detected and attacked by NK cells; however, this process is inhibited by platelets, which coat circulating CTCs and protect them from being attacked through platelet factors that are released. Furthermore, CTCs are also resistant to the anoikis pathway due to an alteration in the expression of integrins and in metabolism. CTCs could also die as a result of the fluidic forces generated in the circulation; to avoid this, CTCs can form clusters that generate resistance to damage [3,13,42,43].
- -
- Extravasation: for CTCs to leave circulation and reach the target organ, it is necessary for the cells to pass through the endothelium in the target tissue. This passage is ensured by platelets, cytokines, and other factors released by cells, which cause weakening of the endothelial barrier. In this way, through the formation of new invadopodia, CTCs can leave the circulation and settle on their target [36,44,45,46].
3. Extracellular Vesicles
4. Role of Extracellular Vesicles in Pre-Metastatic Niche Formation and Metastatic Dissemination
5. Extracellular Vesicles as Biomarkers of Metastatic Diseases
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Stoletov, K.; Beatty, P.H.; Lewis, J.D. Novel Therapeutic Targets for Cancer Metastasis. Expert Rev. Anticancer Ther. 2020, 20, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Suhail, Y.; Cain, M.P.; Vanaja, K.; Kurywchak, P.A.; Levchenko, A.; Kalluri, R. Kshitiz Systems Biology of Cancer Metastasis. Cell Syst. 2019, 9, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Sig. Transduct. Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Chang, W.-H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. In Cancer Cell Signaling; Robles-Flores, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2174, pp. 143–170. ISBN 978-1-07-160758-9. [Google Scholar]
- Celià-Terrassa, T.; Kang, Y. Metastatic Niche Functions and Therapeutic Opportunities. Nat. Cell Biol. 2018, 20, 868–877. [Google Scholar] [CrossRef]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-Metastatic Niches: Organ-Specific Homes for Metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef]
- Ghoroghi, S.; Mary, B.; Asokan, N.; Goetz, J.G.; Hyenne, V. Tumor Extracellular Vesicles Drive Metastasis (It’s a Long Way from Home). FASEB BioAdvances 2021, 3, 930–943. [Google Scholar] [CrossRef]
- Wortzel, I.; Dror, S.; Kenific, C.M.; Lyden, D. Exosome-Mediated Metastasis: Communication from a Distance. Dev. Cell 2019, 49, 347–360. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Gkolfinopoulos, S.; Jones, R.L.; Constantinidou, A. The Emerging Role of Platelets in the Formation of the Micrometastatic Niche: Current Evidence and Future Perspectives. Front. Oncol. 2020, 10, 374. [Google Scholar] [CrossRef]
- Yan, M.; Jurasz, P. The Role of Platelets in the Tumor Microenvironment: From Solid Tumors to Leukemia. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Ding, Y.; Zhuang, R. Platelet-Mediated Tumor Metastasis Mechanism and the Role of Cell Adhesion Molecules. Crit. Rev. Oncol./Hematol. 2021, 167, 103502. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of Platelets to Tumour Metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets Guide the Formation of Early Metastatic Niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef]
- Kerr, B.A.; McCabe, N.P.; Feng, W.; Byzova, T.V. Platelets Govern Pre-Metastatic Tumor Communication to Bone. Oncogene 2013, 32, 4319–4324. [Google Scholar] [CrossRef]
- Leblanc, R.; Lee, S.-C.; David, M.; Bordet, J.-C.; Norman, D.D.; Patil, R.; Miller, D.; Sahay, D.; Ribeiro, J.; Clézardin, P.; et al. Interaction of Platelet-Derived Autotaxin with Tumor Integrin AVβ3 Controls Metastasis of Breast Cancer Cells to Bone. Blood 2014, 124, 3141–3150. [Google Scholar] [CrossRef]
- Żmigrodzka, M.; Witkowska-Piłaszewicz, O.; Winnicka, A. Platelets Extracellular Vesicles as Regulators of Cancer Progression—An Updated Perspective. Int. J. Mol. Sci. 2020, 21, 5195. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in Cancer and Other Diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Maishi, N.; Hida, K. Tumor Endothelial Cells Accelerate Tumor Metastasis. Cancer Sci. 2017, 108, 1921–1926. [Google Scholar] [CrossRef]
- Izraely, S.; Witz, I.P. Site-specific Metastasis: A Cooperation between Cancer Cells and the Metastatic Microenvironment. Int. J. Cancer 2021, 148, 1308–1322. [Google Scholar] [CrossRef]
- Smeda, M.; Przyborowski, K.; Stojak, M.; Chlopicki, S. The Endothelial Barrier and Cancer Metastasis: Does the Protective Facet of Platelet Function Matter? Biochem. Pharmacol. 2020, 176, 113886. [Google Scholar] [CrossRef] [PubMed]
- Abhange, K.; Makler, A.; Wen, Y.; Ramnauth, N.; Mao, W.; Asghar, W.; Wan, Y. Small Extracellular Vesicles in Cancer. Bioact. Mater. 2021, 6, 3705–3743. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ganss, R. Modulation of the Vascular-Immune Environment in Metastatic Cancer. Cancers 2021, 13, 810. [Google Scholar] [CrossRef]
- Todorova, D.; Simoncini, S.; Lacroix, R.; Sabatier, F.; Dignat-George, F. Extracellular Vesicles in Angiogenesis. Circ. Res. 2017, 120, 1658–1673. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Anselmi, M.; Fontana, F.; Marzagalli, M.; Gagliano, N.; Sommariva, M.; Limonta, P. Melanoma Stem Cells Educate Neutrophils to Support Cancer Progression. Cancers 2022, 14, 3391. [Google Scholar] [CrossRef]
- Mills, J.; Capece, M.; Cocucci, E.; Tessari, A.; Palmieri, D. Cancer-Derived Extracellular Vesicle-Associated MicroRNAs in Intercellular Communication: One Cell’s Trash Is Another Cell’s Treasure. Int. J. Mol. Sci. 2019, 20, 6109. [Google Scholar] [CrossRef]
