Levilactobacillus brevis with High Production of Putrescine Isolated from Blue Cheese and Its Application
Abstract
1. Introduction
2. Results
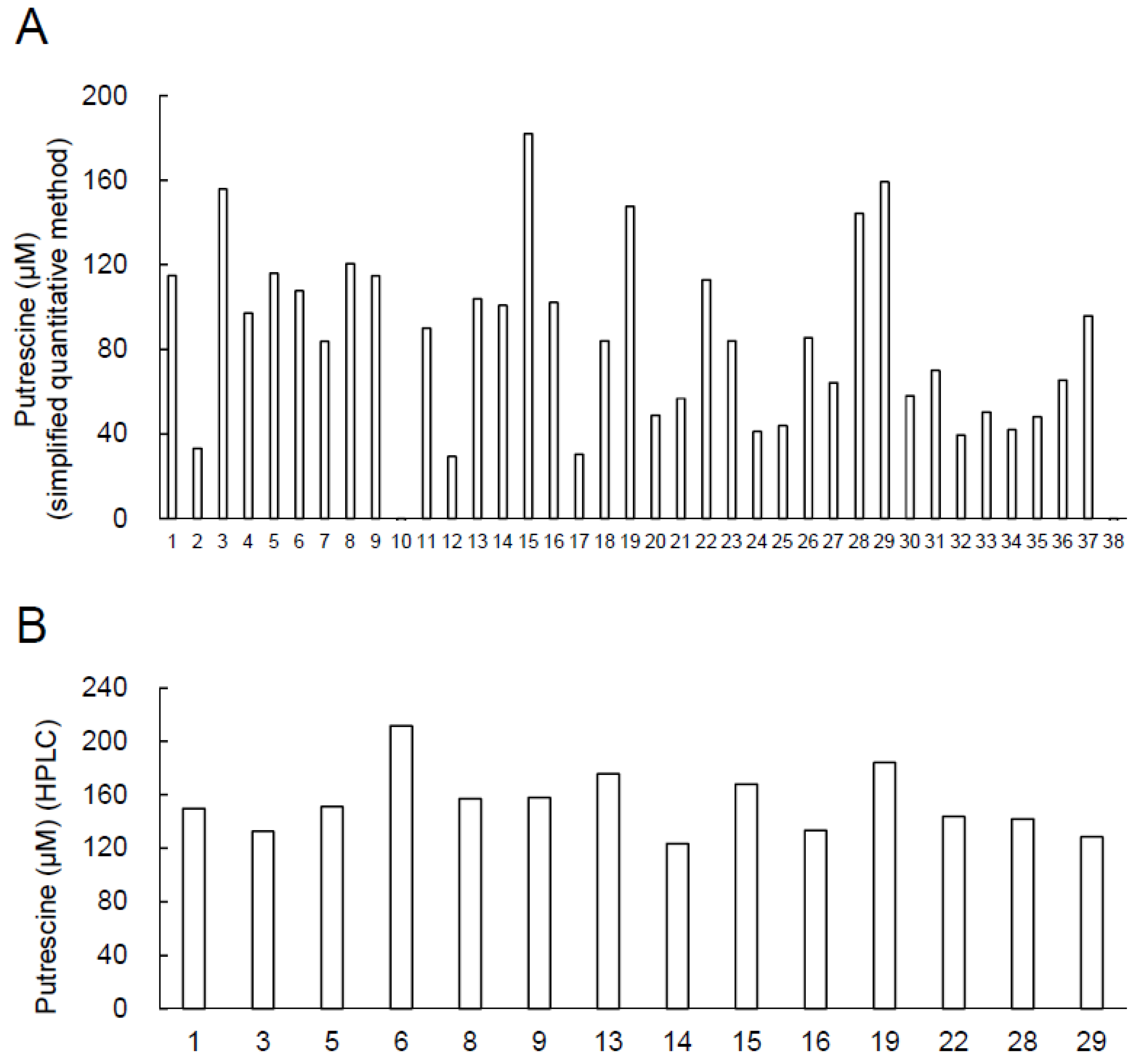
2.1. Screening of High-Putrescine-Producing Bacteria from Blue Cheese
2.2. Putrescine Concentration of Culture Supernatants of Candidate of the High-Putrescine-Producing Bacterial Strains
2.3. Estimation of Bacterial Species by 16S rDNA Sequencing
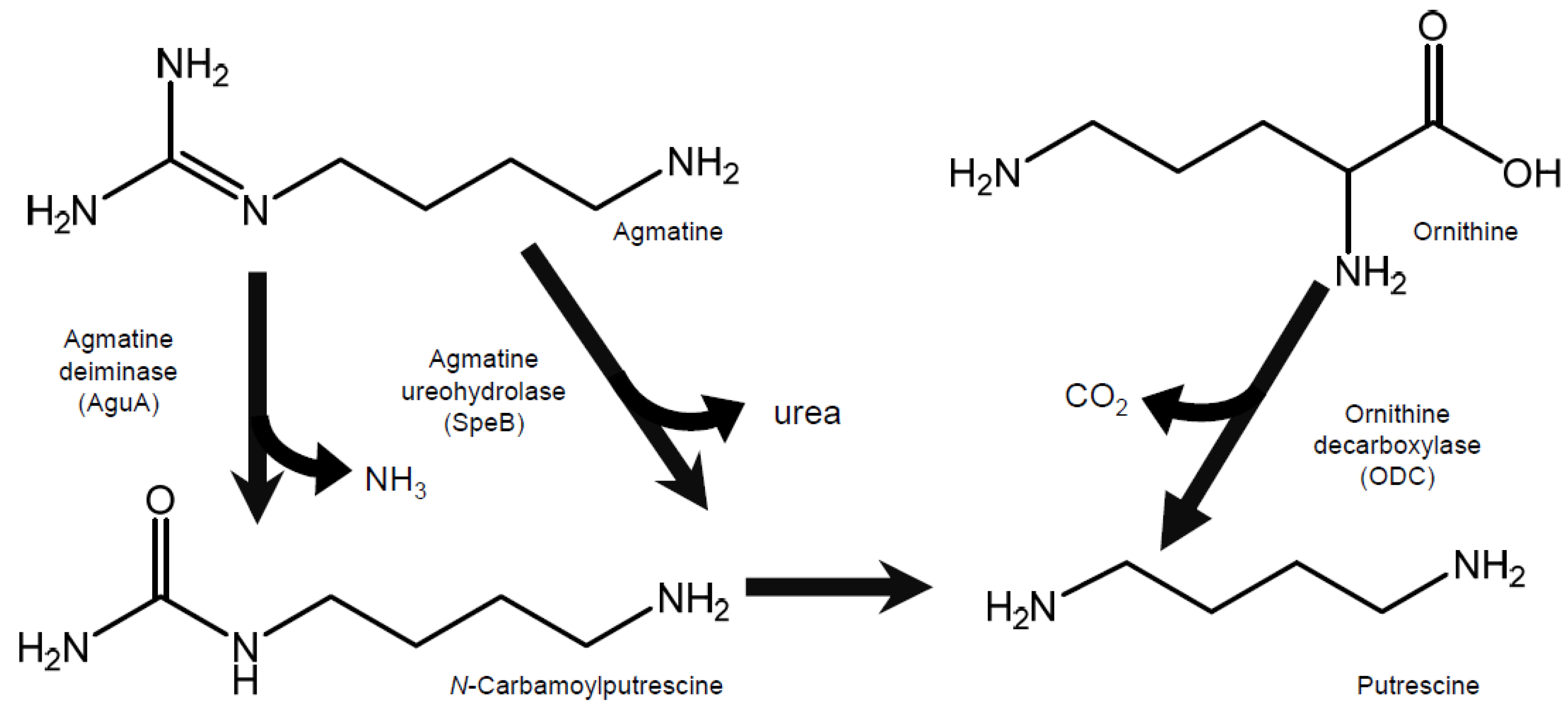
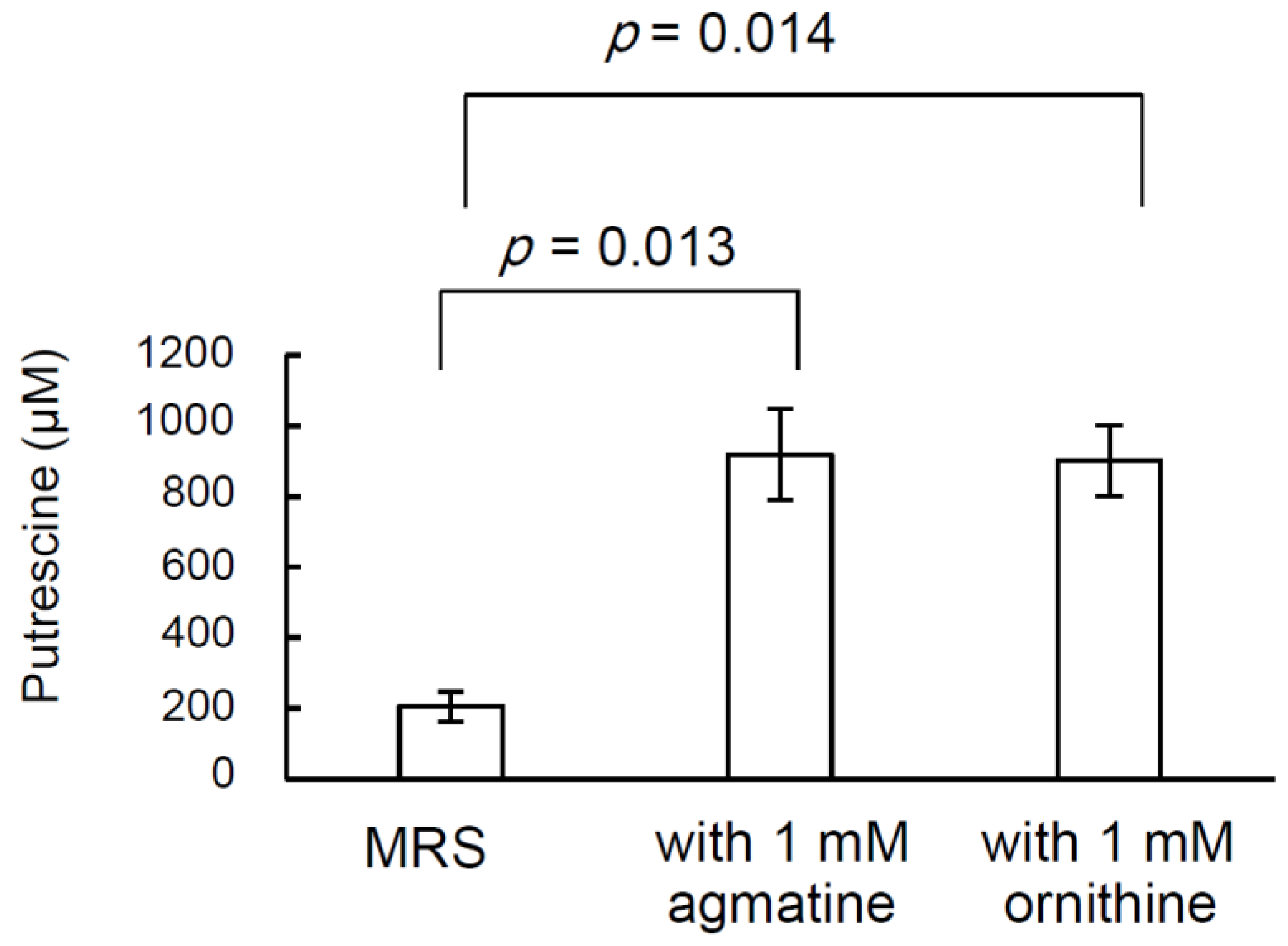
2.4. Effect of the Addition of Putrescine Precursors to the Medium on the Putrescine Productivity of L. brevis FB215
2.5. Determination of Ornithine and Agmatine Concentrations in Sakekasu Extract
2.6. Cultivation of L. brevis FB215 in Sakekasu Extract
3. Discussion
4. Materials and Methods
4.1. Isolation of Polyamine-High-Producing Bacteria from the Blue Cheese
4.2. High-Throughput Determination of Putrescine Concentration in Culture Supernatants of the Blue Cheese-Derived Bacteria
4.3. Determination of Polyamines by HPLC
4.4. Analysis of 16S rDNA
4.5. Gram Staining
4.6. Culture in MRS Medium Supplemented with Putrescine Precursor
4.7. Agmatine and Ornithine Concentration in Sakekasu Extract
4.8. Cultivation of Bacteria in Sakekasu Extract
4.9. Determination of Production of Amines by L. brevis FB215 in Sakekasu Extract
4.10. Measurement of pH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Igarashi, K.; Kashiwagi, K. Polyamines: Mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000, 271, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Chiba, F.; Koizumi, K.; Miyaki, Y. Increased polyamine intake inhibits age-associated alteration in global DNA methylation and 1,2-dimethylhydrazine-induced tumorigenesis. PLoS ONE 2013, 8, e64357. