Mechanisms of Resistance to Antibody-Drug Conjugates
Abstract
:1. Introduction
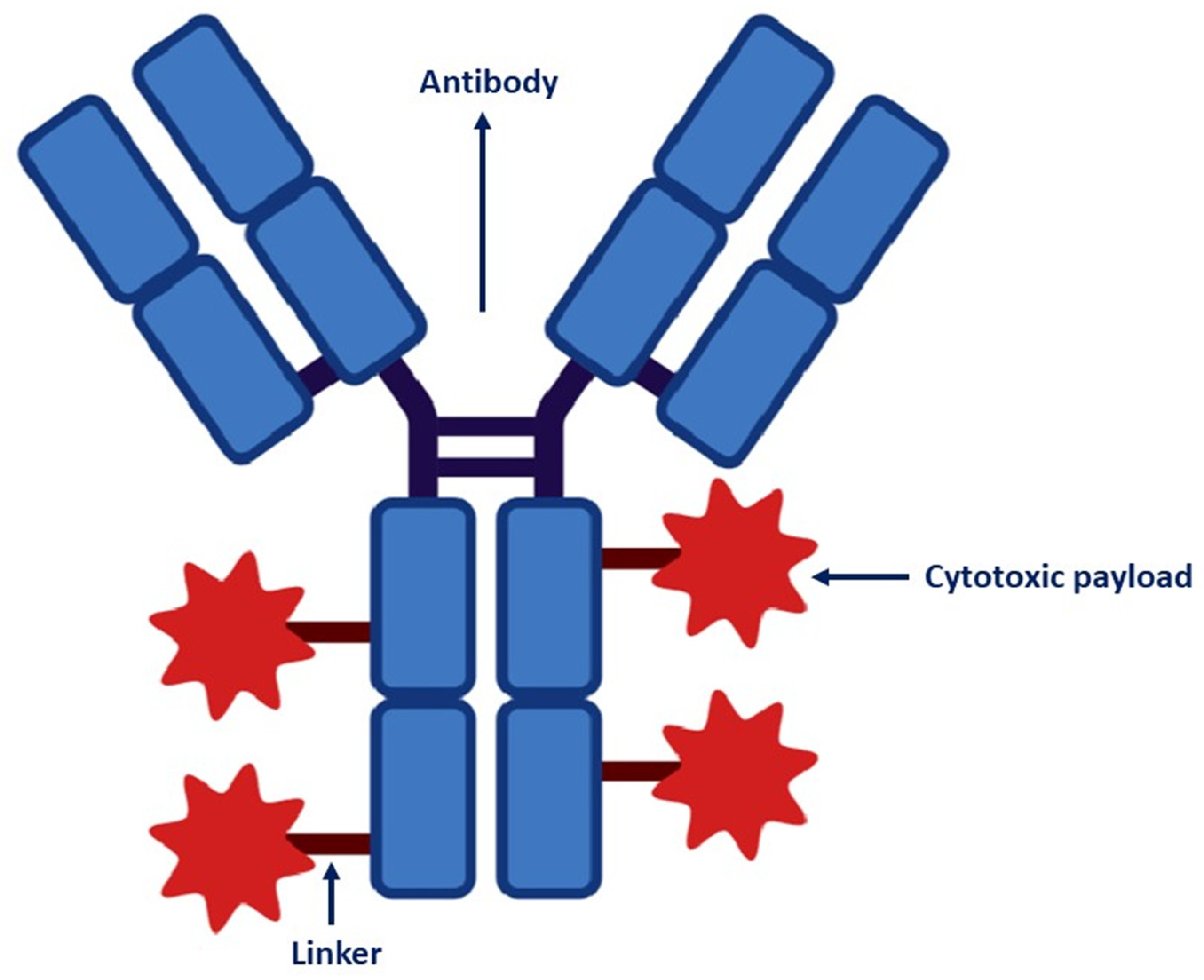
2. ADC Structure
2.1. Target-Antigen Selection and Antibody
2.2. Cytotoxic Payloads
2.3. Linkers
3. Selected ADCs in Solid Tumors: Mechanism of Action
3.1. Trastuzumab Emtansine
3.2. Trastuzumab Deruxtecan
3.3. Sacituzumab Govitecan
3.4. Enfortumab Vedotin
4. Mechanisms of Resistance to ADC
4.1. Antigen-Related Resistance
4.2. Payload-Related Resistance
4.3. Failure in Internalization and Trafficking Pathways
4.4. Impaired Lysosomal Function
4.5. Drug-Efflux Pumps
4.6. Role of Cell Cycle
4.7. Activation of Signaling Pathways
4.8. Apoptotic Dysregulation
5. Overcoming Resistance to ADCs
6. Conclusions and Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Diamantis, N.; Banerji, U. Antibody-Drug Conjugates—An Emerging Class of Cancer Treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef]
- Coiffier, B.; Lepage, E.; Briere, J.; Herbrecht, R.; Tilly, H.; Bouabdallah, R.; Morel, P.; Van Den Neste, E.; Salles, G.; Gaulard, P.; et al. CHOP Chemotherapy plus Rituximab Compared with CHOP Alone in Elderly Patients with Diffuse Large-B-Cell Lymphoma. N. Engl. J. Med. 2002, 346, 235–242. [Google Scholar] [CrossRef]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Marei, H.E.; Cenciarelli, C.; Hasan, A. Potential of Antibody-Drug Conjugates (ADCs) for Cancer Therapy. Cancer Cell Int. 2022, 22, 255. [Google Scholar] [CrossRef]
- Strebhardt, K.; Ullrich, A. Paul Ehrlich’s Magic Bullet Concept: 100 Years of Progress. Nat. Rev. Cancer 2008, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.M.; Coumbe, B.G.T.; Josephs, D.H.; Mele, S.; Ilieva, K.M.; Cheung, A.; Tutt, A.N.; Spicer, J.F.; Thurston, D.E.; Crescioli, S.; et al. Antibody Structure and Engineering Considerations for the Design and Function of Antibody Drug Conjugates (ADCs). Oncoimmunology 2018, 7, e1395127. [Google Scholar] [CrossRef] [PubMed]
- Rowland, G.F.; O’Neill, G.J.; Davies, D.A. Suppression of Tumour Growth in Mice by a Drug-Antibody Conjugate Using a Novel Approach to Linkage. Nature 1975, 255, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Criscitiello, C.; Morganti, S.; Curigliano, G. Antibody-Drug Conjugates in Solid Tumors: A Look into Novel Targets. J. Hematol. Oncol. 2021, 14, 20. [Google Scholar] [CrossRef]
- Trail, P.A.; Willner, D.; Lasch, S.J.; Henderson, A.J.; Hofstead, S.; Casazza, A.M.; Firestone, R.A.; Hellström, I.; Hellström, K.E. Cure of Xenografted Human Carcinomas by BR96-Doxorubicin Immunoconjugates. Science 1993, 261, 212–215. [Google Scholar] [CrossRef]
- Tolcher, A.W. Antibody Drug Conjugates: Lessons from 20 Years of Clinical Experience. Ann. Oncol. 2016, 27, 2168–2172. [Google Scholar] [CrossRef]
