The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects
Abstract
:1. Introduction
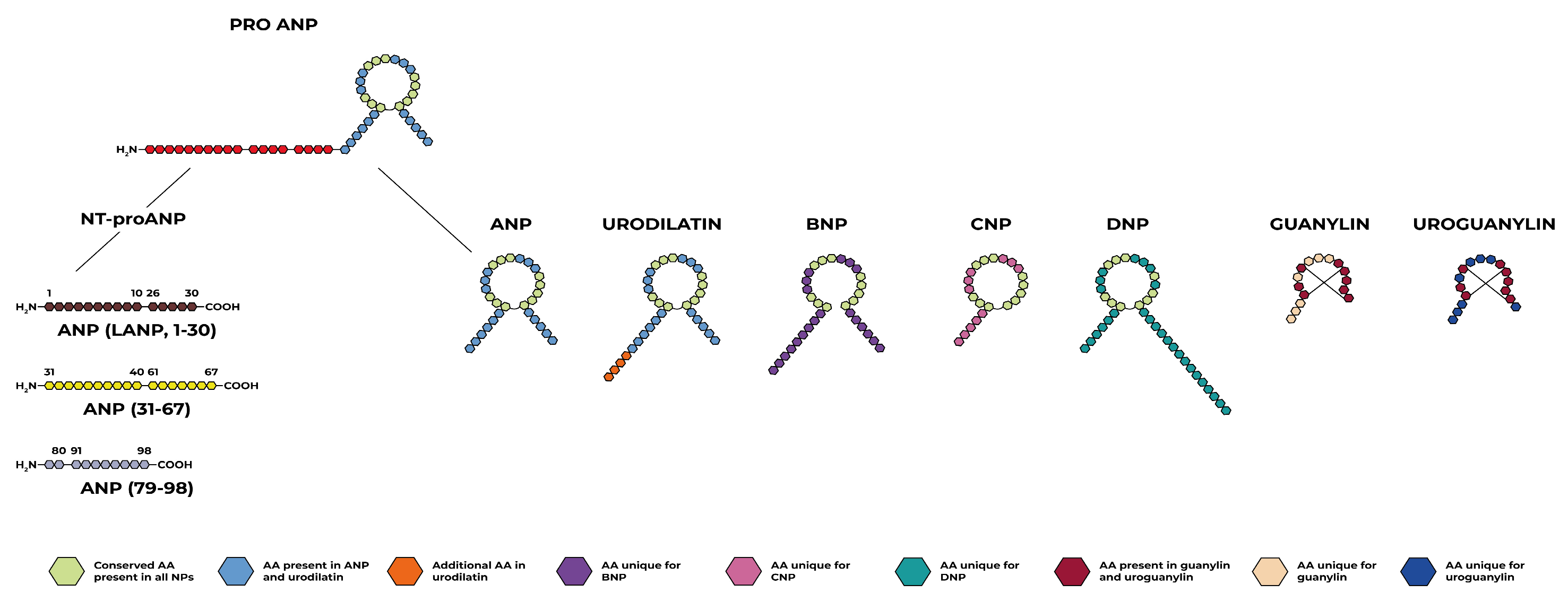
2. Natriuretic Peptides
2.1. Atrial Natriuretic Peptide
2.2. B-Type Natriuretic Peptide
2.3. C-Type Natriuretic Peptide
2.4. Dendroaspis Natriuretic Peptide
2.5. Urodilatin
2.6. Guanylin and Uroguanylin
2.7. Linear ANP Fragments
ANP31–67
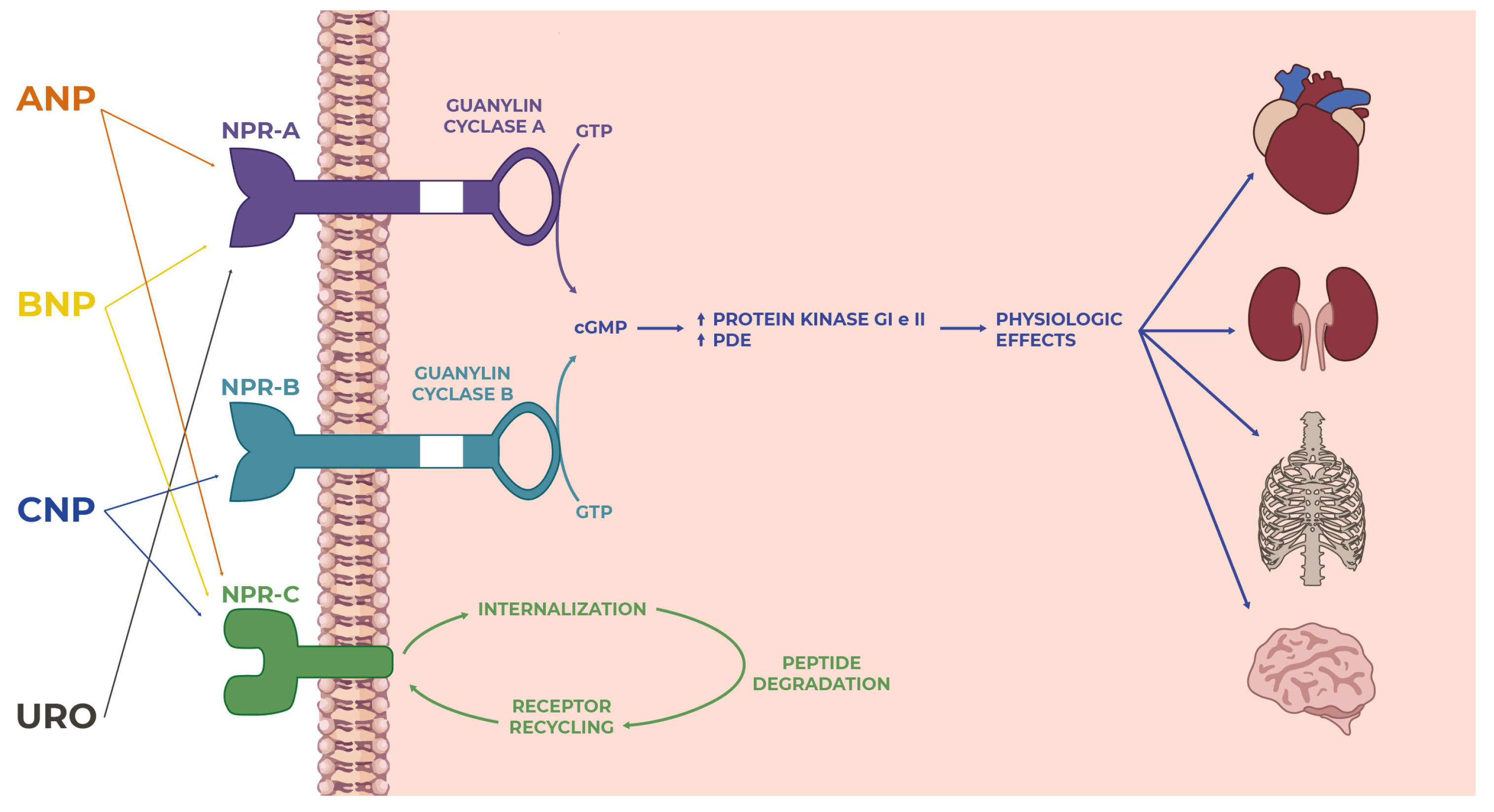
3. Natriuretic Peptide Receptors
3.1. Natriuretic Peptide Receptor-A
NPR-A: Positive Allosteric Modulator
3.2. Natriuretic Peptide Receptor-B
3.3. Natriuretic Peptide Receptor-C
4. Physiologic Effects of Natriuretic Peptides
4.1. Natriuretic Peptide Effects on Cardiovascular System
4.2. Natriuretic Peptide Effects on the Nervous System
4.3. Natriuretic Peptide Effects on Renal System
4.4. Natriuretic Peptide Effects on Musculoskeletal System
5. The Natriuretic Peptide System
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: Their structures, receptors, physiologic functions and therapeutic applications. In Handbook of Experimental Pharmacology; Schmidt, H.H.H.W., Hofmann, F., Stasch, J.-P., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 191, pp. 341–366. ISBN 9783540689607. [Google Scholar]
- Sinđić, A.; Schlatter, E. Mechanisms of action of uroguanylin and guanylin and their role in salt handling. Nephrol. Dial. Transplant. 2006, 21, 3007–3012. [Google Scholar] [CrossRef]
- Richards, A.M.; Lainchbury, J.G.; Nicholls, M.G.; Cameron, A.V.; Yandle, T.G. Dendroaspis natriuretic peptide: Endogenous or dubious? Lancet 2002, 359, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Gunning, M.; Brenner, B.M. Urodilatin: A potent natriuretic peptide of renal origin. Curr. Opin. Nephrol. Hypertens. 1993, 2, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Nagai-Okatani, C.; Nishigori, M.; Kangawa, K.; Minamino, N. Natriuretic peptides in human heart: Novel insight into their molecular forms, functions, and diagnostic use. Peptides 2019, 111, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Nagai-Okatani, C.; Kangawa, K.; Minamino, N. Three molecular forms of atrial natriuretic peptides: Quantitative analysis and biological characterization. J. Pept. Sci. 2017, 23, 486–495. [Google Scholar] [CrossRef]
- Nishikimi, T.; Kuwahara, K.; Nakao, K. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J. Cardiol. 2011, 57, 131–140. [Google Scholar] [CrossRef]
- Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Zoccai, G.B.; Frati, G.; Sciarretta, S. Cardiovascular Pleiotropic Effects of Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 3874. [Google Scholar] [CrossRef]
- Hollister, A.S.; Rodeheffer, R.J.; White, F.J.; Potts, J.R.; Imada, T.; Inagami, T. Clearance of atrial natriuretic factor by lung, liver, and kidney in human subjects and the dog. J. Clin. Investig. 1989, 83, 623–628. [Google Scholar] [CrossRef]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef]
- Mukoyama, M.; Nakao, K.; Hosoda, K.; Suga, S.; Saito, Y.; Ogawa, Y.; Shirakami, G.; Jougasaki, M.; Obata, K.; Yasue, H.; et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Investig. 1991, 87, 1402–1412. [Google Scholar] [CrossRef]
- Sawada, Y.; Inoue, M.; Kanda, T.; Sakamaki, T.; Tanaka, S.; Minamino, N.; Nagai, R.; Takeuchi, T. Co-elevation of brain natriuretic peptide and proprotein-processing endoprotease furin after myocardial infarction in rats. FEBS Lett. 1997, 400, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, T.; Nakagawa, Y.; Minamino, N.; Ikeda, M.; Tabei, K.; Fujishima, A.; Takayama, K.; Akimoto, K.; Yamada, C.; Nakao, K.; et al. Pro-B-type natriuretic peptide is cleaved intracellularly: Impact of distance between O-glycosylation and cleavage sites. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R639–R649. [Google Scholar] [CrossRef] [PubMed]
- Topaz, O.; Shurman, D.L.; Bergman, R.; Indelman, M.; Ratajczak, P.; Mizrachi, M.; Khamaysi, Z.; Behar, D.M.; Petronius, D.; Friedman, V.; et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat. Genet. 2004, 36, 579–581. [Google Scholar] [CrossRef]
- Grépin, C.; Dagnino, L.; Robitaille, L.; Haberstroh, L.; Antakly, T.; Nemer, M. A Hormone-Encoding Gene Identifies a Pathway for Cardiac but Not Skeletal Muscle Gene Transcription. Mol. Cell. Biol. 1994, 14, 3115–3129. [Google Scholar] [CrossRef]
- Mukoyama, M.; Nakao, K.; Saito, Y.; Ogawa, Y.; Hosoda, K.; Suga, S.; Shirakami, G.; Jougasaki, M.; Imura, H. Increased Human Brain Natriuretic Peptide in Congestive Heart Failure. N. Engl. J. Med. 1990, 323, 757–758. [Google Scholar] [CrossRef]
- Pankow, K.; Wang, Y.; Gembardt, F.; Krause, E.; Sun, X.; Krause, G.; Schultheiss, H.-P.; Siems, W.-E.; Walther, T. Successive Action of Meprin A and Neprilysin Catabolizes B-Type Natriuretic Peptide. Circ. Res. 2007, 101, 875–882. [Google Scholar] [CrossRef]