- Chan, I.S.; Knútsdóttir, H.; Ramakrishnan, G.; Padmanaban, V.; Warrier, M.; Ramirez, J.C.; Dunworth, M.; Zhang, H.; Jaffee, E.M.; Bader, J.S.; et al. Cancer Cells Educate Natural Killer Cells to a Metastasis-Promoting Cell State. J. Cell Biol. 2020, 219, e202001134. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X. Characteristics and Significance of the Pre-Metastatic Niche. Cancer Cell 2016, 30, 668–681. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Doglioni, G.; Parik, S.; Fendt, S.-M. Interactions in the (Pre)Metastatic Niche Support Metastasis Formation. Front. Oncol. 2019, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Grigoryeva, E.S.; Savelieva, O.E.; Popova, N.O.; Cherdyntseva, N.V.; Perelmuter, V.M. Do Tumor Exosome Integrins Alone Determine Organotropic Metastasis? Mol. Biol. Rep. 2020, 47, 8145–8157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, X. Organotropic Metastasis: Role of Tumor Exosomes. Cell Res. 2016, 26, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Kuang, G.; Long, R.; Han, Y.; Wang, J. The Overall Process of Metastasis: From Initiation to a New Tumor. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188750. [Google Scholar] [CrossRef]
- Elia, I.; Doglioni, G.; Fendt, S.-M. Metabolic Hallmarks of Metastasis Formation. Trends Cell Biol. 2018, 28, 673–684. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Zavyalova, M.V.; Denisov, E.V.; Tashireva, L.A.; Savelieva, O.E.; Kaigorodova, E.V.; Krakhmal, N.V.; Perelmuter, V.M. Intravasation as a Key Step in Cancer Metastasis. Biochem. Mosc. 2019, 84, 762–772. [Google Scholar] [CrossRef]
- Donato, C.; Kunz, L.; Castro-Giner, F.; Paasinen-Sohns, A.; Strittmatter, K.; Szczerba, B.M.; Scherrer, R.; Di Maggio, N.; Heusermann, W.; Biehlmaier, O.; et al. Hypoxia Triggers the Intravasation of Clustered Circulating Tumor Cells. Cell Rep. 2020, 32, 108105. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Peng, G.; Xiao, Y.; Guo, J.; Wu, B.; Li, X.; Zhou, W.; Li, J.; Li, Z.; et al. Single-Cell RNA Sequencing Reveals a pro-Invasive Cancer-Associated Fibroblast Subgroup Associated with Poor Clinical Outcomes in Patients with Gastric Cancer. Theranostics 2022, 12, 620–638. [Google Scholar] [CrossRef]
- Han, H.; Sung, J.Y.; Kim, S.-H.; Yun, U.-J.; Kim, H.; Jang, E.-J.; Yoo, H.-E.; Hong, E.K.; Goh, S.-H.; Moon, A.; et al. Fibronectin Regulates Anoikis Resistance via Cell Aggregate Formation. Cancer Lett. 2021, 508, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Stock, C. Circulating Tumor Cells: Does Ion Transport Contribute to Intravascular Survival, Adhesion, Extravasation, and Metastatic Organotropism? In From Malignant Transformation to Metastasis; Stock, C., Pardo, L.A., Eds.; Reviews of Physiology, Biochemistry and Pharmacology; Springer International Publishing: Cham, Switzerland, 2021; Volume 182, pp. 139–175. ISBN 978-3-030-99799-1. [Google Scholar]
- Cheng, X.; Cheng, K. Visualizing Cancer Extravasation: From Mechanistic Studies to Drug Development. Cancer Metastasis Rev. 2021, 40, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Steps in Metastasis: An Updated Review. Med. Oncol. 2021, 38, 3. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2021, 8, 2003505. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Gonda, D.; Kim, R.; Carter, B.S.; Chen, C.C. Biogenesis of Extracellular Vesicles (EV): Exosomes, Microvesicles, Retrovirus-like Vesicles, and Apoptotic Bodies. J. Neurooncol. 2013, 113, 1–11. [Google Scholar] [CrossRef]
- Kang, T.; Atukorala, I.; Mathivanan, S. Biogenesis of Extracellular Vesicles. In New Frontiers: Extracellular Vesicles; Mathivanan, S., Fonseka, P., Nedeva, C., Atukorala, I., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2021; Volume 97, pp. 19–43. ISBN 978-3-030-67170-9. [Google Scholar]
- Yokoi, A.; Ochiya, T. Exosomes and Extracellular Vesicles: Rethinking the Essential Values in Cancer Biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.-J.; Tang, Y.-C.; Hao, X.-Y.; Xu, W.-J.; Xiang, D.-X.; Wu, J.-Y. Apoptotic Bodies for Advanced Drug Delivery and Therapy. J. Control Release 2022, 351, 394–406. [Google Scholar] [CrossRef]
- Bazzan, E.; Tinè, M.; Casara, A.; Biondini, D.; Semenzato, U.; Cocconcelli, E.; Balestro, E.; Damin, M.; Radu, C.M.; Turato, G.; et al. Critical Review of the Evolution of Extracellular Vesicles’ Knowledge: From 1946 to Today. Int. J. Mol. Sci. 2021, 22, 6417. [Google Scholar] [CrossRef] [PubMed]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and Function of Extracellular Vesicles in Cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, M.; Winter, C.; Simons, M. Inhibition of Myelin Membrane Sheath Formation by Oligodendrocyte-Derived Exosome-like Vesicles. J. Biol. Chem. 2011, 286, 787–796. [Google Scholar] [CrossRef]
- Frühbeis, C.; Fröhlich, D.; Kuo, W.P.; Amphornrat, J.; Thilemann, S.; Saab, A.S.; Kirchhoff, F.; Möbius, W.; Goebbels, S.; Nave, K.-A.; et al. Neurotransmitter-Triggered Transfer of Exosomes Mediates Oligodendrocyte–Neuron Communication. PLoS Biol. 2013, 11, e1001604. [Google Scholar] [CrossRef]
- Kim, N.-H.; Choi, S.-H.; Kim, C.-H.; Lee, C.H.; Lee, T.R.; Lee, A.-Y. Reduced MiR-675 in Exosome in H19 RNA-Related Melanogenesis via MITF as a Direct Target. J. Investig. Dermatol. 2014, 134, 1075–1082. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Weiss, R.; Gröger, M.; Rauscher, S.; Fendl, B.; Eichhorn, T.; Fischer, M.B.; Spittler, A.; Weber, V. Differential Interaction of Platelet-Derived Extracellular Vesicles with Leukocyte Subsets in Human Whole Blood. Sci. Rep. 2018, 8, 6598. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, L.; Czechowska, E.; Gutowska-Owsiak, D. The Role of Non-Immune Cell-Derived Extracellular Vesicles in Allergy. Front. Immunol. 2021, 12, 702381. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Sun, J.; Qin, G. Extracellular Vesicles in Cardiovascular Disease: Biological Functions and Therapeutic Implications. Pharmacol. Ther. 2021, 233, 108025. [Google Scholar] [CrossRef] [PubMed]