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, H.; Odoko, M.; Grzeskowiak, K.; Hiyama, Y.; Tsukamoto, K.; Maezaki, N.; Ishida, T.; Tanaka, T.; Okabe, N.; Fukuyama, K.; et al. Polyamines stabilize left-handed Z-DNA: Using X-ray crystallographic analysis, we have found a new type of polyamine (PA) that stabilizes left-handed Z-DNA. Biochem. Biophys. Res. Commun. 2008, 366, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kashiwagi, K.; Shigemasa, A.; Taniguchi, S.; Yamamoto, K.; Makinoshima, H.; Ishihama, A.; Igarashi, K. A unifying model for the role of polyamines in bacterial cell growth, the polyamine modulon. J. Biol. Chem. 2004, 279, 46008–46013. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Perry, J.W. Spermidine as a possible mediator of glucocorticoid effect on milk protein synthesis in mouse mammary epithelium in vitro. J. Biol. Chem. 1974, 249, 7647–7652. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef]
- Matsumoto, M.; Kibe, R.; Ooga, T.; Aiba, Y.; Kurihara, S.; Sawaki, E.; Koga, Y.; Benno, Y. Impact of intestinal microbiota on intestinal luminal metabolome. Sci. Rep. 2012, 2, 233. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Nara, M.; Sakanaka, M.; Gotoh, A.; Kitakata, A.; Okuda, S.; Kurihara, S. Comprehensive analysis of polyamine transport and biosynthesis in the dominant human gut bacteria: Potential presence of novel polyamine metabolism and transport genes. Int. J. Biochem. Cell Biol. 2017, 93, 52–61. [Google Scholar] [CrossRef]
- Scalabrino, G.; Ferioli, M.E. Polyamines in mammalian ageing: An oncological problem, too? A review. Mech. Ageing Dev. 1984, 26, 149–164. [Google Scholar] [CrossRef]
- Das, R.; Kanungo, M.S. Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp. Gerontol. 1982, 17, 95–103. [Google Scholar] [CrossRef]
