High VEGF Concentrations Accelerate Human Trabecular Meshwork Fibrosis in a TAZ-Dependent Manner
Abstract
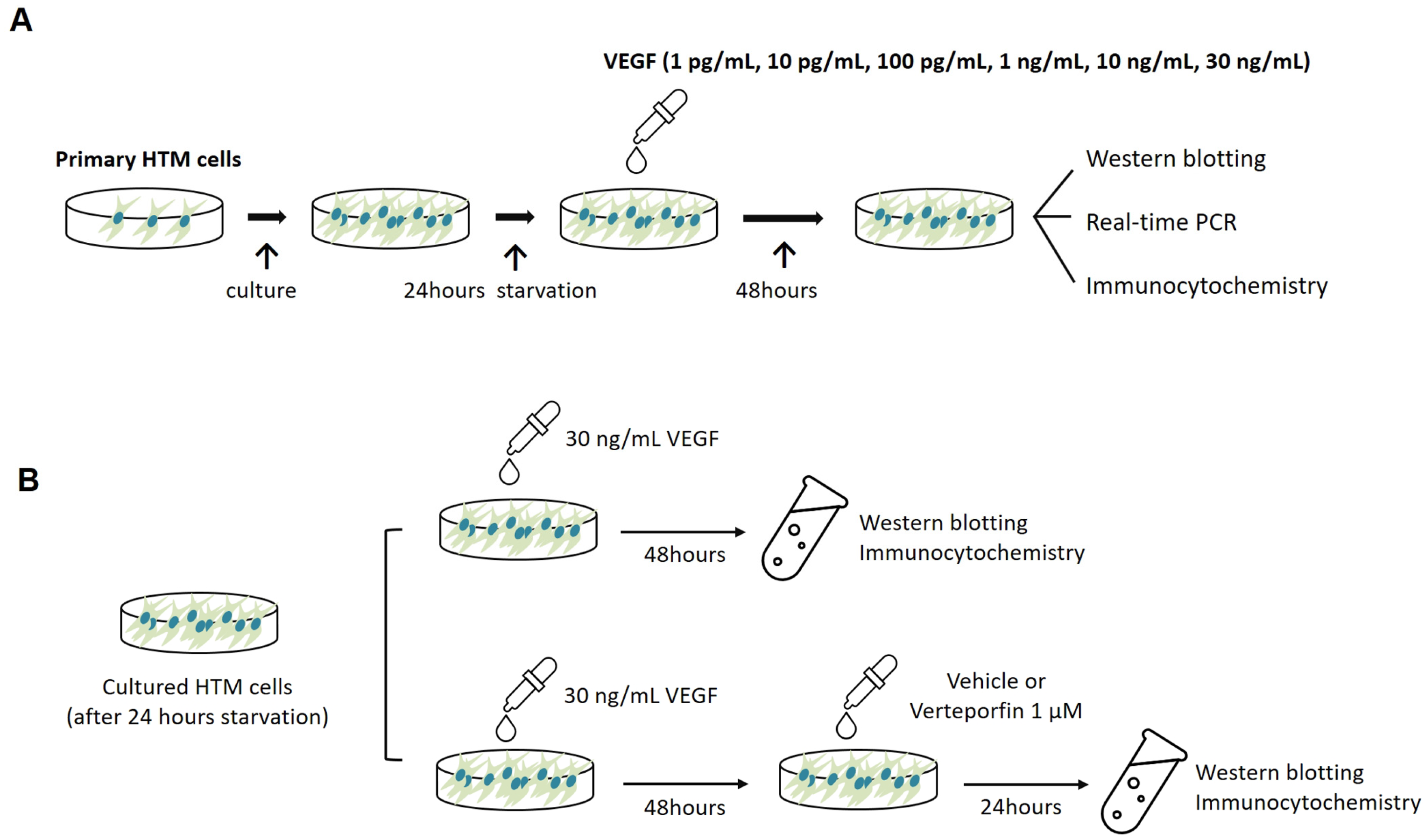
:1. Introduction
2. Results
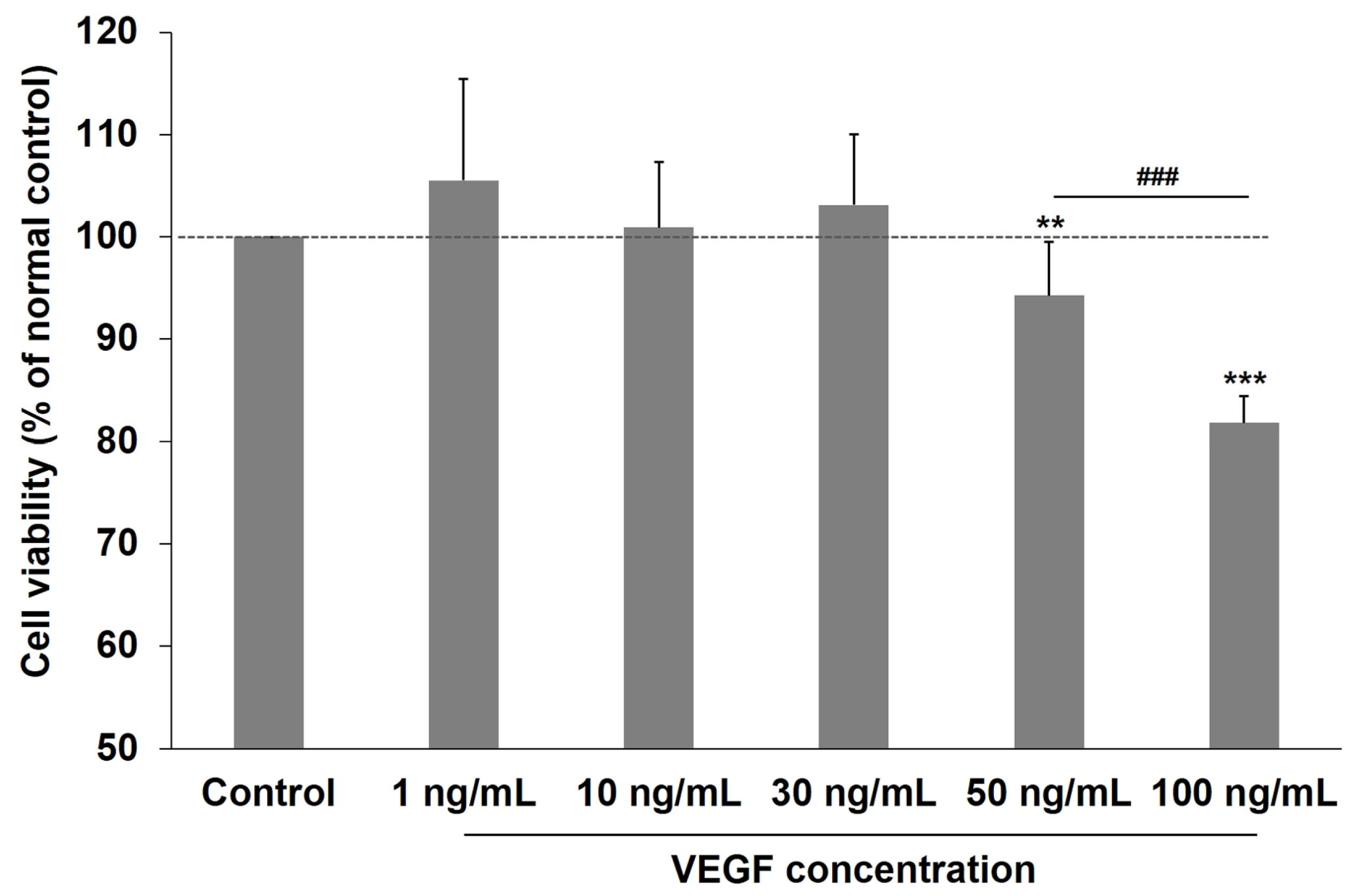
2.1. Effects of VEGF on the Viability of Human TM Cells
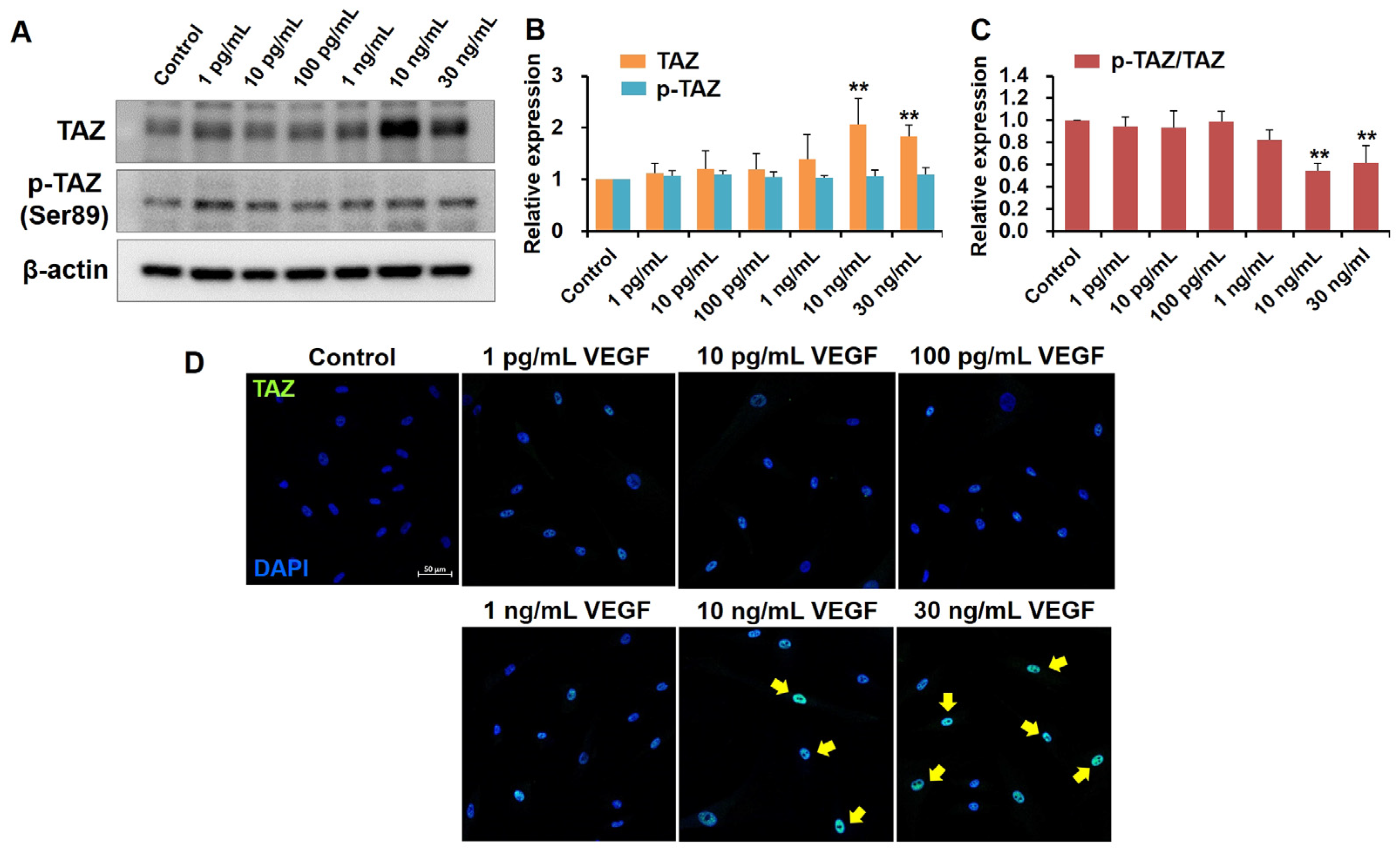
2.2. High VEGF Concentrations Increase TAZ Activity in Human TM Cells
2.3. YAP Expression Unaffected by VEGF Exposure in Human TM Cells
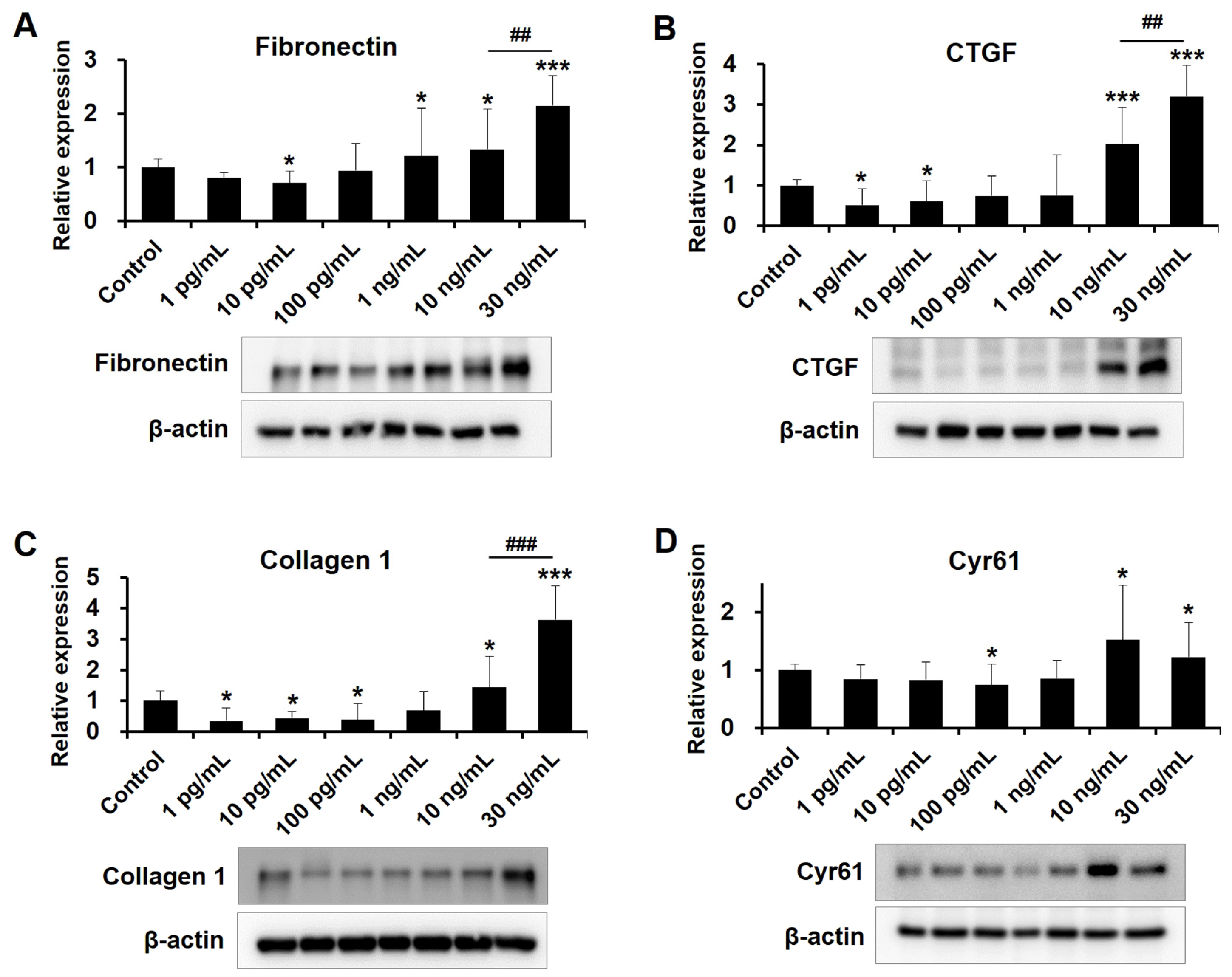
2.4. Dose-Dependent Influences of VEGF on Fibrotic Protein Expression in Human TM Cells
2.5. Exposure to High-Concentration VEGF Promotes CLAN Formation in Human TM Cells
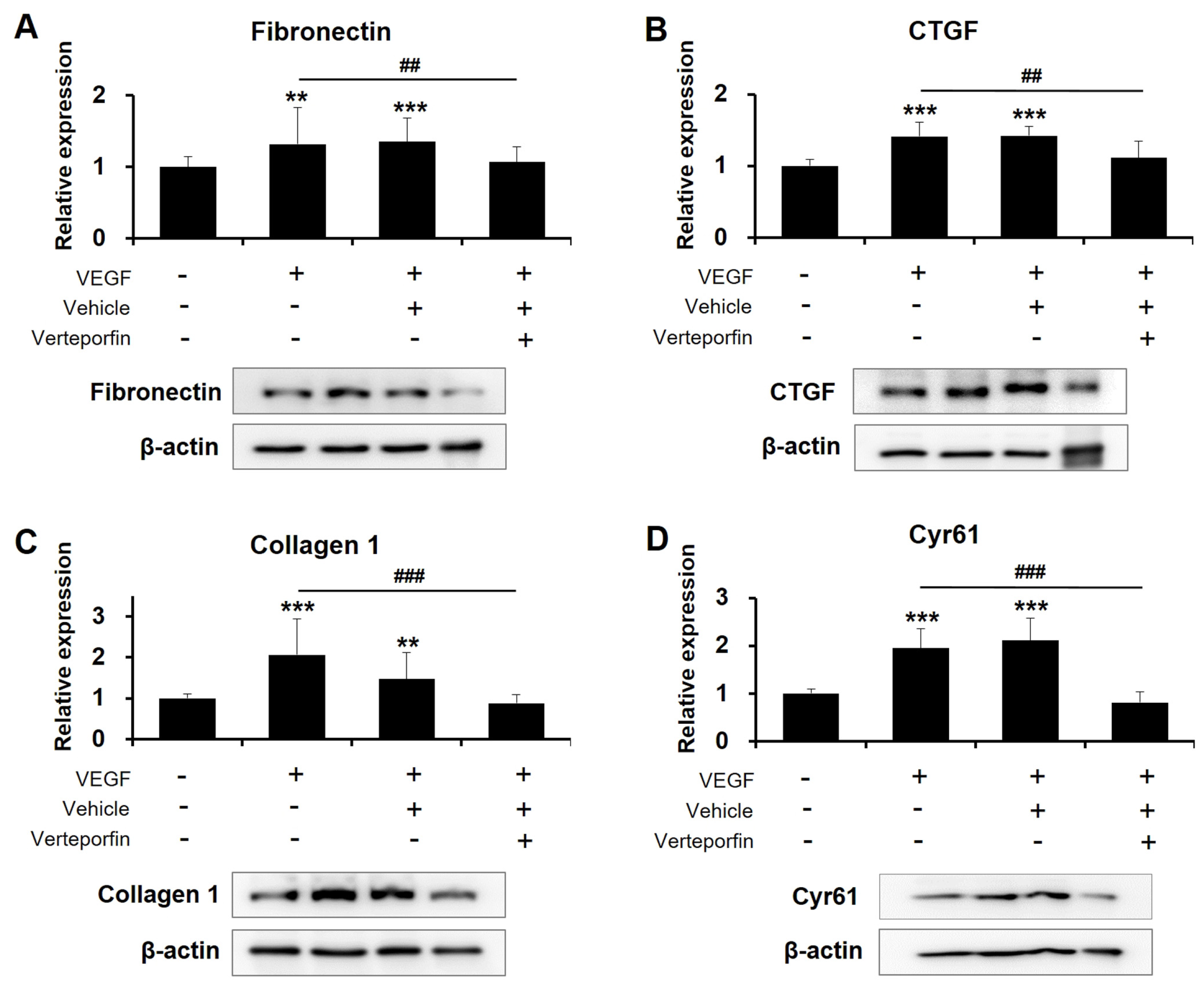
3. Discussion
4. Materials and Methods
4.1. Human Trabecular Meshwork Cell Culture
4.2. Cell Viability
4.3. Cell Treatments
4.4. Quantitative Real-Time Polymerase Chain Reaction
4.5. Western Blot
4.6. Immunocytochemistry
4.7. Cross-Linked Actin Network Evaluation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allingham, R.R.; Liu, Y.; Rhee, D.J. The Genetics of Primary Open-Angle Glaucoma: A review. Exp. Eye Res. 2009, 88, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K.; Wilson, M.R.; Gordon, M.O. The Ocular hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Kelley, M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Stephan, D.A.; Russell, P.; Tamm, E.R. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp. Eye Res. 2009, 88, 1020–1032. [Google Scholar] [CrossRef]