- Ogawa, Y.; Itoh, H.; Tamura, N.; Suga, S.; Yoshimasa, T.; Uehira, M.; Matsuda, S.; Shiono, S.; Nishimoto, H.; Nakao, K. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. J. Clin. Investig. 1994, 93, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, V.; Tuttolomondo, A.; Pecoraro, R.; Pinto, A. Chronic hyponatremia in a patient with renal salt wasting and without cerebral disease: Relationship between RSW, risk of fractures and cognitive impairment. Intern. Emerg. Med. 2018, 13, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Suga, S.; Nakao, K.; Itoh, H.; Komatsu, Y.; Ogawa, Y.; Hama, N.; Imura, H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J. Clin. Investig. 1992, 90, 1145–1149. [Google Scholar] [CrossRef]
- Del Ry, S. C-type natriuretic peptide: A new cardiac mediator. Peptides 2013, 40, 93–98. [Google Scholar] [CrossRef]
- Nakao, K.; Kuwahara, K.; Nishikimi, T.; Nakagawa, Y.; Kinoshita, H.; Minami, T.; Kuwabara, Y.; Yamada, C.; Yamada, Y.; Tokudome, T.; et al. Endothelium-Derived C-Type Natriuretic Peptide Contributes to Blood Pressure Regulation by Maintaining Endothelial Integrity. Hypertension 2017, 69, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Moyes, A.J.; Hobbs, A.J. C-Type Natriuretic Peptide: A Multifaceted Paracrine Regulator in the Heart and Vasculature. Int. J. Mol. Sci. 2019, 20, 2281. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.C.; Barr, C.S.; Struthers, A.D. C-Type Natriuretic Peptide. An endogenous inhibitor of vascular angiotensin-converting enzyme activity. Circulation 1996, 93, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Prickett, T.C.; Espiner, E.A. Circulating products of C-type natriuretic peptide and links with organ function in health and disease. Peptides 2020, 132, 170363. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.J.; Richards, A.M.; Espiner, E.A.; Nicholls, M.G.; Yandle, T.G. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1994, 78, 1428–1435. [Google Scholar] [CrossRef]
- Lisy, O.; Jougasaki, M.; Heublein, D.M.; Schirger, J.A.; Chen, H.H.; Wennberg, P.W.; Burnett, J.C. Renal actions of synthetic Dendroaspis natriuretic peptide. Kidney Int. 1999, 56, 502–508. [Google Scholar] [CrossRef]
- Lisy, O.; Lainchbury, J.G.; Leskinen, H.; Burnett, J.C. Therapeutic actions of a new synthetic vasoactive and natriuretic peptide, dendroaspis natriuretic peptide, in experimental severe congestive heart failure. Hypertension 2001, 37, 1089–1094. [Google Scholar] [CrossRef]
- Khurana, V.G.; Wijdicks, E.F.; Heublein, D.M.; McClelland, R.L.; Meyer, F.B.; Piepgras, D.G.; Burnett, J.C. A Pilot Study of Dendroaspis Natriuretic Peptide in Aneurysmal Subarachnoid Hemorrhage. Neurosurgery 2004, 55, 69–75; discussion 75–76. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.W. Dendroaspis Natriuretic Peptide Administered Intracerebroventricularly Increases Renal Water Excretion. Clin. Exp. Pharmacol. Physiol. 2002, 29, 195–197. [Google Scholar] [CrossRef]
- Schulz-Knappe, P.; Forssmann, K.; Herbst, F.; Hock, D.; Pipkorn, R.; Forssmann, W.G. Isolation and structural analysis of “Urodilatin”, a new peptide of the cardiodilatin-(ANP)-family, extracted from human urine. Klin. Wochenschr. 1988, 66, 752–759. [Google Scholar] [CrossRef]
- Vesely, B.A.; Eichelbaum, E.J.; Alli, A.A.; Sun, Y.; Gower, W.R.; Vesely, D.L. Urodilatin and four cardiac hormones decrease human renal carcinoma cell numbers. Eur. J. Clin. Investig. 2006, 36, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Feller, S.; Gagelmann, M.; Forssmann, W. Urodilatin: A newly described member of the ANP family. Trends Pharmacol. Sci. 1989, 10, 93–94. [Google Scholar] [CrossRef]
- Abassi, Z.A.; Golomb, E.; Klein, H.; Keiser, H.R. Urodilatin: A Natriuretic Peptide of Renal Origin. Cardiovasc. Drug Rev. 1992, 10, 199–210. [Google Scholar] [CrossRef]
- Greenwald, J.E.; Ritter, D.; Tetens, E.; Rotwein, P.S. Renal expression of the gene for atrial natriuretic factor. Am. J. Physiol. 1992, 263 Pt 2, F974–F978. [Google Scholar] [CrossRef] [PubMed]
- Heim, J.-M.; Kiefersauer, S.; Fülle, H.-J.; Gerzer, R.; Heim, J.-M.; Kiefersauer, S.; Fülle, H.-J.; Gerzer, R. Urodilatin and β-ANF: Binding properties and activation of particulate guanylate cyclase. Biochem. Biophys. Res. Commun. 1989, 163, 37–41. [Google Scholar] [CrossRef]
- Forssmann, W.-G.; Meyer, M.; Forssmann, K. The renal urodilatin system: Clinical implications. Cardiovasc. Res. 2001, 51, 450–462. [Google Scholar] [CrossRef]
- Gagelmann, M.; Hock, D.; Forssmann, W.-G. Urodilatin (CDD/ANP-95-126) is not biologically inactivated by a peptidase from dog kidney cortex membranes in contrast to atrial natriuretic peptide/cardiodilatin (α-hANP/CDD-99-126). FEBS Lett. 1988, 233, 249–254. [Google Scholar] [CrossRef]
- Koehn, J.A.; Norman, J.A.; Jones, B.N.; LeSueur, L.; Sakane, Y.; Ghai, R.D. Degradation of atrial natriuretic factor by kidney cortex membranes. Isolation and characterization of the primary proteolytic product. J. Biol. Chem. 1987, 262, 11623–11627. [Google Scholar] [CrossRef]