- Vandendriessche, C.; Bruggeman, A.; Van Cauwenberghe, C.; Vandenbroucke, R.E. Extracellular Vesicles in Alzheimer’s and Parkinson’s Disease: Small Entities with Large Consequences. Cells 2020, 9, 2485. [Google Scholar] [CrossRef] [PubMed]
- Mazzariol, M.; Camussi, G.; Brizzi, M.F. Extracellular Vesicles Tune the Immune System in Renal Disease: A Focus on Systemic Lupus Erythematosus, Antiphospholipid Syndrome, Thrombotic Microangiopathy and ANCA-Vasculitis. Int. J. Mol. Sci. 2021, 22, 4194. [Google Scholar] [CrossRef]
- Shehzad, A.; Islam, S.U.; Shahzad, R.; Khan, S.; Lee, Y.S. Extracellular Vesicles in Cancer Diagnostics and Therapeutics. Pharmacol. Ther. 2021, 223, 107806. [Google Scholar] [CrossRef]
- Beltraminelli, T.; Perez, C.R.; De Palma, M. Disentangling the Complexity of Tumor-Derived Extracellular Vesicles. Cell Rep. 2021, 35, 108960. [Google Scholar] [CrossRef]
- Borzi, C.; Calzolari, L.; Ferretti, A.M.; Caleca, L.; Pastorino, U.; Sozzi, G.; Fortunato, O. C-Myc Shuttled by Tumour-Derived Extracellular Vesicles Promotes Lung Bronchial Cell Proliferation through MiR-19b and MiR-92a. Cell Death Dis. 2019, 10, 759. [Google Scholar] [CrossRef]
- Pan, J.; Sheng, S.; Ye, L.; Xu, X.; Ma, Y.; Feng, X.; Qiu, L.; Fan, Z.; Wang, Y.; Xia, X.; et al. Extracellular Vesicles Derived from Glioblastoma Promote Proliferation and Migration of Neural Progenitor Cells via PI3K-Akt Pathway. Cell Commun. Signal 2022, 20, 7. [Google Scholar] [CrossRef]
- Yang, L.; Wu, X.-H.; Wang, D.; Luo, C.-L.; Chen, L.-X. Bladder Cancer Cell-Derived Exosomes Inhibit Tumor Cell Apoptosis and Induce Cell Proliferation In Vitro. Mol. Med. Rep. 2013, 8, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-C.; Chang, Y.-A.; Chen, Y.-J.; Sung, H.-M.; Bogeski, I.; Su, H.-L.; Hsu, Y.-L.; Wang, H.-M.D. The Roles of Extracellular Vesicles in Malignant Melanoma. Cells 2021, 10, 2740. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, M.; Huang, D.; Ma, Y.; Ye, G.; Wen, Q.; Li, Y.; Deng, L.; Qi, Q.; Liu, T.; et al. Tumor Perivascular Cell-Derived Extracellular Vesicles Promote Angiogenesis via the Gas6/Axl Pathway. Cancer Lett. 2022, 524, 131–143. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Z.; Chen, W.; Wang, X.; Cao, M.; Han, X.; Zhang, K.; Teng, B.; Cao, J.; Wu, W.; et al. M2 Macrophage-Derived Exosomes Promote Angiogenesis and Growth of Pancreatic Ductal Adenocarcinoma by Targeting E2F2. Mol. Ther. 2021, 29, 1226–1238. [Google Scholar] [CrossRef]
- Giannandrea, D.; Platonova, N.; Colombo, M.; Mazzola, M.; Citro, V.; Adami, R.; Maltoni, F.; Ancona, S.; Dolo, V.; Giusti, I.; et al. Extracellular Vesicles Mediate the Communication between Multiple Myeloma and Bone Marrow Microenvironment in a NOTCH Dependent Way. Haematol 2022, 107, 2183–2194. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular Vesicles in Immunomodulation and Tumor Progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Pucci, M.; Raimondo, S.; Urzì, O.; Moschetti, M.; Di Bella, M.A.; Conigliaro, A.; Caccamo, N.; La Manna, M.P.; Fontana, S.; Alessandro, R. Tumor-Derived Small Extracellular Vesicles Induce Pro-Inflammatory Cytokine Expression and PD-L1 Regulation in M0 Macrophages via IL-6/STAT3 and TLR4 Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 12118. [Google Scholar] [CrossRef]
- Ringuette Goulet, C.; Bernard, G.; Tremblay, S.; Chabaud, S.; Bolduc, S.; Pouliot, F. Exosomes Induce Fibroblast Differentiation into Cancer-Associated Fibroblasts through TGFβ Signaling. Mol. Cancer Res. 2018, 16, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Di Francesco, M.; D’Ascenzo, S.; Palmerini, M.G.; Macchiarelli, G.; Carta, G.; Dolo, V. Ovarian Cancer-Derived Extracellular Vesicles Affect Normal Human Fibroblast Behavior. Cancer Biol. Ther. 2018, 19, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Di Francesco, M.; Poppa, G.; Esposito, L.; D’Ascenzo, S.; Dolo, V. Tumor-Derived Extracellular Vesicles Activate Normal Human Fibroblasts to a Cancer-Associated Fibroblast-Like Phenotype, Sustaining a Pro-Tumorigenic Microenvironment. Front. Oncol. 2022, 12, 839880. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Dean, D.C.; Hornicek, F.J.; Shi, H.; Duan, Z. Exosomes Promote Pre-Metastatic Niche Formation in Ovarian Cancer. Mol. Cancer 2019, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Battafarano, G.; D’Agostini, M.; Del Fattore, A. The Role of Extracellular Vesicles in Bone Metastasis. Int. J. Mol. Sci. 2018, 19, 1136. [Google Scholar] [CrossRef]
- Tang, D.; Liu, S.; Shen, H.; Deng, G.; Zeng, S. Extracellular Vesicles Promote the Formation of Pre-Metastasis Niche in Gastric Cancer. Front. Immunol. 2022, 13, 813015. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E. Extracellular Vesicles and Metastasis. Cold Spring Harb. Perspect Med. 2020, 10, a037275. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Nawrocki, A.; Jensen, S.G.; Thorsen, K.; Whitehead, B.; Howard, K.A.; Dyrskjøt, L.; Ørntoft, T.F.; Larsen, M.R.; Ostenfeld, M.S. Quantitative Proteomics of Fractionated Membrane and Lumen Exosome Proteins from Isogenic Metastatic and Nonmetastatic Bladder Cancer Cells Reveal Differential Expression of EMT Factors. Proteomics 2014, 14, 699–712. [Google Scholar] [CrossRef]
- Xiao, D.; Barry, S.; Kmetz, D.; Egger, M.; Pan, J.; Rai, S.N.; Qu, J.; McMasters, K.M.; Hao, H. Melanoma Cell–Derived Exosomes Promote Epithelial–Mesenchymal Transition in Primary Melanocytes through Paracrine/Autocrine Signaling in the Tumor Microenvironment. Cancer Lett. 2016, 376, 318–327. [Google Scholar] [CrossRef]