- Fujimoto, T.; Inoue, T.; Maki, K.; Inoue-Mochita, M.; Tanihara, H. Vascular endothelial growth factor-A increases the aqueous humor outflow facility. PLoS ONE 2016, 11, e0161332. [Google Scholar] [CrossRef]
- Reina-Torres, E.; Wen, J.C.; Liu, K.C.; Li, G.; Sherwood, J.M.; Chang, J.Y.; Challa, P.; Flügel-Koch, C.M.; Stamer, W.D.; Allingham, R.R.; et al. VEGF as a paracrine regulator of conventional outflow facility. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1899–1908. [Google Scholar] [CrossRef]
- Rogers, M.E.; Navarro, I.D.; Perkumas, K.M.; Niere, S.M.; Allingham, R.R.; Crosson, C.E.; Stamer, W.D. Pigment epithelium-derived factor decreases outflow facility. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6655–6661. [Google Scholar] [CrossRef]
- Lazzara, F.; Fidilio, A.; Platania, C.B.M.; Giurdanella, G.; Salomone, S.; Leggio, G.M.; Tarallo, V.; Cicatiello, V.; De Falco, S.; Eandi, C.M.; et al. Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochem. Pharmacol. 2019, 168, 341–351. [Google Scholar] [CrossRef]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Itoga, K.; Okano, T.; Yonemura, S.; Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 2011, 138, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Chen, W.S.; Cao, Z.; Krishnan, C.; Panjwani, N. Verteporfin without light stimulation inhibits YAP activation in trabecular meshwork cells: Implications for glaucoma treatment. Biochem. Biophys. Res. Commun. 2015, 466, 221–225. [Google Scholar] [CrossRef]