- Heringlake, M.; Wagner, K.; Schumacher, J.; Pagel, H. Urinary excretion of urodilatin is increased during pressure natriuresis in the isolated perfused rat kidney. Am. J. Physiol. 1999, 277, F347–F351. [Google Scholar] [CrossRef]
- Goetz, K.; Drummer, C.; Zhu, J.L.; Leadley, R.; Fiedler, F.; Gerzer, R. Evidence that urodilatin, rather than ANP, regulates renal sodium excretion. J. Am. Soc. Nephrol. 1990, 1, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Emmeluth, C.; Drummer, C.; Gerzer, R.; Bie, P. Roles of cephalic Na+ concentration and urodilatin in control of renal Na+ excretion. Am. J. Physiol. 1992, 262 Pt 2, F513–F516. [Google Scholar] [CrossRef] [PubMed]
- Emmeluth, C.; Goetz, K.L.; Drummer, C.; Gerzer, R.; Forssmann, W.G.; Bie, P. Natriuresis caused by increased carotid Na+ concentration after renal denervation. Am. J. Physiol. 1996, 270 Pt 2, F510–F517. [Google Scholar] [CrossRef] [PubMed]
- Norsk, P.; Drummer, C.; Johansen, L.B.; Gerzer, R. Effect of water immersion on renal natriuretic peptide (urodilatin) excretion in humans. J. Appl. Physiol. 1993, 74, 2881–2885. [Google Scholar] [CrossRef]
- Mitrovic, V.; Lüss, H.; Nitsche, K.; Forssmann, K.; Maronde, E.; Fricke, K.; Forssmann, W.-G.; Meyer, M. Effects of the renal natriuretic peptide urodilatin (ularitide) in patients with decompensated chronic heart failure: A double-blind, placebo-controlled, ascending-dose trial. Am. Heart J. 2005, 150, 1239.e1–1239.e8. [Google Scholar] [CrossRef]
- Samanta, S.; Chaudhuri, A.G. Guanylin and uroguanylin: A promising nexus in intestinal electrolyte and fluid homeostasis. J. Physiol. Pharmacol. 2021, 72, 667–676. [Google Scholar] [CrossRef]
- Kita, T.; Kitamura, K.; Sakata, J.; Eto, T. Marked increase of guanylin secretion in response to salt loading in the rat small intestine. Am. J. Physiol.-Ren. Physiol. 1999, 277, G960–G966. [Google Scholar] [CrossRef]
- Fellner, R.C.; Moss, N.G.; Goy, M.F. Dietary salt regulates uroguanylin expression and signaling activity in the kidney, but not in the intestine. Physiol. Rep. 2016, 4, e12782. [Google Scholar] [CrossRef]
- Dye, F.S.; Larraufie, P.; Kay, R.; Darwish, T.; Rievaj, J.; Goldspink, D.A.; Meek, C.L.; Middleton, S.J.; Hardwick, R.H.; Roberts, G.P.; et al. Characterisation of proguanylin expressing cells in the intestine—evidence for constitutive luminal secretion. Sci. Rep. 2019, 9, 15574. [Google Scholar] [CrossRef]
- de Sauvage, F.J.; Keshav, S.; Kuang, W.J.; Gillett, N.; Henzel, W.; Goeddel, D.V. Precursor structure, expression, and tissue distribution of human guanylin. Proc. Natl. Acad. Sci. USA 1992, 89, 9089–9093. [Google Scholar] [CrossRef]
- Kita, T.; Smith, C.E.; Fok, K.F.; Duffin, K.L.; Moore, W.M.; Karabatsos, P.J.; Kachur, J.F.; Hamra, F.K.; Pidhorodeckyj, N.V.; Forte, L.R.; et al. Characterization of human uroguanylin: A member of the guanylin peptide family. Am. J. Physiol. 1994, 266 Pt 2, F342–F348. [Google Scholar] [CrossRef] [PubMed]
- Vesely, D.L. Which of the cardiac natriuretic peptides is most effective for the treatment of congestive heart failure, renal failure and cancer? Clin. Exp. Pharmacol. Physiol. 2006, 33, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Burnett, J.C., Jr. Atrial Natriuretic Peptide—Old But New Therapeutic in Cardiovascular Diseases—. Circ. J. 2017, 81, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Vesely, D.L.; Douglass, M.A.; Dietz, J.R.; Gower, W.R., Jr.; McCormick, M.T.; Rodriguez-Paz, G.; Schocken, D.D. Three peptides from the atrial natriuretic factor prohormone amino terminus lower blood pressure and produce diuresis, natriuresis, and/or kaliuresis in humans. Circulation 1994, 90, 1129–1140. [Google Scholar] [CrossRef]
- Gower, W.R.; Chiou, S.; Skolnick, K.A.; Vesely, D.L. Molecular forms of circulating atrial natriuretic peptides in human plasma and their metabolites. Peptides 1994, 15, 861–867. [Google Scholar] [CrossRef]
- Greenwald, J.E.; Needleman, P.; Siegel, N.; Tetens, E.; Biel, B.; Ritter, D. Processing of atriopeptin prohormone by nonmyocytic atrial cells. Biochem. Biophys. Res. Commun. 1992, 188, 644–654. [Google Scholar] [CrossRef]
- Hartter, E.; Khalafpour, S.; Mißbichler, A.; Hawa, G.; Woloszczuk, W. Enzyme Immunoassays for Fragments (Epitopes) of Human Proatrial Natriuretic Peptides. Clin. Chem. Lab. Med. 2000, 38, 27–32. [Google Scholar] [CrossRef]
- Gunning, M.E.; Brady, H.R.; Otuechere, G.; Brenner, B.M.; Zeidel, M.L. Atrial natriuretic peptide(31-67) inhibits Na+ transport in rabbit inner medullary collecting duct cells. Role of prostaglandin E2. J. Clin. Investig. 1992, 89, 1411–1417. [Google Scholar] [CrossRef]
- Luce, M.; Barba, C.; Yi, D.; Mey, A.; Roussel, D.; Bres, E.; Benoit, B.; Pastural, M.; Granjon, S.; Szelag, J.C.; et al. Accumulation of natriuretic peptides is associated with protein energy wasting and activation of browning in white adipose tissue in chronic kidney disease. Kidney Int. 2020, 98, 663–672. [Google Scholar] [CrossRef]
- Bordicchia, M.; Liu, D.; Amri, E.-Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef]