- Bigagli, E.; Luceri, C.; Guasti, D.; Cinci, L. Exosomes Secreted from Human Colon Cancer Cells Influence the Adhesion of Neighboring Metastatic Cells: Role of MicroRNA-210. Cancer Biol. Ther. 2016, 17, 1062–1069. [Google Scholar] [CrossRef]
- Wei, F.; Ma, C.; Zhou, T.; Dong, X.; Luo, Q.; Geng, L.; Ding, L.; Zhang, Y.; Zhang, L.; Li, N.; et al. Exosomes Derived from Gemcitabine-Resistant Cells Transfer Malignant Phenotypic Traits via Delivery of MiRNA-222-3p. Mol. Cancer 2017, 16, 132. [Google Scholar] [CrossRef]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of Extracellular Hsp90α via Exosomes Increases Cancer Cell Motility: A Role for Plasminogen Activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef]
- Singh, R.; Pochampally, R.; Watabe, K.; Lu, Z.; Mo, Y.-Y. Exosome-Mediated Transfer of MiR-10b Promotes Cell Invasion in Breast Cancer. Mol. Cancer 2014, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.A.; Patel, S.H.; Gucek, M.; Hendrix, A.; Westbroek, W.; Taraska, J.W. Exosomes Released from Breast Cancer Carcinomas Stimulate Cell Movement. PLoS ONE 2015, 10, e0117495. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Kirkbride, K.C.; Costello, K.; Clark, E.S.; Sinha, S.; Grega-Larson, N.; Tyska, M.J.; Weaver, A.M. Exosome Secretion Is Enhanced by Invadopodia and Drives Invasive Behavior. Cell Rep. 2013, 5, 1159–1168. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, K.; Hu, L.; Zhou, C.; Lu, Z.; Zhan, G.; Pan, X.; Pan, C.; Wang, J.; Wen, W.; et al. Exosome-mediated Transfer of MiR-1260b Promotes Cell Invasion through Wnt/β–Catenin Signaling Pathway in Lung Adenocarcinoma. J. Cell. Physiol. 2020, 235, 6843–6853. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, V.; Yang, X.; Ma, Y.; Wu, M.H.; Yuan, S.Y. Extracellular Vesicles: New Players in Regulating Vascular Barrier Function. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H1181–H1196. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, S.; Yoshioka, Y.; Prieto-Vila, M.; Ochiya, T. Involvement of Extracellular Vesicles in Vascular-Related Functions in Cancer Progression and Metastasis. Int. J. Mol. Sci. 2019, 20, 2584. [Google Scholar] [CrossRef]
- Di Modica, M.; Regondi, V.; Sandri, M.; Iorio, M.V.; Zanetti, A.; Tagliabue, E.; Casalini, P.; Triulzi, T. Breast Cancer-Secreted MiR-939 Downregulates VE-Cadherin and Destroys the Barrier Function of Endothelial Monolayers. Cancer Lett. 2017, 384, 94–100. [Google Scholar] [CrossRef]
- Zhou, W.; Fong, M.Y.; Min, Y.; Somlo, G.; Liu, L.; Palomares, M.R.; Yu, Y.; Chow, A.; O’Connor, S.T.F.; Chin, A.R.; et al. Cancer-Secreted MiR-105 Destroys Vascular Endothelial Barriers to Promote Metastasis. Cancer Cell 2014, 25, 501–515. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung Cancer-Secreted Exosomal MiR-23a Increased Angiogenesis and Vascular Permeability by Targeting Prolyl Hydroxylase and Tight Junction Protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain Metastatic Cancer Cells Release MicroRNA-181c-Containing Extracellular Vesicles Capable of Destructing Blood–Brain Barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Z.; Shang, L.; Luo, Y.; Lin, Y.; Yuan, Y.; Zhuang, S. Hepatoma Cell-secreted Exosomal MicroRNA-103 Increases Vascular Permeability and Promotes Metastasis by Targeting Junction Proteins. Hepatology 2018, 68, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Treps, L.; Perret, R.; Edmond, S.; Ricard, D.; Gavard, J. Glioblastoma Stem-like Cells Secrete the pro-Angiogenic VEGF-A Factor in Extracellular Vesicles. J. Extracell. Vesicles 2017, 6, 1359479. [Google Scholar] [CrossRef] [PubMed]
- Treps, L.; Edmond, S.; Harford-Wright, E.; Galan-Moya, E.M.; Schmitt, A.; Azzi, S.; Citerne, A.; Bidère, N.; Ricard, D.; Gavard, J. Extracellular Vesicle-Transported Semaphorin3A Promotes Vascular Permeability in Glioblastoma. Oncogene 2016, 35, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.G.; Leal, A.C.; Vargas, G.; Porto-Carreiro, I.; Monteiro, R.Q. Intercellular Transfer of Tissue Factor via the Uptake of Tumor-Derived Microvesicles. Thromb. Res. 2013, 132, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.G.; Sandim, V.; Almeida, V.H.; Rondon, A.M.R.; Succar, B.B.; Hottz, E.D.; Leal, A.C.; Verçoza, B.R.F.; Rodrigues, J.C.F.; Bozza, P.T.; et al. Breast-Cancer Extracellular Vesicles Induce Platelet Activation and Aggregation by Tissue Factor-Independent and -Dependent Mechanisms. Thromb. Res. 2017, 159, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.C.; Mizurini, D.M.; Gomes, T.; Rochael, N.C.; Saraiva, E.M.; Dias, M.S.; Werneck, C.C.; Sielski, M.S.; Vicente, C.P.; Monteiro, R.Q. Tumor-Derived Exosomes Induce the Formation of Neutrophil Extracellular Traps: Implications For The Establishment of Cancer-Associated Thrombosis. Sci. Rep. 2017, 7, 6438. [Google Scholar] [CrossRef]
- Maus, R.L.G.; Jakub, J.W.; Nevala, W.K.; Christensen, T.A.; Noble-Orcutt, K.; Sachs, Z.; Hieken, T.J.; Markovic, S.N. Human Melanoma-Derived Extracellular Vesicles Regulate Dendritic Cell Maturation. Front. Immunol. 2017, 8, 358. [Google Scholar] [CrossRef]
- Wen, S.W.; Sceneay, J.; Lima, L.G.; Wong, C.S.F.; Becker, M.; Krumeich, S.; Lobb, R.J.; Castillo, V.; Wong, K.N.; Ellis, S.; et al. The Biodistribution and Immune Suppressive Effects of Breast Cancer–Derived Exosomes. Cancer Res. 2016, 76, 6816–6827. [Google Scholar] [CrossRef]
- Yang, P.; Qin, H.; Li, Y.; Xiao, A.; Zheng, E.; Zeng, H.; Su, C.; Luo, X.; Lu, Q.; Liao, M.; et al. CD36-Mediated Metabolic Crosstalk between Tumor Cells and Macrophages Affects Liver Metastasis. Nat. Commun. 2022, 13, 5782. [Google Scholar] [CrossRef]