- Dhamodaran, K.; Baidouri, H.; Sandoval, L.; Raghunathan, V. Wnt activation after inhibition restores trabecular meshwork cells toward a normal phenotype. Investig. Ophthalmol. Vis. Sci. 2020, 61, 30. [Google Scholar] [CrossRef]
- Ho, L.T.Y.; Skiba, N.; Ullmer, C.; Rao, P.V. Lysophosphatidic acid induces ECM production via activation of the mechanosensitive YAP/TAZ transcriptional pathway in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1969–1984. [Google Scholar] [CrossRef]
- Honjo, M.; Igarashi, N.; Nishida, J.; Kurano, M.; Yatomi, Y.; Igarashi, K.; Kano, K.; Aoki, J.; Aihara, M. Role of the autotaxin-LPA pathway in dexamethasone-induced fibrotic responses and extracellular matrix production in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2018, 59, 21–30. [Google Scholar] [CrossRef]
- Li, H.; Raghunathan, V.; Stamer, W.D.; Ganapathy, P.S.; Herberg, S. Extracellular matrix stiffness and TGFβ2 regulate YAP/TAZ activity in human trabecular meshwork cells. Front. Cell Dev. Biol. 2022, 10, 844342. [Google Scholar] [CrossRef]
- Peng, J.; Wang, H.; Wang, X.; Sun, M.; Deng, S.; Wang, Y. YAP and TAZ mediate steroid-induced alterations in the trabecular meshwork cytoskeleton in human trabecular meshwork cells. Int. J. Mol. Med. 2018, 41, 164–172. [Google Scholar] [CrossRef]