- Cappellin, E.; De Palo, E.F.; Gatti, R.; Soldà, G.; Woloszczuk, W.; Spinella, P. Effect of prolonged physical exercise on urinary proANP1-30 and proANP31-67. Clin. Chem. Lab. Med. 2004, 42, 1058–1062. [Google Scholar] [CrossRef] [PubMed]
- De Palo, E.F.; Woloszczuk, W.; Meneghetti, M.; De Palo, C.B.; Nielsen, H.B.; Secher, N.H. Circulating Immunoreactive proANP(1-30) and proANP(31-67) in Sedentary Subjects and Athletes. Clin. Chem. 2000, 46, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Vesely, D.L.; Cornett, L.E.; MacLeod, S.L.; Nash, A.A.; Norris, J.S. Specific binding sites for prohormone atrial natriuretic peptides 1–30, 31–67 and 99–126. Peptides 1990, 11, 193–197. [Google Scholar] [CrossRef]
- Vesely, D.L.; Perez-Lamboy, G.I.; Schocken, D.D. Vessel dilator, long acting natriuretic peptide, and kaliuretic peptide increase circulating prostaglandin E2. Life Sci. 2000, 66, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Freeman, R.H.; Taraben, A.; Reams, G.P. Modulation of Renin Secretion by Atrial Natriuretic Factor Prohormone Fragment 31–67. Am. J. Med. Sci. 1999, 318, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Purdy, K.E.; Arendshorst, W.J. EP1 and EP4 receptors mediate prostaglandin E2 actions in the microcirculation of rat kidney. Am. J. Physiol.-Ren. Physiol. 2000, 279, F755–F764. [Google Scholar] [CrossRef]
- Makino, H.; Tanaka, I.; Mukoyama, M.; Sugawara, A.; Mori, K.; Muro, S.; Suganami, T.; Yahata, K.; Ishibashi, R.; Ohuchida, S.; et al. Prevention of Diabetic Nephropathy in Rats by Prostaglandin E Receptor EP1-Selective Antagonist. J. Am. Soc. Nephrol. 2002, 13, 1757–1765. [Google Scholar] [CrossRef]
- Wang, Q.; Oka, T.; Yamagami, K.; Lee, J.-K.; Akazawa, H.; Naito, A.T.; Yasui, T.; Ishizu, T.; Nakaoka, Y.; Sakata, Y.; et al. An EP4 Receptor Agonist Inhibits Cardiac Fibrosis Through Activation of PKA Signaling in Hypertrophied Heart. Int. Heart J. 2017, 58, 107–114. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Harding, P.; Liu, Y.; Shesely, E.; Yang, X.-P.; LaPointe, M.C. Reduced Cardiac Remodeling and Function in Cardiac-Specific EP4 Receptor Knockout Mice with Myocardial Infarction. Hypertension 2008, 51, 560–566. [Google Scholar] [CrossRef]
- Umemura, M.; Osawa, K.; Tanaka, R.; Hikichi, M.; Nakakaji, R.; Rafikul, I.; Ishikawa, Y. Abstract 13193: Prostaglandin E2 Receptor Ep4 Regulates Fibrotic Changes via Intracellular Calcium in Cardiac Fibroblast Cells. Circulation 2019, 140 (Suppl. S1), A13193. [Google Scholar]
- Harding, P.; LaPointe, M.C. Prostaglandin E2 increases cardiac fibroblast proliferation and increases cyclin D expression via EP1 receptor. Prostaglandins Leukot. Essent. Fat. Acids 2011, 84, 147–152. [Google Scholar] [CrossRef]
- Xu, H.; Fu, J.-L.; Miao, Y.-F.; Wang, C.-J.; Han, Q.-F.; Li, S.; Huang, S.-Z.; Du, S.-N.; Qiu, Y.-X.; Yang, J.-C.; et al. Prostaglandin E2 receptor EP3 regulates both adipogenesis and lipolysis in mouse white adipose tissue. J. Mol. Cell Biol. 2016, 8, 518–529. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, V.; López-Vicario, C.; Titos, E.; Morán-Salvador, E.; González-Périz, A.; Rius, B.; Párrizas, M.; Werz, O.; Arroyo, V.; Clària, J. Coordinate Functional Regulation between Microsomal Prostaglandin E Synthase-1 (mPGES-1) and Peroxisome Proliferator-activated Receptor γ (PPARγ) in the Conversion of White-to-brown Adipocytes. J. Biol. Chem. 2013, 288, 28230–28242. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, R.P.; Lee, D.; Maulis, M.F.; Carboneau, B.A.; Threadgill, D.W.; Poffenberger, G.; Milne, G.; Boyd, K.L.; Powers, A.C.; McGuinness, O.P.; et al. The PGE2 EP3 Receptor Regulates Diet-Induced Adiposity in Male Mice. Endocrinology 2016, 157, 220–232. [Google Scholar] [CrossRef]
- Reginauld, S.H.; Cannone, V.; Iyer, S.; Scott, C.; Bailey, K.; Schaefer, J.; Chen, Y.; Sangaralingham, S.J.; Burnett, J.C. Differential Regulation of ANP and BNP in Acute Decompensated Heart Failure: Deficiency of ANP. JACC Heart Fail. 2019, 7, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Winters, C.J.; Sallman, A.L.; Baker, B.J.; Meadows, J.; Rico, D.M.; Vesely, D.L. The N-terminus and a 4000-MW peptide from the midportion of the N-terminus of the atrial natriuretic factor prohormone each circulate in humans and increase in congestive heart failure. Circulation 1989, 80, 438–449. [Google Scholar] [CrossRef]
- Altara, R.; Da Silva, G.J.; Frisk, M.; Spelta, F.; Zouein, F.A.; Louch, W.E.; Booz, G.W.; Cataliotti, A. Cardioprotective Effects of the Novel Compound Vastiras in a Preclinical Model of End-Organ Damage. Hypertension 2020, 75, 1195–1204. [Google Scholar] [CrossRef]
- Rose, R.; Giles, W.R. Natriuretic peptide C receptor signalling in the heart and vasculature. J. Physiol. 2008, 586, 353–366. [Google Scholar] [CrossRef]
- Phosphorylation of the Kinase Homology Domain is Essential for Activation of the A-Type Natriuretic Peptide Receptor-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/9528788/ (accessed on 24 May 2023).