- Morrissey, S.M.; Zhang, F.; Ding, C.; Montoya-Durango, D.E.; Hu, X.; Yang, C.; Wang, Z.; Yuan, F.; Fox, M.; Zhang, H.; et al. Tumor-Derived Exosomes Drive Immunosuppressive Macrophages in a Pre-Metastatic Niche through Glycolytic Dominant Metabolic Reprogramming. Cell Metab. 2021, 33, 2040–2058. [Google Scholar] [CrossRef]
- Dou, D.; Ren, X.; Han, M.; Xu, X.; Ge, X.; Gu, Y.; Wang, X. Cancer-Associated Fibroblasts-Derived Exosomes Suppress Immune Cell Function in Breast Cancer via the MiR-92/PD-L1 Pathway. Front. Immunol. 2020, 11, 2026. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Tu, Y.; Xu, Y.; Guo, Y.; Yao, F.; Zhang, X. Endoplasmic Reticulum Stress-induced Exosomal MiR-27a-3p Promotes Immune Escape in Breast Cancer via Regulating PD-L1 Expression in Macrophages. J. Cell Mol. Med. 2020, 24, 9560–9573. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor Exosomal RNAs Promote Lung Pre-Metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Barranco, A.; Nogués, L.; Peinado, H. Could Extracellular Vesicles Contribute to Generation or Awakening of “Sleepy” Metastatic Niches? Front. Cell Dev. Biol. 2021, 9, 625221. [Google Scholar] [CrossRef]
- Phan, T.G.; Croucher, P.I. The Dormant Cancer Cell Life Cycle. Nat. Rev. Cancer 2020, 20, 398–411. [Google Scholar] [CrossRef]
- Bliss, S.A.; Sinha, G.; Sandiford, O.A.; Williams, L.M.; Engelberth, D.J.; Guiro, K.; Isenalumhe, L.L.; Greco, S.J.; Ayer, S.; Bryan, M.; et al. Mesenchymal Stem Cell–Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res. 2016, 76, 5832–5844. [Google Scholar] [CrossRef]
- Lim, P.K.; Bliss, S.A.; Patel, S.A.; Taborga, M.; Dave, M.A.; Gregory, L.A.; Greco, S.J.; Bryan, M.; Patel, P.S.; Rameshwar, P. Gap Junction–Mediated Import of MicroRNA from Bone Marrow Stromal Cells Can Elicit Cell Cycle Quiescence in Breast Cancer Cells. Cancer Res. 2011, 71, 1550–1560. [Google Scholar] [CrossRef]
- Zhang, B.; Nguyen, L.X.T.; Li, L.; Zhao, D.; Kumar, B.; Wu, H.; Lin, A.; Pellicano, F.; Hopcroft, L.; Su, Y.-L.; et al. Bone Marrow Niche Trafficking of MiR-126 Controls the Self-Renewal of Leukemia Stem Cells in Chronic Myelogenous Leukemia. Nat. Med. 2018, 24, 450–462. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, D.; Xie, M. Tumor-Derived Extracellular Vesicles Promote Activation of Carcinoma-Associated Fibroblasts and Facilitate Invasion and Metastasis of Ovarian Cancer by Carrying MiR-630. Front. Cell Dev. Biol. 2021, 9, 652322. [Google Scholar] [CrossRef]
- Sansone, P.; Berishaj, M.; Rajasekhar, V.K.; Ceccarelli, C.; Chang, Q.; Strillacci, A.; Savini, C.; Shapiro, L.; Bowman, R.L.; Mastroleo, C.; et al. Evolution of Cancer Stem-like Cells in Endocrine-Resistant Metastatic Breast Cancers Is Mediated by Stromal Microvesicles. Cancer Res. 2017, 77, 1927–1941. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Fiore, D.; Nappa, M.; Roscigno, G.; Adamo, A.; Iaboni, M.; Russo, V.; Affinito, A.; Puoti, I.; Quintavalle, C.; et al. Cancer-Associated Fibroblasts Release Exosomal MicroRNAs That Dictate an Aggressive Phenotype in Breast Cancer. Oncotarget 2017, 8, 19592–19608. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, C.; Zhan, Y.; Wang, H.; Jiang, X.; Li, W. Aberrant Low Expression of P85α in Stromal Fibroblasts Promotes Breast Cancer Cell Metastasis through Exosome-Mediated Paracrine Wnt10b. Oncogene 2017, 36, 4692–4705. [Google Scholar] [CrossRef]
- Shu, S.L.; Yang, Y.; Allen, C.L.; Maguire, O.; Minderman, H.; Sen, A.; Ciesielski, M.J.; Collins, K.A.; Bush, P.J.; Singh, P.; et al. Metabolic Reprogramming of Stromal Fibroblasts by Melanoma Exosome MicroRNA Favours a Pre-Metastatic Microenvironment. Sci. Rep. 2018, 8, 12905. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, W.; Hua, S.; Liu, L. Roles of Extracellular Vesicles in Metastatic Breast Cancer. Breast Cancer 2018, 12, 117822341876766. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Warshall, C.; Bandyopadhyay, C.; Dutta, D.; Chandran, B. Interactions between Exosomes from Breast Cancer Cells and Primary Mammary Epithelial Cells Leads to Generation of Reactive Oxygen Species Which Induce DNA Damage Response, Stabilization of P53 and Autophagy in Epithelial Cells. PLoS ONE 2014, 9, e97580. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R.; et al. Breast-Cancer-Secreted MiR-122 Reprograms Glucose Metabolism in Premetastatic Niche to Promote Metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef]
- Ji, Q.; Zhou, L.; Sui, H.; Yang, L.; Wu, X.; Song, Q.; Jia, R.; Li, R.; Sun, J.; Wang, Z.; et al. Primary Tumors Release ITGBL1-Rich Extracellular Vesicles to Promote Distal Metastatic Tumor Growth through Fibroblast-Niche Formation. Nat. Commun. 2020, 11, 1211. [Google Scholar] [CrossRef]
- Wang, W.; Mu, W.; Wang, H. Breast Tumor-Derived Exosomal MicroRNA-200b-3p Promotes Specific Organ Metastasis through Regulating CCL2 Expression in Lung Epithelial Cells. Front. Cell Dev. Biol. 2021, 9, 657158. [Google Scholar]
- Keklikoglou, I.; Cianciaruso, C.; Güç, E.; Squadrito, M.L.; Spring, L.M.; Tazzyman, S.; Lambein, L.; Poissonnier, A.; Ferraro, G.B.; Baer, C.; et al. Chemotherapy Elicits Pro-Metastatic Extracellular Vesicles in Breast Cancer Models. Nat. Cell Biol. 2019, 21, 190–202. [Google Scholar] [CrossRef]
- Barenholz-Cohen, T.; Merkher, Y.; Haj, J.; Shechter, D.; Kirchmeier, D.; Shaked, Y.; Weihs, D. Lung Mechanics Modifications Facilitating Metastasis Are Mediated in Part by Breast Cancer-derived Extracellular Vesicles. Int. J. Cancer 2020, 147, 2924–2933. [Google Scholar] [CrossRef] [PubMed]