- Raghunathan, V.K.; Morgan, J.T.; Dreier, B.; Reilly, C.M.; Thomasy, S.M.; Wood, J.A.; Ly, I.; Tuyen, B.C.; Hughbanks, M.; Murphy, C.J.; et al. Role of substratum stiffness in modulating genes associated with extracellular matrix and mechanotransducers YAP and TAZ. Investig. Ophthalmol. Vis. Sci. 2013, 54, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Yemanyi, F.; Raghunathan, V. Lysophosphatidic acid and IL-6 trans-signaling interact via YAP/TAZ and STAT3 signaling pathways in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 29. [Google Scholar] [CrossRef] [PubMed]
- Yemanyi, F.; Vranka, J.; Raghunathan, V.K. Crosslinked extracellular matrix Stiffens Human Trabecular Meshwork Cells via dysregulating β-catenin and YAP/TAZ Signaling Pathways. Investig. Ophthalmol. Vis. Sci. 2020, 61, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Freire Valls, A.; Schermann, G.; Shen, Y.; Moya, I.M.; Castro, L.; Urban, S.; Solecki, G.M.; Winkler, F.; Riedemann, L.; et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev. Cell. 2017, 42, 462–478.e7. [Google Scholar] [CrossRef]
- Reggiani, F.; Gobbi, G.; Ciarrocchi, A.; Sancisi, V. YAP and TAZ are not identical twins. Trends. Biochem. Sci. 2021, 46, 154–168. [Google Scholar] [CrossRef]
- Shepard, A.R.; Millar, J.C.; Pang, I.H.; Jacobson, N.; Wang, W.H.; Clark, A.F. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2067–2076. [Google Scholar] [CrossRef]