- Potter, L.; Garbers, D. Dephosphorylation of the guanylyl cyclase—A receptor causes desensitization. J. Biol. Chem. 1992, 267, 14531–14534. [Google Scholar] [CrossRef]
- Potter, L.; Garbers, D. Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J. Biol. Chem. 1994, 269, 14636–14642. [Google Scholar] [CrossRef]
- Chang, C.-H.; Kohse, K.P.; Chang, B.; Hirata, M.; Jiang, B.; Douglas, J.E.; Murad, F. Characterization of ATP-stimulated guanylate cyclase activation in rat lung membranes. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1990, 1052, 159–165. [Google Scholar] [CrossRef]
- Larose, L.; McNicoll, N.; Ong, H.; De Lean, A. Allosteric modulation by ATP of the bovine adrenal natriuretic factor R1 receptor functions. Biochemistry 1991, 30, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- De Léan, A. Amiloride potentiates atrial natriuretic factor inhibitory action by increasing receptor binding in bovine adrenal zona glomerulosa. Life Sci. 1986, 39, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Goy, M.F.; Oliver, P.M.; Purdy, K.E.; Knowles, J.W.; Fox, J.E.; Mohler, P.J.; Qian, X.; Smithies, O.; Maeda, N. Evidence for a novel natriuretic peptide receptor that prefers brain natriuretic peptide over atrial natriuretic peptide. Biochem. J. 2001, 358, 379–387. [Google Scholar] [CrossRef]
- Kuhn, M.; Holtwick, R.; Baba, H.A.; Perriard, J.C.; Schmitz, W.; Ehler, E. Progressive cardiac hypertrophy and dysfunction in atrial natriuretic peptide receptor (GC-A) deficient mice. Heart 2002, 87, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Soma, M.; Takahashi, Y.; Rehemudula, D.; Kanmatsuse, K.; Furuya, K. Functional Deletion Mutation of the 5′-Flanking Region of Type A Human Natriuretic Peptide Receptor Gene and Its Association with Essential Hypertension and Left Ventricular Hypertrophy in the Japanese. Circ. Res. 2000, 86, 841–845. [Google Scholar] [CrossRef]
- Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous Nesiritide vs Nitroglycerin for Treatment of Decompensated Congestive Heart Failure: A randomized controlled trial. JAMA 2002, 287, 1531–1540. [Google Scholar] [CrossRef]
- Saito, Y. Roles of atrial natriuretic peptide and its therapeutic use. J. Cardiol. 2010, 56, 262–270. [Google Scholar] [CrossRef]
- Meems, L.M.; Andersen, I.A.; Pan, S.; Harty, G.; Chen, Y.; Zheng, Y.; Harders, G.E.; Ichiki, T.; Heublein, D.M.; Iyer, S.R.; et al. Design, Synthesis, and Actions of an Innovative Bispecific Designer Peptide. Hypertension 2019, 73, 900–909. [Google Scholar] [CrossRef]
- Gentry, P.R.; Sexton, P.M.; Christopoulos, A. Novel Allosteric Modulators of G Protein-coupled Receptors. J. Biol. Chem. 2015, 290, 19478–19488. [Google Scholar] [CrossRef]
- Ogawa, H.; Qiu, Y.; Ogata, C.M.; Misono, K.S. Crystal Structure of Hormone-bound Atrial Natriuretic Peptide Receptor Extracellular Domain: Rotation mechanism for transmembrane signal transduction. J. Biol. Chem. 2004, 279, 28625–28631. [Google Scholar] [CrossRef] [PubMed]
- Sangaralingham, S.J.; Whig, K.; Peddibhotla, S.; Kirby, R.J.; Sessions, H.E.; Maloney, P.R.; Hershberger, P.M.; Mose-Yates, H.; Hood, B.L.; Vasile, S.; et al. Discovery of small molecule guanylyl cyclase A receptor positive allosteric modulators. Proc. Natl. Acad. Sci. USA 2021, 118, e2109386118. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.; Singh, S.; Bellet, R.A.; Singh, G.; Tubb, D.; Chin, H.; Garbers, D.L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell 1989, 58, 1155–1162. [Google Scholar] [CrossRef]
- Potthast, R.; Abbey-Hosch, S.E.; Antos, L.K.; Marchant, J.; Kuhn, M.; Potter, L.R. Calcium-dependent Dephosphorylation Mediates the Hyperosmotic and Lysophosphatidic Acid-dependent Inhibition of Natriuretic Peptide Receptor-B/Guanylyl Cyclase-B. J. Biol. Chem. 2004, 279, 48513–48519. [Google Scholar] [CrossRef] [PubMed]
- Dickey, D.M.; Flora, D.R.; Bryan, P.M.; Xu, X.; Chen, Y.; Potter, L.R. Differential Regulation of Membrane Guanylyl Cyclases in Congestive Heart Failure: Natriuretic Peptide Receptor (NPR)-B, Not NPR-A, Is the Predominant Natriuretic Peptide Receptor in the Failing Heart. Endocrinology 2007, 148, 3518–3522. [Google Scholar] [CrossRef]
- Bryan, P.M.; Smirnov, D.; Smolenski, A.; Feil, S.; Feil, R.; Hofmann, F.; Lohmann, S.; Potter, L.R. A Sensitive Method for Determining the Phosphorylation Status of Natriuretic Peptide Receptors: cGK-Iα Does Not Regulate NPR-A. Biochemistry 2006, 45, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Doolittle, L.K.; Hammer, R.E.; Shelton, J.M.; Richardson, J.A.; Garbers, D.L. Critical roles of the guanylyl cyclase B receptor in endochondral ossification and development of female reproductive organs. Proc. Natl. Acad. Sci. USA 2004, 101, 17300–17305. [Google Scholar] [CrossRef]
- Tsuji, T.; Kunieda, T. A Loss-of-Function Mutation in Natriuretic Peptide Receptor 2 (Npr2) Gene Is Responsible for Disproportionate Dwarfism in cn/cn Mouse. J. Biol. Chem. 2005, 280, 14288–14292. [Google Scholar] [CrossRef]
- Bartels, C.F.; Bükülmez, H.; Padayatti, P.; Rhee, D.K.; van Ravenswaaij-Arts, C.; Pauli, R.M.; Mundlos, S.; Chitayat, D.; Shih, L.-Y.; Al-Gazali, L.I.; et al. Mutations in the Transmembrane Natriuretic Peptide Receptor NPR-B Impair Skeletal Growth and Cause Acromesomelic Dysplasia, Type Maroteaux. Am. J. Hum. Genet. 2004, 75, 27–34. [Google Scholar] [CrossRef]
- Ammarguellat, F.; Larouche, I.; Schiffrin, E.L. Myocardial Fibrosis in DOCA-Salt Hypertensive Rats: Effect of endothelin ET(A) receptor antagonism. Circulation 2001, 103, 319–324. [Google Scholar] [CrossRef]
- Koh, G.; Nussenzveig, D.; Okolicany, J.; Price, D.; Maack, T. Dynamics of atrial natriuretic factor-guanylate cyclase receptors and receptor-ligand complexes in cultured glomerular mesangial and renomedullary interstitial cells. J. Biol. Chem. 1992, 267, 11987–11994. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, P.; Thomas, G.; Sellin, K.; Bessette, M.-C.; Lafrenière, F.; Akhouayri, O.; St-Arnaud, R.; Lanctôt, C. Osteocrin Is a Specific Ligand of the Natriuretic Peptide Clearance Receptor That Modulates Bone Growth. J. Biol. Chem. 2007, 282, 36454–36462. [Google Scholar] [CrossRef] [PubMed]