- Armacki, M.; Polaschek, S.; Waldenmaier, M.; Morawe, M.; Ruhland, C.; Schmid, R.; Lechel, A.; Tharehalli, U.; Steup, C.; Bektas, Y.; et al. Protein Kinase D1, Reduced in Human Pancreatic Tumors, Increases Secretion of Small Extracellular Vesicles From Cancer Cells That Promote Metastasis to Lung in Mice. Gastroenterology 2020, 159, 1019–1035. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, F.; Guo, L.; Shi, J.; Wu, M.; Cheng, S.; Guo, W. Tumor Cells Derived-Extracellular Vesicles Transfer MiR-3129 to Promote Hepatocellular Carcinoma Metastasis by Targeting TXNIP. Dig. Liver Dis. 2021, 53, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, C.; Trojanowicz, B.; Li, X.; Shi, D.; Zhan, C.; Wang, Z.; Chen, L. CD97 Promotion of Gastric Carcinoma Lymphatic Metastasis Is Exosome Dependent. Gastric Cancer 2016, 19, 754–766. [Google Scholar] [CrossRef]
- Henrich, S.E.; McMahon, K.M.; Plebanek, M.P.; Calvert, A.E.; Feliciano, T.J.; Parrish, S.; Tavora, F.; Mega, A.; De Souza, A.; Carneiro, B.A.; et al. Prostate Cancer Extracellular Vesicles Mediate Intercellular Communication with Bone Marrow Cells and Promote Metastasis in a Cholesterol-dependent Manner. J. Extracell. Vesicles 2020, 10, e12042. [Google Scholar] [CrossRef]
- Kogure, A.; Yoshioka, Y.; Ochiya, T. Extracellular Vesicles in Cancer Metastasis: Potential as Therapeutic Targets and Materials. IJMS 2020, 21, 4463. [Google Scholar] [CrossRef]
- Nogués, L.; Benito-Martin, A.; Hergueta-Redondo, M.; Peinado, H. The Influence of Tumour-Derived Extracellular Vesicles on Local and Distal Metastatic Dissemination. Mol. Asp. Med. 2018, 60, 15–26. [Google Scholar] [CrossRef]
- Seibold, T.; Waldenmaier, M.; Seufferlein, T.; Eiseler, T. Small Extracellular Vesicles and Metastasis—Blame the Messenger. Cancers 2021, 13, 4380. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, Y.; Ho, C.; Li, L.; Jin, C.; Wang, X.; Zou, C.; Mao, Y.; Wang, X.; Li, Q.; et al. Exosome-Delivered CD44v6/C1QBP Complex Drives Pancreatic Cancer Liver Metastasis by Promoting Fibrotic Liver Microenvironment. Gut 2022, 71, 568–579. [Google Scholar] [CrossRef]
- Ohzawa, H.; Kumagai, Y.; Yamaguchi, H.; Miyato, H.; Sakuma, Y.; Horie, H.; Hosoya, Y.; Kawarai Lefor, A.; Sata, N.; Kitayama, J. Exosomal MicroRNA in Peritoneal Fluid as a Biomarker of Peritoneal Metastases from Gastric Cancer. Ann. Gastroent Surg. 2020, 4, 84–93. [Google Scholar] [CrossRef]
- Fu, F.; Jiang, W.; Zhou, L.; Chen, Z. Circulating Exosomal MiR-17-5p and MiR-92a-3p Predict Pathologic Stage and Grade of Colorectal Cancer. Transl. Oncol. 2018, 11, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mi, Y.; Guan, B.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Cai, S.; Xu, Y.; Li, X.; et al. Tumor-Derived Exosomal MiR-934 Induces Macrophage M2 Polarization to Promote Liver Metastasis of Colorectal Cancer. J. Hematol. Oncol. 2020, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chen, T.; Zheng, X.; Yang, S.; Xu, K.; Chen, X.; Xu, F.; Wang, L.; Shen, Y.; Wang, T.; et al. Colorectal Cancer-Derived Small Extracellular Vesicles Establish an Inflammatory Premetastatic Niche in Liver Metastasis. Carcinogenesis 2018, 39, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mi, Y.; Zheng, B.; Wei, P.; Gu, Y.; Zhang, Z.; Xu, Y.; Cai, S.; Li, X.; Li, D. Highly-metastatic Colorectal Cancer Cell Released MiR-181a-5p-rich Extracellular Vesicles Promote Liver Metastasis by Activating Hepatic Stellate Cells and Remodelling the Tumour Microenvironment. J. Extracell. Vesicle 2022, 11, e12186. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast Cancer Exosomes Contribute to Pre-Metastatic Niche Formation and Promote Bone Metastasis of Tumor Cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Q.; Zhang, R.; Lu, H.; Yue, X.-M.; Huang, Y.-F. Extracellular Vesicle–Packaged CDH11 and ITGA5 Induce the Premetastatic Niche for Bone Colonization of Breast Cancer Cells. Cancer Res. 2022, 82, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Godinho-Pereira, J.; Galego, S.; Maia, J.; Haskó, J.; Molnár, K.; Malhó, R.; Costa-Silva, B.; Wilhelm, I.; Krizbai, I.A.; et al. MicroRNAs and Extracellular Vesicles as Distinctive Biomarkers of Precocious and Advanced Stages of Breast Cancer Brain Metastases Development. Int. J. Mol. Sci. 2021, 22, 5214. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Zhi, C.; Liang, W.; Wang, X.; Chen, X.; Lv, T.; Shen, Q.; Song, Y.; Lin, D.; et al. Clinical Significance of PD-L1 Expression in Serum-Derived Exosomes in NSCLC Patients. J. Transl. Med. 2019, 17, 355. [Google Scholar] [CrossRef]
- Wang, N.; Song, X.; Liu, L.; Niu, L.; Wang, X.; Song, X.; Xie, L. Circulating Exosomes Contain Protein Biomarkers of Metastatic Non-small-cell Lung Cancer. Cancer Sci. 2018, 109, 1701–1709. [Google Scholar] [CrossRef]
- Kong, J.; Tian, H.; Zhang, F.; Zhang, Z.; Li, J.; Liu, X.; Li, X.; Liu, J.; Li, X.; Jin, D.; et al. Extracellular Vesicles of Carcinoma-Associated Fibroblasts Creates a Pre-Metastatic Niche in the Lung through Activating Fibroblasts. Mol. Cancer 2019, 18, 175. [Google Scholar] [CrossRef]
- Ozawa, P.M.M.; Jucoski, T.S.; Vieira, E.; Carvalho, T.M.; Malheiros, D.; Ribeiro, E.M.d.S.F. Liquid Biopsy for Breast Cancer Using Extracellular Vesicles and Cell-Free MicroRNAs as Biomarkers. Transl. Res. 2020, 223, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Forder, A.; Hsing, C.-Y.; Trejo Vazquez, J.; Garnis, C. Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis. Cells 2021, 10, 3429. [Google Scholar] [CrossRef] [PubMed]