- Beyer, H.S.; Ellefson, M.; Sherman, R.; Zieve, L. Aging alters ornithine decarboxylase and decreases polyamines in regenerating rat liver but putrescine replacement has no effect. J. Lab. Clin. Med. 1992, 119, 38–47. [Google Scholar]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Yamamoto, T.; Hinoi, E.; Fujita, H.; Iezaki, T.; Takahata, Y.; Takamori, M.; Yoneda, Y. The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br. J. Pharmacol. 2012, 166, 1084–1096. [Google Scholar] [CrossRef]
- Wirth, M.; Benson, G.; Schwarz, C.; Kobe, T.; Grittner, U.; Schmitz, D.; Sigrist, S.J.; Bohlken, J.; Stekovic, S.; Madeo, F.; et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 2018, 109, 181–188. [Google Scholar] [CrossRef]
- Zoumas-Morse, C.; Rock, C.L.; Quintana, E.L.; Neuhouser, M.L.; Gerner, E.W.; Meyskens, F.L., Jr. Development of a polyamine database for assessing dietary intake. J. Am. Diet. Assoc. 2007, 107, 1024–1027. [Google Scholar] [CrossRef]
- Buyukuslu, N.; Hizli, H.; Esin, K.; Garipagaoglu, M. A Cross-Sectional Study: Nutritional Polyamines in Frequently Consumed Foods of the Turkish Population. Foods 2014, 3, 541–557. [Google Scholar] [CrossRef]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2007, 100, 491–497. [Google Scholar] [CrossRef]
- Ali, M.A.; Poortvliet, E.; Stromberg, R.; Yngve, A. Polyamines: Total daily intake in adolescents compared to the intake estimated from the Swedish Nutrition Recommendations Objectified (SNO). Food Nutr. Res. 2011, 55, 5455. [Google Scholar] [CrossRef]
- Okamoto, A.; Sugi, E.; Koizumi, Y.; Yanagida, F.; Udaka, S. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci. Biotechnol. Biochem. 1997, 61, 1582–1584. [Google Scholar] [CrossRef]
- Hirano, R.; Kume, A.; Nishiyama, C.; Honda, R.; Shirasawa, H.; Ling, Y.; Sugiyama, Y.; Nara, M.; Shimokawa, H.; Kawada, H.; et al. Putrescine Production by Latilactobacillus curvatus KP 3-4 Isolated from Fermented Foods. Microorganisms 2022, 10, 697. [Google Scholar] [CrossRef]