- Chau, C.H.; Steeg, P.S.; Figg, W.D. Antibody-Drug Conjugates for Cancer. Lancet 2019, 394, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody Drug Conjugate: The “Biological Missile” for Targeted Cancer Therapy. Signal Transduct. Target. Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Damelin, M.; Zhong, W.; Myers, J.; Sapra, P. Evolving Strategies for Target Selection for Antibody-Drug Conjugates. Pharm. Res. 2015, 32, 3494–3507. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, M.; Galbraith, D.N.; McDermott, L.L. What Makes a Good Antibody-Drug Conjugate? Expert Opin. Biol. Ther. 2021, 21, 841–847. [Google Scholar] [CrossRef]
- Carter, P.J. Potent Antibody Therapeutics by Design. Nat. Rev. Immunol. 2006, 6, 343–357. [Google Scholar] [CrossRef]
- Saber, H.; Leighton, J.K. An FDA Oncology Analysis of Antibody-Drug Conjugates. Regul. Toxicol. Pharmacol. 2015, 71, 444–452. [Google Scholar] [CrossRef]
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current ADC Linker Chemistry. Pharm. Res. 2015, 32, 3526–3540. [Google Scholar] [CrossRef] [Green Version]
- Drago, J.Z.; Modi, S.; Chandarlapaty, S. Unlocking the Potential of Antibody-Drug Conjugates for Cancer Therapy. Nat. Rev. Clin. Oncol. 2021, 18, 327–344. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, F.; Lu, A.; Zhang, G. Linkers Having a Crucial Role in Antibody-Drug Conjugates. Int. J. Mol. Sci. 2016, 17, 561. [Google Scholar] [CrossRef]
- Sun, X.; Ponte, J.F.; Yoder, N.C.; Laleau, R.; Coccia, J.; Lanieri, L.; Qiu, Q.; Wu, R.; Hong, E.; Bogalhas, M.; et al. Effects of Drug-Antibody Ratio on Pharmacokinetics, Biodistribution, Efficacy, and Tolerability of Antibody-Maytansinoid Conjugates. Bioconjug. Chem. 2017, 28, 1371–1381. [Google Scholar] [CrossRef]
- Abelman, R.O.; Wu, B.; Spring, L.M.; Ellisen, L.W.; Bardia, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Cancers 2023, 15, 1278. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Hee Park, Y.; Kim, S.-B.; Takano, T.; Im, S.-A.; Borges, G.; Lima, J.P.; Aksoy, S.; Gavila Gregori, J.; De Laurentiis, M.; et al. Trastuzumab Deruxtecan versus Treatment of Physician’s Choice in Patients with HER2-Positive Metastatic Breast Cancer (DESTINY-Breast02): A Randomised, Open-Label, Multicentre, Phase 3 Trial. Lancet 2023, 401, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Flaig, T.W.; Milowsky, M.I.; Friedlander, T.W.; Bilen, M.A.; Gupta, S.; Srinivas, S.; Merchan, J.R.; McKay, R.R.; Petrylak, D.P.; et al. Enfortumab Vedotin Plus Pembrolizumab in Previously Untreated Advanced Urothelial Cancer. J. Clin. Oncol. 2023, 41, 22–31. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junttila, T.T.; Li, G.; Parsons, K.; Phillips, G.L.; Sliwkowski, M.X. Trastuzumab-DM1 (T-DM1) Retains All the Mechanisms of Action of Trastuzumab and Efficiently Inhibits Growth of Lapatinib Insensitive Breast Cancer. Breast Cancer Res. Treat. 2011, 128, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody-Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, F.W.; Barker, H.R.; Lipert, B.; Rothé, F.; Gebhart, G.; Piccart-Gebhart, M.J.; Sotiriou, C.; Jamieson, S.M.F. Mechanisms of Resistance to Trastuzumab Emtansine (T-DM1) in HER2-Positive Breast Cancer. Br. J. Cancer 2020, 122, 603–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab Emtansine: Mechanisms of Action and Drug Resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef] [Green Version]