- de Miguel Pérez, D.; Rodriguez Martínez, A.; Ortigosa Palomo, A.; Delgado Ureña, M.; Garcia Puche, J.L.; Robles Remacho, A.; Exposito Hernandez, J.; Lorente Acosta, J.A.; Ortega Sánchez, F.G.; Serrano, M.J. Extracellular Vesicle-MiRNAs as Liquid Biopsy Biomarkers for Disease Identification and Prognosis in Metastatic Colorectal Cancer Patients. Sci. Rep. 2020, 10, 3974. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New Insights Into Tumor Immune Escape Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 569219. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular Vesicles: Structure, Function, and Potential Clinical Uses in Renal Diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Sonoda, H.; Yokota-Ikeda, N.; Oshikawa, S.; Kanno, Y.; Yoshinaga, K.; Uchida, K.; Ueda, Y.; Kimiya, K.; Uezono, S.; Ueda, A.; et al. Decreased Abundance of Urinary Exosomal Aquaporin-1 in Renal Ischemia-Reperfusion Injury. Am. J. Physiol.-Ren. Physiol. 2009, 297, F1006–F1016. [Google Scholar] [CrossRef]
- Upadhya, R.; Shetty, A.K. Extracellular Vesicles for the Diagnosis and Treatment of Parkinson’s Disease. Aging Dis. 2021, 12, 1438. [Google Scholar] [CrossRef]
- Kodam, S.P.; Ullah, M. Diagnostic and Therapeutic Potential of Extracellular Vesicles. Technol. Cancer Res. Treat. 2021, 20, 153303382110412. [Google Scholar] [CrossRef]
- Kral, J.; Korenkova, V.; Novosadova, V.; Langerova, L.; Schneiderova, M.; Liska, V.; Levy, M.; Veskrnova, V.; Spicak, J.; Opattova, A.; et al. Expression Profile of MiR-17/92 Cluster Is Predictive of Treatment Response in Rectal Cancer. Carcinogenesis 2018, 39, 1359–1367. [Google Scholar] [CrossRef]
- Cammarata, G.; Barraco, N.; Giusti, I.; Gristina, V.; Dolo, V.; Taverna, S. Extracellular Vesicles-CeRNAs as Ovarian Cancer Biomarkers: Looking into CircRNA-MiRNA-MRNA Code. Cancers 2022, 14, 3404. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; D’Ascenzo, S.; Dolo, V. Microvesicles as Potential Ovarian Cancer Biomarkers. BioMed. Res. Int. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Dolo, V. Extracellular Vesicles in Prostate Cancer: New Future Clinical Strategies? BioMed. Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Giusti, I.; Francesco, M.; Dolo, V. Extracellular Vesicles in Glioblastoma: Role in Biological Processes and in Therapeutic Applications. Curr. Cancer Drug Targets 2017, 17, 221–235. [Google Scholar] [CrossRef]
- Taverna, S.; Giusti, I.; D’Ascenzo, S.; Pizzorno, L.; Dolo, V. Breast Cancer Derived Extracellular Vesicles in Bone Metastasis Induction and Their Clinical Implications as Biomarkers. IJMS 2020, 21, 3573. [Google Scholar] [CrossRef] [PubMed]
- Skog, J.; Würdinger, T.; Van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Yu, S.; Wang, Z.; He, X.; Su, Y.; Guo, T.; Sheng, H.; Chen, J.; Zheng, Q.; et al. Extracellular Vesicles Long RNA Sequencing Reveals Abundant MRNA, CircRNA, and LncRNA in Human Blood as Potential Biomarkers for Cancer Diagnosis. Clin. Chem. 2019, 65, 798–808. [Google Scholar] [CrossRef]
- Hu, D.; Zhan, Y.; Zhu, K.; Bai, M.; Han, J.; Si, Y.; Zhang, H.; Kong, D. Plasma Exosomal Long Non-Coding RNAs Serve as Biomarkers for Early Detection of Colorectal Cancer. Cell Physiol. Biochem. 2018, 51, 2704–2715. [Google Scholar] [CrossRef]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front. Neurosci. 2019, 13, 163. [Google Scholar] [CrossRef]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles Derived from Human Adult Mesenchymal Stem Cells Protect against Ischaemia-Reperfusion-Induced Acute and Chronic Kidney Injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles Derived from Mesenchymal Stem Cells Enhance Survival in a Lethal Model of Acute Kidney Injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Liver Diseases. Exp Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Yeo, R.W.Y.; Tan, K.H.; Lim, S.K. Exosomes for Drug Delivery—A Novel Application for the Mesenchymal Stem Cell. Biotechnol. Adv. 2013, 31, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Liu, M.; Tan, T.; Yang, Q.; Wang, Y.; Men, L.; Zhao, L.; Zhang, H.; Wang, S.; Xie, T.; et al. Emerging Significance and Therapeutic Potential of Extracellular Vesicles. Int. J. Biol. Sci. 2021, 17, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Chaput, N.; Théry, C. Exosomes: Immune Properties and Potential Clinical Implementations. Semin. Immunopathol. 2011, 33, 419–440. [Google Scholar] [CrossRef]
- Sabanovic, B.; Piva, F.; Cecati, M.; Giulietti, M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. [Google Scholar] [CrossRef]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef]
- Pordanjani, P.M.; Bolhassani, A.; Milani, A.; Pouriayevali, M.H. Extracellular Vesicles in Vaccine Development and Therapeutic Approaches for Viral Diseases. Process Biochem. 2023, 128, 167–180. [Google Scholar] [CrossRef]
- Mustajab, T.; Kwamboka, M.S.; Choi, D.A.; Kang, D.W.; Kim, J.; Han, K.R.; Han, Y.; Lee, S.; Song, D.; Chwae, Y.-J. Update on Extracellular Vesicle-Based Vaccines and Therapeutics to Combat COVID-19. Int. J. Mol. Sci. 2022, 23, 11247. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological FeatuRes. of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef] [PubMed]
- Enderle, D.; Noerholm, M. Are Extracellular Vesicles Ready for the Clinical Laboratory? J. Lab. Med. 2022, 46, 273–282. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical Challenges of Working with Extracellular Vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef] [PubMed]
| EVs or EVs Associated Molecules | Cancer Type | Changes Detected | Refs. |
|---|---|---|---|
| casein kinase II α annexin A2 | Bladder carcinoma | EMT induction | [89] |
| miR-191 let-7a | Melanoma | EMT induction | [90] |
| miR-210 | Colorectal cancer | Resistance to death | [91] |
| miR-222-3p | NSCLC | Resistance to death | [92] |
| miR-10b | Breast cancer | Increased invasion | [94] |
| EVs | Breast cancer | Increased invasion | [95] |
| Proteinases | Breast cancer | Increased invasion | [96] |
| miR-1260b | Lung adenocarcinoma | Increased invasion | [97] |