- Khattab, A.R.; Guirguis, H.A.; Tawfik, S.M.; Farag, M.A. Cheese ripening: A review on modern technologies towards flavor enhancement, process acceleration and improved quality assessment. Trends Food Sci. Technol. 2019, 88, 343–360. [Google Scholar] [CrossRef]
- Novella-Rodriguez, S.; Veciana-Nogues, M.T.; Izquierdo-Pulido, M.; Vidal-Carou, M.C. Distribution of biogenic amines and polyamines in cheese. J. Food Sci. 2003, 68, 750–755. [Google Scholar] [CrossRef]
- Vardjan, T.; Lorbeg, P.M.; Rogelj, I.; Majhenic, A.C. Characterization and stability of lactobacilli and yeast microbiota in kefir grains. J. Dairy Sci. 2013, 96, 2729–2736. [Google Scholar] [CrossRef] [PubMed]
- Takii, Y.; Nishimura, S.; Yoshida-Yamamoto, S.; Kobayashi, Y.; Nagayoshi, E. Effects of intake of pickles containing Lactobacillus brevis on immune activity and bowel symptoms in female students. J. Nutr. Sci. Vitaminol. 2013, 59, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Du, R.P.; Yu, L.S.; Yu, N.X.; Ping, W.X.; Song, G.; Ge, J.P. Characterization of exopolysaccharide produced by Levilactobacillus brevis HDE-9 and evaluation of its potential use in dairy products. Int. J. Biol. Macromol. 2022, 217, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Shah, N.P. High gamma-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2017, 57, 3661–3672. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.M.; Blancato, V.S.; Claisse, O.; Magni, C.; Lolkema, J.S.; Lonvaud-Funel, A. Agmatine deiminase pathway genes in Lactobacillus brevis are linked to the tyrosine decarboxylation operon in a putative acid resistance locus. Microbiology 2007, 153 Pt 7, 2221–2230. [Google Scholar] [CrossRef]