- Leitman, D.C.; Andresen, J.W.; Kuno, T.; Kamisaki, Y.; Chang, J.K.; Murad, F. Identification of multiple binding sites for atrial natriuretic factor by affinity cross-linking in cultured endothelial cells. J. Biol. Chem. 1986, 261, 11650–11655. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, N.; Grzesik, W.J.; Takahashi, N.; Pandey, K.N.; Pang, S.; Yamauchi, M.; Smithies, O. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc. Natl. Acad. Sci. USA 1999, 96, 7403–7408. [Google Scholar] [CrossRef]
- Jaubert, J.; Jaubert, F.; Martin, N.; Washburn, L.L.; Lee, B.K.; Eicher, E.M.; Guénet, J.-L. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3). Proc. Natl. Acad. Sci. USA 1999, 96, 10278–10283. [Google Scholar] [CrossRef]
- Sangaralingham, S.J.; Kuhn, M.; Cannone, V.; Chen, H.H.; Burnett, J.C. Natriuretic peptide pathways in heart failure: Further therapeutic possibilities. Cardiovasc. Res. 2023, 118, 3416–3433. [Google Scholar] [CrossRef]
- John, S.W.M.; Krege, J.H.; Oliver, P.M.; Hagaman, J.R.; Hodgin, J.B.; Pang, S.C.; Flynn, T.G.; Smithies, O. Genetic Decreases in Atrial Natriuretic Peptide and Salt-Sensitive Hypertension. Science 1995, 267, 679–681. [Google Scholar] [CrossRef]
- Kishimoto, I.; Dubois, S.K.; Garbers, D.L. The heart communicates with the kidney exclusively through the guanylyl cyclase-A receptor: Acute handling of sodium and water in response to volume expansion. Proc. Natl. Acad. Sci. USA 1996, 93, 6215–6219. [Google Scholar] [CrossRef]
- Holtwick, R.; Gotthardt, M.; Skryabin, B.; Steinmetz, M.; Potthast, R.; Zetsche, B.; Hammer, R.E.; Herz, J.; Kuhn, M. Smooth muscle-selective deletion of guanylyl cyclase-A prevents the acute but not chronic effects of ANP on blood pressure. Proc. Natl. Acad. Sci. USA 2002, 99, 7142–7147. [Google Scholar] [CrossRef]
- Cataliotti, A.; Boerrigter, G.; Costello-Boerrigter, L.C.; Schirger, J.A.; Tsuruda, T.; Heublein, D.M.; Chen, H.H.; Malatino, L.S.; Burnett, J.C., Jr. Brain Natriuretic Peptide Enhances Renal Actions of Furosemide and Suppresses Furosemide-Induced Aldosterone Activation in Experimental Heart Failure. Circulation 2004, 109, 1680–1685. [Google Scholar] [CrossRef]
- Siragy, H.M.; Lamb, N.E.; Rose, C.E.; Peach, M.J.; Carey, R.M. Angiotensin II modulates the intrarenal effects of atrial natriuretic peptide. Am. J. Physiol.-Ren. Physiol. 1988, 255, F545–F551. [Google Scholar] [CrossRef] [PubMed]
- Belluardo, P.; Cataliotti, A.; Bonaiuto, L.; Giuffrè, E.; Maugeri, E.; Noto, P.; Orlando, G.; Raspa, G.; Piazza, B.; Babuin, L.; et al. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H1529–H1535. [Google Scholar] [CrossRef] [PubMed]
- Macheret, F.; Heublein, D.; Costello-Boerrigter, L.C.; Boerrigter, G.; McKie, P.; Bellavia, D.; Mangiafico, S.; Ikeda, Y.; Bailey, K.; Scott, C.G.; et al. Human Hypertension Is Characterized by a Lack of Activation of the Antihypertensive Cardiac Hormones ANP and BNP. J. Am. Coll. Cardiol. 2012, 60, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Wan, S.-H.; Iyer, S.R.; Cannone, V.; Sangaralingham, S.J.; Nuetel, J.; Burnett, J.C., Jr. First-in-Human Study of MANP: A Novel ANP (Atrial Natriuretic Peptide) Analog in Human Hypertension. Hypertension 2021, 78, 1859–1867. [Google Scholar] [CrossRef]
- Sabrane, K.; Kruse, M.N.; Fabritz, L.; Zetsche, B.; Mitko, D.; Skryabin, B.V.; Zwiener, M.; Baba, H.A.; Yanagisawa, M.; Kuhn, M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J. Clin. Investig. 2005, 115, 1666–1674. [Google Scholar] [CrossRef]
- Knowles, J.W.; Esposito, G.; Mao, L.; Hagaman, J.R.; Fox, J.E.; Smithies, O.; Rockman, H.A.; Maeda, N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A–deficient mice. J. Clin. Investig. 2001, 107, 975–984. [Google Scholar] [CrossRef]
- Patel, J.B.; Valencik, M.L.; Pritchett, A.M.; Burnett, J.J.C.; McDonald, J.A.; Redfield, M.M. Cardiac-specific attenuation of natriuretic peptide A receptor activity accentuates adverse cardiac remodeling and mortality in response to pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H777–H784. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef]
- Li, Y.; Kishimoto, I.; Saito, Y.; Harada, M.; Kuwahara, K.; Izumi, T.; Takahashi, N.; Kawakami, R.; Tanimoto, K.; Nakagawa, Y.; et al. Guanylyl Cyclase-A Inhibits Angiotensin II Type 1A Receptor-Mediated Cardiac Remodeling, an Endogenous Protective Mechanism in the Heart. Circulation 2002, 106, 1722–1728. [Google Scholar] [CrossRef]
- Kishimoto, I.; Rossi, K.; Garbers, D.L. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 2703–2706. [Google Scholar] [CrossRef]
- Holtwick, R.; van Eickels, M.; Skryabin, B.V.; Baba, H.A.; Bubikat, A.; Begrow, F.; Schneider, M.D.; Garbers, D.L.; Kuhn, M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J. Clin. Investig. 2003, 111, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gardner, D.G. Natriuretic Peptides Inhibit DNA Synthesis in Cardiac Fibroblasts. Hypertension 1995, 25, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Bigatti, G.; Evangelista, A.; Lanzani, C.; Stanzione, R.; Zagato, L.; Manunta, P.; Marchitti, S.; Venturelli, V.; Bianchi, G.; et al. Association of Atrial Natriuretic Peptide and Type A Natriuretic Peptide Receptor Gene Polymorphisms with Left Ventricular Mass in Human Essential Hypertension. J. Am. Coll. Cardiol. 2006, 48, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Ciavarella, G.M.; Venturelli, V.; De Paolis, P.; Tocci, G.; De Biase, L.; Ferrucci, A.; Volpe, M. Reduced levels of N-terminal-proatrial natriuretic peptide in hypertensive patients with metabolic syndrome and their relationship with left ventricular mass. J. Hypertens. 2007, 25, 833–839. [Google Scholar] [CrossRef]
- Cataliotti, A.; Malatino, L.S.; Jougasaki, M.; Zoccali, C.; Castellino, P.; Giacone, G.; Bellanuova, I.; Tripepi, R.; Seminara, G.; Parlongo, S.; et al. Circulating Natriuretic Peptide Concentrations in Patients with End-Stage Renal Disease: Role of Brain Natriuretic Peptide as a Biomarker for Ventricular Remodeling. Mayo Clin. Proc. 2001, 76, 1111–1119. [Google Scholar] [CrossRef]
- Zoccali, C.; Mallamaci, F.; Benedetto, F.A.; Tripepi, G.; Parlongo, S.; Cataliotti, A.; Cutrupi, S.; Giacone, G.; Bellanuova, I.; Cottini, E.; et al. Cardiac Natriuretic Peptides Are Related to Left Ventricular Mass and Function and Predict Mortality in Dialysis Patients. J. Am. Soc. Nephrol. 2001, 12, 1508–1515. [Google Scholar] [CrossRef]