- Erickson, H.K.; Park, P.U.; Widdison, W.C.; Kovtun, Y.V.; Garrett, L.M.; Hoffman, K.; Lutz, R.J.; Goldmacher, V.S.; Blättler, W.A. Antibody-Maytansinoid Conjugates Are Activated in Targeted Cancer Cells by Lysosomal Degradation and Linker-Dependent Intracellular Processing. Cancer Res. 2006, 66, 4426–4433. [Google Scholar] [CrossRef] [Green Version]
- Issell, B.F.; Crooke, S.T. Maytansine. Cancer Treat. Rev. 1978, 5, 199–207. [Google Scholar] [CrossRef]
- Hudis, C.A. Trastuzumab—Mechanism of Action and Use in Clinical Practice. N. Engl. J. Med. 2007, 357, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Cooley, S.; Burns, L.J.; Repka, T.; Miller, J.S. Natural Killer Cell Cytotoxicity of Breast Cancer Targets Is Enhanced by Two Distinct Mechanisms of Antibody-Dependent Cellular Cytotoxicity against LFA-3 and HER2/Neu. Exp. Hematol. 1999, 27, 1533–1541. [Google Scholar] [CrossRef]
- Spector, N.L.; Blackwell, K.L. Understanding the Mechanisms behind Trastuzumab Therapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer. J. Clin. Oncol. 2009, 27, 5838–5847. [Google Scholar] [CrossRef] [PubMed]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, A Novel HER2-Targeting ADC with a Novel DNA Topoisomerase I Inhibitor, Demonstrates a Promising Antitumor Efficacy with Differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakada, T.; Sugihara, K.; Jikoh, T.; Abe, Y.; Agatsuma, T. The Latest Research and Development into the Antibody-Drug Conjugate, [Fam-] Trastuzumab Deruxtecan (DS-8201a), for HER2 Cancer Therapy. Chem. Pharm. Bull. 2019, 67, 173–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander Killing Effect of DS-8201a, a Novel Anti-Human Epidermal Growth Factor Receptor 2 Antibody-Drug Conjugate, in Tumors with Human Epidermal Growth Factor Receptor 2 Heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Arrojo, R.; Liu, D.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody-Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjug. Chem. 2015, 26, 919–931. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-Hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Shih, L.B.; Xuan, H.; Aninipot, R.; Stein, R.; Goldenberg, D.M. In Vitro and in Vivo Reactivity of an Internalizing Antibody, RS7, with Human Breast Cancer. Cancer Res. 1995, 55, 5857s–5863s. [Google Scholar]
- Kawato, Y.; Aonuma, M.; Hirota, Y.; Kuga, H.; Sato, K. Intracellular Roles of SN-38, a Metabolite of the Camptothecin Derivative CPT-11, in the Antitumor Effect of CPT-11. Cancer Res. 1991, 51, 4187–4191. [Google Scholar]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 Is a Novel Target for Solid Cancer Therapy with Sacituzumab Govitecan (IMMU-132), an Antibody-Drug Conjugate (ADC). Oncotarget 2015, 6, 22496–22512. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.; Glode, A.; Messersmith, W.A.; Diamond, J. Sacituzumab Govitecan: Breakthrough Targeted Therapy for Triple-Negative Breast Cancer. Expert Rev. Anticancer Ther. 2019, 19, 673–679. [Google Scholar] [CrossRef]