- Hoare, M.J.; Grierson, I.; Brotchie, D.; Pollock, N.; Cracknell, K.; Clark, A.F. Cross-linked actin networks (CLANs) in the trabecular meshwork of the normal and glaucomatous human eye in situ. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1255–1263. [Google Scholar] [CrossRef]
- Clark, A.F.; Wordinger, R.J. The role of steroids in outflow resistance. Exp. Eye Res. 2009, 88, 752–759. [Google Scholar] [CrossRef]
- Gardel, M.L.; Shin, J.H.; MacKintosh, F.C.; Mahadevan, L.; Matsudaira, P.; Weitz, D.A. Elastic behavior of cross-linked and bundled actin networks. Science 2004, 304, 1301–1305. [Google Scholar] [CrossRef]
- Lui, J.W.; Xiao, S.; Ogomori, K.; Hammarstedt, J.E.; Little, E.C.; Lang, D. The efficiency of verteporfin as a therapeutic option in pre-clinical models of melanoma. J. Cancer 2019, 10, 1. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, Y.; Ji, X.; Xiao, X.; Li, Z.; Yi, X.; Zhu, Y.; Guo, T.; Wang, Y.; Chen, L.; et al. The Hippo-TAZ axis mediates vascular endothelial growth factor C in glioblastoma-derived exosomes to promote angiogenesis. Cancer Lett. 2021, 513, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mao, L.; Xiong, J.; Wen, J.; Wang, Y.; Geng, D.; Liu, Y. TAZ expression on endothelial cells is closely related to blood vascular density and VEGFR2 expression in astrocytomas. J. Neuropathol. Exp. Neurol. 2019, 78, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Keller, K.E.; Peters, D.M. Pathogenesis of glaucoma: Extracellular matrix dysfunction in the trabecular meshwork-A review. Clin. Exp. Ophthalmol. 