- Tsuji, A.; Kozawa, M.; Tokuda, K.; Enomoto, T.; Koyanagi, T. Robust Domination of Lactobacillus sakei in Microbiota during Traditional Japanese Sake Starter Yamahai-Moto Fermentation and the Accompanying Changes in Metabolites. Curr. Microbiol. 2018, 75, 1498–1505. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Ohta, H.; Hirano, R.; Shimokawa, H.; Sakanaka, M.; Koyanagi, T.; Kurihara, S. Development of a new chromogenic method for putrescine quantification using coupling reactions involving putrescine oxidase. Anal. Biochem. 2020, 593, 113607. [Google Scholar] [CrossRef]
- Tabor, C.W.; Tabor, H. Polyamines in microorganisms. Microbiol. Rev. 1985, 49, 81–99. [Google Scholar] [CrossRef]
- Shirasawa, H.; Nishiyama, C.; Hirano, R.; Koyanagi, T.; Okuda, S.; Takagi, H.; Kurihara, S. Isolation of the high polyamine-producing bacterium Staphylococcus epidermidis FB146 from fermented foods and identification of polyamine-related genes. Biosci. Microbiota Food Health 2023, 42, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kibe, R.; Kurihara, S.; Sakai, Y.; Suzuki, H.; Ooga, T.; Sawaki, E.; Muramatsu, K.; Nakamura, A.; Yamashita, A.; Kitada, Y.; et al. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014, 4, 4548. [Google Scholar] [CrossRef] [PubMed]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef]
- Allen, D.G., Jr.; Green, D.P.; Bolton, G.E.; Jaykus, L.A.; Cope, W.G. Detection and identification of histamine-producing bacteria associated with harvesting and processing mahimahi and yellowfin tuna. J. Food Prot. 2005, 68, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef]
- Ralph, A.; Englyst, K.; Bardócz, S. Polyamine Content of the Human Diet; Kluwer Academic Publishers: London, UK, 1999; pp. 123–137. [Google Scholar]
- Sugiyama, Y.; Mori, Y.; Nara, M.; Kotani, Y.; Nagai, E.; Kawada, H.; Kitamura, M.; Hirano, R.; Shimokawa, H.; Nakagawa, A.; et al. Gut bacterial aromatic amine production: Aromatic amino acid decarboxylase and its effects on peripheral serotonin production. Gut Microbes 2022, 14, 2128605. [Google Scholar] [CrossRef]