- Chang, E.; Weinstock, C.; Zhang, L.; Charlab, R.; Dorff, S.E.; Gong, Y.; Hsu, V.; Li, F.; Ricks, T.K.; Song, P.; et al. FDA Approval Summary: Enfortumab Vedotin for Locally Advanced or Metastatic Urothelial Carcinoma. Clin. Cancer Res. 2021, 27, 922–927. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alt, M.; Stecca, C.; Tobin, S.; Jiang, D.M.; Sridhar, S.S. Enfortumab Vedotin in Urothelial Cancer. Ther. Adv. Urol. 2020, 12, 1756287220980192. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Rosenberg, J.E. The Biology and Rationale of Targeting Nectin-4 in Urothelial Carcinoma. Nat. Rev. Urol. 2021, 18, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Loganzo, F.; Tan, X.; Sung, M.; Jin, G.; Myers, J.S.; Melamud, E.; Wang, F.; Diesl, V.; Follettie, M.T.; Musto, S.; et al. Tumor Cells Chronically Treated with a Trastuzumab-Maytansinoid Antibody-Drug Conjugate Develop Varied Resistance Mechanisms but Respond to Alternate Treatments. Mol. Cancer Ther. 2015, 14, 952–963. [Google Scholar] [CrossRef] [Green Version]
- Filho, O.M.; Viale, G.; Stein, S.; Trippa, L.; Yardley, D.A.; Mayer, I.A.; Abramson, V.G.; Arteaga, C.L.; Spring, L.M.; Waks, A.G.; et al. Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov. 2021, 11, 2474–2487. [Google Scholar] [CrossRef]
- Gebhart, G.; Lamberts, L.E.; Wimana, Z.; Garcia, C.; Emonts, P.; Ameye, L.; Stroobants, S.; Huizing, M.; Aftimos, P.; Tol, J.; et al. Molecular Imaging as a Tool to Investigate Heterogeneity of Advanced HER2-Positive Breast Cancer and to Predict Patient Outcome under Trastuzumab Emtansine (T-DM1): The ZEPHIR Trial. Ann. Oncol. 2016, 27, 619–624. [Google Scholar] [CrossRef]
- Scaltriti, M.; Rojo, F.; Ocaña, A.; Anido, J.; Guzman, M.; Cortes, J.; Di Cosimo, S.; Matias-Guiu, X.; Ramon y Cajal, S.; Arribas, J.; et al. Expression of p95HER2, a Truncated Form of the HER2 Receptor, and Response to Anti-HER2 Therapies in Breast Cancer. J. Natl. Cancer Inst. 2007, 99, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Nagy, P.; Friedländer, E.; Tanner, M.; Kapanen, A.I.; Carraway, K.L.; Isola, J.; Jovin, T.M. Decreased Accessibility and Lack of Activation of ErbB2 in JIMT-1, a Herceptin-Resistant, MUC4-Expressing Breast Cancer Cell Line. Cancer Res. 2005, 65, 473–482. [Google Scholar] [CrossRef]
- Phillips, G.D.L.; Fields, C.T.; Li, G.; Dowbenko, D.; Schaefer, G.; Miller, K.; Andre, F.; Burris, H.A.; Albain, K.S.; Harbeck, N.; et al. Dual Targeting of HER2-Positive Cancer with Trastuzumab Emtansine and Pertuzumab: Critical Role for Neuregulin Blockade in Antitumor Response to Combination Therapy. Clin. Cancer Res. 2014, 20, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Coates, J.T.; Sun, S.; Leshchiner, I.; Thimmiah, N.; Martin, E.E.; McLoughlin, D.; Danysh, B.P.; Slowik, K.; Jacobs, R.A.; Rhrissorrakrai, K.; et al. Parallel Genomic Alterations of Antigen and Payload Targets Mediate Polyclonal Acquired Clinical Resistance to Sacituzumab Govitecan in Triple-Negative Breast Cancer. Cancer Discov. 