| miR-939 | Breast cancer | Down-regulation of VE-cadherin, increasing endothelial barrier permeability and cell migration | [100] |
| miR-105 | Breast cancer | Dysfunction of the endothelial barrier due to ZO-1 alteration | [101] |
| miR-23a | Lung cancer | Dysfunction of the endothelial barrier due to ZO-1 alteration | [102] |
| miR-181c | Breast cancer | Downregulation of PDPK1 and ensuing the alteration of the BBB | [103] |
| miR-103 | Hepatocellular carcinoma | Endothelial cell permeability and trans-endothelial invasion | [104] |
| VEGF-A | Glioblastoma | Endothelial permeability | [105] |
| Semaphorin3A | Glioblastoma | Vascular permeability | [106] |
| TF | Breast cancer | Accelerated coagulation platelet activation | [107,108] |
| tEVs | Mammary carcinoma | NETs formation in G-CSF-primed neutrophils, supporting a thrombotic state | [109] |
| EVs | Melanoma | Incorrect maturation of dendritic cells through the downregulation of chemokines | [110] |
| EXOs | Breast cancer | Immune system alteration by decreasing the CD8 T cells and NK cells frequency, altering the relative composition of CD4 cells, and increasing the gMDSC population | [111] |
| EXOs | Breast cancer | Alteration of T-cell and NK-cell functions by suppressing the proliferation of CD8 and CD4 T-cells and reducing the cytotoxic activity of NK cells against target tumor cells | [111] |
| LCFA | Liver metastasis | Immunosuppressive activity in the TME mediated by macrophage | [112] |
| EXOs | Subcutaneous tumors | Reduced immune response due to PD-L1 increasing and T-cell function inhibition | [113] |
| EVs | Breast cancer | Immune suppression driven by PD-L1 | [114,115] |
| tEVs | Lung cancer | Upregulation of TLR3 in host lung epithelial cells, neutrophils recruitment, and formation of a pro-inflammatory state | [116] |
| tEVs | Breast cancer | Increasing tissue permeability by modifying the ECM, through increasing in several proteins, such as fibronectin | [134] |
| tEVs | Prostate cancer | Upregulation of the NF-kB pathway, enhancing the differentiation of osteoclasts in bone in response to the high amount of cholesterol | [138] |
| miR-222 miR-223 | Breast cancer | Establishment of a dormant state and resistance to pharmacological treatments | [119] |
| miR-127 miR-197 | Breast cancer | Cycle arrest into the G0-phase | [120] |
| miR-126 | Chronic myelogenous leukemia | Dormant stage of leukemia stem cells | [121] |
| EXOs | Pancreatic ductal adenocarcinoma | Mestastatic burden in the liver induced by the production of TGF-β and other factors and the recruitment of macrophages and neutrophils | [31] |
| TGF-β | Primary tumors | Stromal fibroblast activation into CAFs | [82,83,123] |
| miR-155 miR-210 | Melanoma | Acidification of the extracellular environment due to the increase in aerobic glycolysis and decrease in oxidative phosphorylation in human adult dermal fibroblasts | [127] |
| miR-122 | Breast cancer | Suppression of glucose metabolism in receiving cells through the downregulation of pyruvate kinase and the GLUT1 | [130] |
| ITGBL1 | Colorectal cancer | EMT caused by fibroblasts activation in CAFs | [131] |
| miR-200b-3p | Breast cancer | Cellular microenvironment modification by the inhibition of PTEN, causing an increase in CCL2 chemokines | [132] |
| Annexin-A6 | Breast cancer | Increased expression of CCL2, and Ly6C + CCR2+ monocytes levels | [133] |
| ITGα6β4 ITGα6β1 | Lung cancer | Activation of the Src–S100A4 axis in targeted lung fibroblasts | [33] |
| ITGα6β4 | Pancreatic ductal adenocarcinoma | Increase in sEVs secretion related to reduced PRKD1 concentration | [135] |
| miR-3129 | Hepatocellular carcinoma | Inhibition of the tumor suppressor TXNIP activity | [136] |
| mir-105 | Breast Cancer | Vascular permeability | [101] |
| CD97 | Gastric cancer | Cell migration | [137] |
| Primary Cancer and Related Metastasis | Biomarker | Refs. |
|---|---|---|
| Liver metastasis in pancreatic cancer | ITGαv | [33] |
| Lung metastasis in breast cancer | ITGβ4 | [35] |
| Breast Cancer metastasis | miR-105 | [101] |
| Liver metastasis in pancreatic ductal adenocarcinoma | MIF CD44v6/C1QBP | [31,142] |
| Peritoneal metastasis in gastric cancer | miR-21-5p miR-92a-3p miR-342-3p miR-223-3p | [143] |
| Colorectal cancer metastasis | miR-17a-5p miR-17-92 miR-934 | [144,145] |
| Liver metastasis in colorectal cancer | miR-21 miR-181a-5p | [146,147] |
| Breast Cancer bone metastasis | miR-21 CDH11 ITGA5 | [148,149] |
| Breast Cancer brain metastasis | miR-92a-1-5p miR-205-5p miR-181a-1-3p miR-802-5p miR-194-5p | [150] |
| Non-small cell lung cancer metastasis | Liposaccharide-binding proteins PD-L1 | [151,152] |
| Melanoma metastasis | PD-L1 | [151] |
| Bone metastasis in melanoma | MET | [10] |
| Lung metastasis in salivary cystic adenoid carcinoma | α2β1 | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giusti, I.; Poppa, G.; Di Fazio, G.; D’Ascenzo, S.; Dolo, V. Metastatic Dissemination: Role of Tumor-Derived Extracellular Vesicles and Their Use as Clinical Biomarkers. Int. J. Mol. Sci. 2023, 24, 9590. https://doi.org/10.3390/ijms24119590
Giusti I, Poppa G, Di Fazio G, D’Ascenzo S, Dolo V. Metastatic Dissemination: Role of Tumor-Derived Extracellular Vesicles and Their Use as Clinical Biomarkers. International Journal of Molecular Sciences. 2023; 24(11):9590. https://doi.org/10.3390/ijms24119590
Chicago/Turabian StyleGiusti, Ilaria, Giuseppina Poppa, Giulia Di Fazio, Sandra D’Ascenzo, and Vincenza Dolo. 2023. "Metastatic Dissemination: Role of Tumor-Derived Extracellular Vesicles and Their Use as Clinical Biomarkers" International Journal of Molecular Sciences 24, no. 11: 9590. https://doi.org/10.3390/ijms24119590