2022, 50, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Villarreal, G., Jr.; Rhee, D.J. Matricellular proteins in the trabecular meshwork: Review and update. J. Ocul. Pharmacol. Ther. 2014, 30, 447–463. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Montecchi-Palmer, M.; Bermudez, J.Y.; Webber, H.C.; Patel, G.C.; Clark, A.F.; Mao, W. TGFβ2 induces the formation of cross-linked actin networks (CLANs) in human trabecular meshwork cells through the Smad and non-Smad dependent pathways. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1288–1295. [Google Scholar] [CrossRef]
- Liu, F.; Lagares, D.; Choi, K.M.; Stopfer, L.; Marinković, A.; Vrbanac, V.; Probst, C.K.; Hiemer, S.E.; Sisson, T.H.; Horowitz, J.C.; et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L344–L357. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, K.; Li, J.M.; Shen, J.; Xu, W. Elevated levels of endoglin, endostatin, FGF-α, HGF, and Thrombospondin-2 in aqueous humor of nAMD patients. Ocul. Immunol. Inflamm. 2022, 30, 1092–1098. [Google Scholar] [CrossRef]
- Hu, D.N.; Ritch, R.; Liebmann, J.; Liu, Y.; Cheng, B.; Hu, M.S. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J. Glaucoma 2002, 11, 406–410. [Google Scholar] [CrossRef]
- Kwon, J.W.; Jee, D. Aqueous humor cytokine levels in patients with diabetic macular edema refractory to anti-VEGF treatment. PLoS ONE 2018, 13, e0203408. [Google Scholar] [CrossRef]