- Cataliotti, A.; Tonne, J.M.; Bellavia, D.; Martin, F.L.; Oehler, E.A.; Harders, G.E.; Campbell, J.M.; Peng, K.-W.; Russell, S.J.; Malatino, L.S.; et al. Long-Term Cardiac pro-B-Type Natriuretic Peptide Gene Delivery Prevents the Development of Hypertensive Heart Disease in Spontaneously Hypertensive Rats. Circulation 2011, 123, 1297–1305. [Google Scholar] [CrossRef]
- Wang, Y.; De Waard, M.C.; Sterner-Kock, A.; Stepan, H.; Schultheiss, H.-P.; Duncker, D.J.; Walther, T. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur. J. Heart Fail. 2007, 9, 548–557. [Google Scholar] [CrossRef]
- Sarzani, R.; Allevi, M.; Di Pentima, C.; Schiavi, P.; Spannella, F.; Giulietti, F. Role of Cardiac Natriuretic Peptides in Heart Structure and Function. Int. J. Mol. Sci. 2022, 23, 14415. [Google Scholar] [CrossRef]
- Kuhn, M.; Völker, K.; Schwarz, K.; Carbajo-Lozoya, J.; Flögel, U.; Jacoby, C.; Stypmann, J.; van Eickels, M.; Gambaryan, S.; Hartmann, M.; et al. The natriuretic peptide/guanylyl cyclase–A system functions as a stress-responsive regulator of angiogenesis in mice. J. Clin. Investig. 2009, 119, 2019–2030. [Google Scholar] [CrossRef]
- Oliver, P.M.; Fox, J.E.; Kim, R.; Rockman, H.A.; Kim, H.-S.; Reddick, R.L.; Pandey, K.N.; Milgram, S.L.; Smithies, O.; Maeda, N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc. Natl. Acad. Sci. USA 1997, 94, 14730–14735. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.M.; John, S.W.M.; Purdy, K.E.; Kim, R.; Maeda, N.; Goy, M.F.; Smithies, O. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc. Natl. Acad. Sci. USA 1998, 95, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Clavell, A.L.; Stingo, A.J.; Wei, C.M.; Heublein, D.M.; Burnett, J.C. C-type natriuretic peptide: A selective cardiovascular peptide. Am. J. Physiol. 1993, 264 Pt 2, R290–R295. [Google Scholar] [CrossRef] [PubMed]
- Mahinrad, S.; Bulk, M.; van der Velpen, I.; Mahfouz, A.; van Roon-Mom, W.; Fedarko, N.; Yasar, S.; Sabayan, B.; van Heemst, D.; van der Weerd, L. Natriuretic Peptides in Post-mortem Brain Tissue and Cerebrospinal Fluid of Non-demented Humans and Alzheimer’s Disease Patients. Front. Neurosci. 2018, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R.; Frank, H.J. Natriuretic peptides inhibit rat astroglial proliferation: Mediation by C receptor. Am. J. Physiol. 1991, 261 Pt 2, R453–R457. [Google Scholar] [CrossRef]
- Langub, M.C.; Dolgas, C.M.; Watson, R.E.; Herman, J.P. The C-Type Natriuretic Peptide Receptor is the Predominant Natriuretic Peptide Receptor mRNA Expressed in Rat Hypothalamus. J. Neuroendocr. 1995, 7, 305–309. [Google Scholar] [CrossRef]
- Huang, W.; Lee, D.; Yang, Z.; Copolov, D.L.; Lim, A.T. Norepinephrine stimulates immunoreactive (ir) atrial natriuretic peptide (ANP) secretion and pro-ANP mRNA expression from rat hypothalamic neurons in culture: Effects of alpha 2-adrenoceptors. Endocrinology 1992, 130, 2426–2428. [Google Scholar] [CrossRef]
- Blackburn, R.E.; Samson, W.K.; Fulton, R.J.; Stricker, E.M.; Verbalis, J.G. Central oxytocin and ANP receptors mediate osmotic inhibition of salt appetite in rats. Am. J. Physiol. Integr. Comp. Physiol. 1995, 269, R245–R251. [Google Scholar] [CrossRef]
- Samson, W.K. Natriuretic peptides: A family of hormones. Trends Endocrinol. Metab. 1992, 3, 86–90. [Google Scholar] [CrossRef]
- Yang, R.H.; Jin, H.K.; Wyss, J.M.; Chen, Y.F.; Oparil, S. Pressor effect of blocking atrial natriuretic peptide in nucleus tractus solitarii. Hypertension 1992, 19, 198–205. [Google Scholar] [CrossRef]
- Marin-Grez, M.; Fleming, J.T.; Steinhausen, M. Atrial natriuretic peptide causes pre-glomerular vasodilatation and post-glomerular vasoconstriction in rat kidney. Nature 1986, 324, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Stockand, J.D.; Sansom, S.C. Regulation of filtration rate by glomerular mesangial cells in health and diabetic renal disease. Am. J. Kidney Dis. 1997, 29, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.J.; Thomas, D.; Morgan, T.O. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 1987, 326, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Dillingham, M.A.; Anderson, R.J. Inhibition of Vasopressin Action by Atrial Natriuretic Factor. Science 1986, 231, 1572–1573. [Google Scholar] [CrossRef]
- Zeidel, M.L. Regulation of collecting duct Na+ reabsorption by ANP 31–67. Clin. Exp. Pharmacol. Physiol. 1995, 22, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Wijeyaratne, C.N.; Moult, P.J. The effect of alpha human atrial natriuretic peptide on plasma volume and vascular permeability in normotensive subjects. J. Clin. Endocrinol. Metab. 1993, 76, 343–346. [Google Scholar] [CrossRef]
- Cataliotti, A.; Giordano, M.; De Pascale, E.; Giordano, G.; Castellino, P.; Jougasaki, M.; Costello, L.C.; Boerrigter, G.; Tsuruda, T.; Belluardo, P.; et al. CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome. Am. J. Physiol.-Ren. Physiol. 2002, 283, F464–F472. [Google Scholar] [CrossRef]
- Goetz, K.L.; Wang, B.C.; Geer, P.G.; Leadley, R.J.; Reinhardt, H.W. Atrial stretch increases sodium excretion independently of release of atrial peptides. Am. J. Physiol. 1986, 250 Pt 2, R946–R950. [Google Scholar] [CrossRef]
- Leppäluoto, J.; Ruskoaho, H. Atrial natriuretic peptide, renin activity, aldosterone, urine volume and electrolytes during a 24-h sleep-wake cycle in man. Acta Physiol. Scand. 1990, 139, 47–53. [Google Scholar] [CrossRef]
- Saxenhofer, H.; Raselli, A.; Weidmann, P.; Forssmann, W.G.; Bub, A.; Ferrari, P.; Shaw, S.G. Urodilatin, a natriuretic factor from kidneys, can modify renal and cardiovascular function in men. Am. J. Physiol. 1990, 259 Pt 2, F832–F838. [Google Scholar] [CrossRef]