| Animals | Low Polyamine Group (μg/g Body Weight/Day) | High Polyamine Group (μg/g Body Weight/Day) | Administration Method | Literature |
|---|---|---|---|---|
| Mouse | 19.66 a | 73.58 a | diet | [14] |
| Mouse | 10.44 b | 104.40 b | ad libitum drinking water | [15] |
| Mouse | 11.23 b | 112.34 b | ad libitum drinking water | [16] |
| Human | 0.48 c | 0.50 c | supplement | [17] |
| Human | 0.48 d | 0.93 d | fermented soy bean (Natto) | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ami, Y.; Kodama, N.; Umeda, M.; Nakamura, H.; Shirasawa, H.; Koyanagi, T.; Kurihara, S. Levilactobacillus brevis with High Production of Putrescine Isolated from Blue Cheese and Its Application. Int. J. Mol. Sci. 2023, 24, 9668. https://doi.org/10.3390/ijms24119668
Ami Y, Kodama N, Umeda M, Nakamura H, Shirasawa H, Koyanagi T, Kurihara S. Levilactobacillus brevis with High Production of Putrescine Isolated from Blue Cheese and Its Application. International Journal of Molecular Sciences. 2023; 24(11):9668. https://doi.org/10.3390/ijms24119668
Chicago/Turabian StyleAmi, Yuta, Narumi Kodama, Masahiro Umeda, Hanae Nakamura, Hideto Shirasawa, Takashi Koyanagi, and Shin Kurihara. 2023. "Levilactobacillus brevis with High Production of Putrescine Isolated from Blue Cheese and Its Application" International Journal of Molecular Sciences 24, no. 11: 9668. https://doi.org/10.3390/ijms24119668
APA StyleAmi, Y., Kodama, N., Umeda, M., Nakamura, H., Shirasawa, H., Koyanagi, T., & Kurihara, S. (2023). Levilactobacillus brevis with High Production of Putrescine Isolated from Blue Cheese and Its Application. International Journal of Molecular Sciences, 24(11), 9668. https://doi.org/10.3390/ijms24119668