- Obadă, O.; Pantalon, A.D.; Rusu-Zota, G.; Hăisan, A.; Lupuşoru, S.I.; Constantinescu, D.; Chiseliţă, D. Aqueous humor cytokines in non-proliferative diabetic retinopathy. Medicina 2022, 58, 909. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Nam, W.H.; Park, S.E.; Kim, J.H.; Kim, H.K. Aqueous humor concentrations of vascular endothelial growth factor and pigment epithelium-derived factor in high myopic patients. Mol. Vis. 2012, 18, 2265–2270. [Google Scholar]
- Kuo, Y.Z.; Kang, Y.R.; Chang, W.L.; Sim, L.C.; Hsieh, T.C.; Chang, C.H.; Wang, Y.C.; Tsai, C.J.; Huang, L.C.; Tsai, S.T.; et al. YAP1 acts as a negative regulator of pro-tumor TAZ expression in esophageal squamous cell carcinoma. Cell. Oncol. 2022, 45, 893–909. [Google Scholar] [CrossRef] [PubMed]
- Makita, R.; Uchijima, Y.; Nishiyama, K.; Amano, T.; Chen, Q.; Takeuchi, T.; Mitani, A.; Nagase, T.; Yatomi, Y.; Aburatani, H.; et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am. J. Physiol. Renal Physiol. 2008, 294, F542–F553. [Google Scholar] [CrossRef] [PubMed]
- Morin-Kensicki, E.M.; Boone, B.N.; Howell, M.; Stonebraker, J.R.; Teed, J.; Alb, J.G.; Magnuson, T.R.; O’Neal, W.; Milgram, S.L. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol. Cell. Biol. 2006, 26, 77–87. [Google Scholar] [CrossRef]
- Dimtsas, G.S.; Tsiogka, A.; Moschos, M.M. VEGF levels in the aqueous humor of patients with primary open angle glaucoma: A systematic review and a meta-analysis. Eur. J. Ophthalmol. 2023. [Google Scholar] [CrossRef]