- Drummer, C.; Franck, W.; Heer, M.; Forssmann, W.G.; Gerzer, R.; Goetz, K. Postprandial natriuresis in humans: Further evidence that urodilatin, not ANP, modulates sodium excretion. Am. J. Physiol.-Ren. Physiol. 1996, 270, F301–F310. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Sano, T.; Kase, H.; Yamada, K.; Inagami, T.; Matsuda, Y. HS-142-1, a novel nonpeptide atrial natriuretic peptide (ANP) antagonist, blocks ANP-induced renal responses through a specific interaction with guanylyl cyclase-linked receptors. Eur. J. Pharmacol. Mol. Pharmacol. 1992, 225, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Wada, A.; Tsutamoto, T.; Matsuda, Y.; Kinoshita, M. Cardiorenal and neurohumoral effects of endogenous atrial natriuretic peptide in dogs with severe congestive heart failure using a specific antagonist for guanylate cyclase-coupled receptors. Circulation 1994, 89, 2232–2240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.L.; Mackenzie, H.S.; Troy, J.L.; Brenner, B.M. Effects of an atrial natriuretic peptide receptor antagonist on glomerular hyperfiltration in diabetic rats. J. Am. Soc. Nephrol. 1994, 4, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, A.; Filvaroff, E.H.; Gitelman, S.E.; Derynck, R. Toward a molecular understanding of skeletal development. Cell 1995, 80, 371–378. [Google Scholar] [CrossRef]
- Chung, U.-I.; Kronenberg, H.M. Parathyroid hormone-related peptide and Indian hedgehog. Curr. Opin. Nephrol. Hypertens. 2000, 9, 357–362. [Google Scholar] [CrossRef]
- Vortkamp, A.; Lee, K.; Lanske, B.; Segre, G.V.; Kronenberg, H.M.; Tabin, C.J. Regulation of Rate of Cartilage Differentiation by Indian Hedgehog and PTH-Related Protein. Science 1996, 273, 613–622. [Google Scholar] [CrossRef]
- Minina, E.; Wenzel, H.M.; Kreschel, C.; Karp, S.; Gaffield, W.; McMahon, A.P.; Vortkamp, A. BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation. Development 2001, 128, 4523–4534. [Google Scholar] [CrossRef]
- Karp, S.J.; Schipani, E.; St-Jacques, B.; Hunzelman, J.; Kronenberg, H.; McMahon, A.P. Indian Hedgehog coordinates endochondral bone growth and morphogenesis via Parathyroid Hormone related-Protein-dependent and -independent pathways. Development 2000, 127, 543–548. [Google Scholar] [CrossRef]
- Pines, M.; Hurwitz, S. The Effect of Parathyroid Hormone and Atrial Natriuretic Peptide on Cyclic Nucleotides Production and Proliferation of Avian Epiphyseal Growth Plate Chondroprogenitor Cells. Endocrinology 1988, 123, 360–365. [Google Scholar] [CrossRef]
- Hagiwara, H.; Sakaguchi, H.; Itakura, M.; Yoshimoto, T.; Furuya, M.; Tanaka, S.; Hirose, S. Autocrine regulation of rat chondrocyte proliferation by natriuretic peptide C and its receptor, natriuretic peptide receptor-B. J. Biol. Chem. 1994, 269, 10729–10733. [Google Scholar] [CrossRef] [PubMed]
- Suda, M.; Ogawa, Y.; Tanaka, K.; Tamura, N.; Yasoda, A.; Takigawa, T.; Uehira, M.; Nishimoto, H.; Itoh, H.; Saito, Y.; et al. Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 1998, 95, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Chusho, H.; Tamura, N.; Ogawa, Y.; Yasoda, A.; Suda, M.; Miyazawa, T.; Nakamura, K.; Nakao, K.; Kurihara, T.; Komatsu, Y.; et al. Dwarfism and early death in mice lacking C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 2001, 98, 4016–4021. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Matsuda, M.; Yamada, Y.; Kawai, K.; Suzuki, E.; Makishima, M.; Kitamura, T.; Shimomura, I. Musclin, a Novel Skeletal Muscle-derived Secretory Factor. J. Biol. Chem. 2004, 279, 19391–19395. [Google Scholar] [CrossRef]
- Thomas, G.; Moffatt, P.; Salois, P.; Gaumond, M.-H.; Gingras, R.; Godin, E.; Miao, D.; Goltzman, D.; Lanctôt, C. Osteocrin, a Novel Bone-specific Secreted Protein That Modulates the Osteoblast Phenotype. J. Biol. Chem. 2003, 278, 50563–50571. [Google Scholar] [CrossRef]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.K.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef]
- Szaroszyk, M.; Kattih, B.; Martin-Garrido, A.; Trogisch, F.A.; Dittrich, G.M.; Grund, A.; Abouissa, A.; Derlin, K.; Meier, M.; Holler, T.; et al. Skeletal muscle derived Musclin protects the heart during pathological overload. Nat. Commun. 2022, 13, 149. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Ni, W.; Yuan, X.; Zhang, H.; Li, P.; Xu, J.; Zhao, Z. Sarcopenia in heart failure: A systematic review and meta-analysis. ESC Heart Fail. 2021, 8, 1007–1017. [Google Scholar] [CrossRef]
- Takei, Y. Does the natriuretic peptide system exist throughout the animal and plant kingdom? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 559–573. [Google Scholar] [CrossRef]
- Inoue, K.; Sakamoto, T.; Yuge, S.; Iwatani, H.; Yamagami, S.; Tsutsumi, M.; Hori, H.; Cerra, M.C.; Tota, B.; Suzuki, N.; et al. Structural and Functional Evolution of Three Cardiac Natriuretic Peptides. Mol. Biol. Evol. 2005, 22, 2428–2434. [Google Scholar] [CrossRef]
- Potter, L.R. Guanylyl cyclase structure, function and regulation. Cell. Signal. 2011, 23, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Kengne, F.G.; Andres, C.; Sattar, L.; Melot, C.; Decaux, G. Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM Int. J. Med. 2008, 101, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Renneboog, B.; Musch, W.; Vandemergel, X.; Manto, M.U.; Decaux, G. Mild Chronic Hyponatremia Is Associated with Falls, Unsteadiness, and Attention Deficits. Am. J. Med. 2006, 119, 71.e1–71.e8. [Google Scholar] [CrossRef]
- Fujisawa, H.; Sugimura, Y.; Takagi, H.; Mizoguchi, H.; Takeuchi, H.; Izumida, H.; Nakashima, K.; Ochiai, H.; Takeuchi, S.; Kiyota, A.; et al. Chronic Hyponatremia Causes Neurologic and Psychologic Impairments. J. Am. Soc. Nephrol. 2016, 27, 766–780. [Google Scholar] [CrossRef]
- Barsony, J.; Sugimura, Y.; Verbalis, J.G. Osteoclast Response to Low Extracellular Sodium and the Mechanism of Hyponatremia-induced Bone Loss. J. Biol. Chem. 2011, 286, 10864–10875. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Corte, V.; Pacinella, G.; Todaro, F.; Pecoraro, R.; Tuttolomondo, A. The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects. Int. J. Mol. Sci. 2023, 24, 9642. https://doi.org/10.3390/ijms24119642
Della Corte V, Pacinella G, Todaro F, Pecoraro R, Tuttolomondo A. The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects. International Journal of Molecular Sciences. 2023; 24(11):9642. https://doi.org/10.3390/ijms24119642
Chicago/Turabian StyleDella Corte, Vittoriano, Gaetano Pacinella, Federica Todaro, Rosaria Pecoraro, and Antonino Tuttolomondo. 2023. "The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects" International Journal of Molecular Sciences 24, no. 11: 9642. https://doi.org/10.3390/ijms24119642
APA StyleDella Corte, V., Pacinella, G., Todaro, F., Pecoraro, R., & Tuttolomondo, A. (2023). The Natriuretic Peptide System: A Single Entity, Pleiotropic Effects. International Journal of Molecular Sciences, 24(11), 9642. https://doi.org/10.3390/ijms24119642