- Bhisitkul, R.B. Vascular endothelial growth factor biology: Clinical implications for ocular treatments. Br. J. Ophthalmol. 2006, 90, 1542–1547. [Google Scholar] [CrossRef]
- Djordjevic, S.; Driscoll, P.C. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov. Today 2013, 18, 447–455. [Google Scholar] [CrossRef]
- Stamer, W.D.; Clark, A.F. The many faces of the trabecular meshwork cell. Exp. Eye Res. 2017, 158, 112–123. [Google Scholar] [CrossRef]
- Futakuchi, A.; Inoue, T.; Wei, F.Y.; Inoue-Mochita, M.; Fujimoto, T.; Tomizawa, K.; Tanihara, H. YAP/TAZ are essential for TGF-β2-mediated conjunctival fibrosis. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3069–3078. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, M.S.; Kim, S.Y.; Eom, G.H.; Park, S.W. High VEGF Concentrations Accelerate Human Trabecular Meshwork Fibrosis in a TAZ-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 9625. https://doi.org/10.3390/ijms24119625
Sung MS, Kim SY, Eom GH, Park SW. High VEGF Concentrations Accelerate Human Trabecular Meshwork Fibrosis in a TAZ-Dependent Manner. International Journal of Molecular Sciences. 2023; 24(11):9625. https://doi.org/10.3390/ijms24119625
Chicago/Turabian StyleSung, Mi Sun, So Young Kim, Gwang Hyeon Eom, and Sang Woo Park. 2023. "High VEGF Concentrations Accelerate Human Trabecular Meshwork Fibrosis in a TAZ-Dependent Manner" International Journal of Molecular Sciences 24, no. 11: 9625. https://doi.org/10.3390/ijms24119625