2021, 11, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Klümper, N.; Ralser, D.J.; Ellinger, J.; Roghmann, F.; Albrecht, J.; Below, E.; Alajati, A.; Sikic, D.; Breyer, J.; Bolenz, C.; et al. Membranous NECTIN-4 Expression Frequently Decreases during Metastatic Spread of Urothelial Carcinoma and Is Associated with Enfortumab Vedotin Resistance. Clin. Cancer Res. 2023, 29, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-F.; Zheng, B.; Go, M.; Lau, J.; Spencer, S.; Raab, H.; Soriano, R.; Jhunjhunwala, S.; Cohen, R.; Caruso, M.; et al. A Novel Anti-CD22 Anthracycline-Based Antibody-Drug Conjugate (ADC) That Overcomes Resistance to Auristatin-Based ADCs. Clin. Cancer Res. 2015, 21, 3298–3306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takegawa, N.; Nonagase, Y.; Yonesaka, K.; Sakai, K.; Maenishi, O.; Ogitani, Y.; Tamura, T.; Nishio, K.; Nakagawa, K.; Tsurutani, J. DS-8201a, a New HER2-Targeting Antibody-Drug Conjugate Incorporating a Novel DNA Topoisomerase I Inhibitor, Overcomes HER2-Positive Gastric Cancer T-DM1 Resistance. Int. J. Cancer 2017, 141, 1682–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conilh, L.; Sadilkova, L.; Viricel, W.; Dumontet, C. Payload Diversification: A Key Step in the Development of Antibody-Drug Conjugates. J. Hematol. Oncol. 2023, 16, 3. [Google Scholar] [CrossRef]
- Qiu, D.; Huang, Y.; Chennamsetty, N.; Miller, S.A.; Hay, M. Characterizing and Understanding the Formation of Cysteine Conjugates and Other By-Products in a Random, Lysine-Linked Antibody Drug Conjugate. MAbs 2021, 13, 1974150. [Google Scholar] [CrossRef]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.C.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [Green Version]
- Yoder, N.C.; Bai, C.; Tavares, D.; Widdison, W.C.; Whiteman, K.R.; Wilhelm, A.; Wilhelm, S.D.; McShea, M.A.; Maloney, E.K.; Ab, O.; et al. A Case Study Comparing Heterogeneous Lysine- and Site-Specific Cysteine-Conjugated Maytansinoid Antibody-Drug Conjugates (ADCs) Illustrates the Benefits of Lysine Conjugation. Mol. Pharm. 2019, 16, 3926–3937. [Google Scholar] [CrossRef]
- Bai, C.; Reid, E.E.; Wilhelm, A.; Shizuka, M.; Maloney, E.K.; Laleau, R.; Harvey, L.; Archer, K.E.; Vitharana, D.; Adams, S.; et al. Site-Specific Conjugation of the Indolinobenzodiazepine DGN549 to Antibodies Affords Antibody-Drug Conjugates with an Improved Therapeutic Index as Compared with Lysine Conjugation. Bioconjug Chem. 2020, 31, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Kalim, M.; Chen, J.; Wang, S.; Lin, C.; Ullah, S.; Liang, K.; Ding, Q.; Chen, S.; Zhan, J. Intracellular Trafficking of New Anticancer Therapeutics: Antibody-Drug Conjugates. Drug Des. Dev. Ther. 2017, 11, 2265–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, M.; Tan, X.; Lu, B.; Golas, J.; Hosselet, C.; Wang, F.; Tylaska, L.; King, L.; Zhou, D.; Dushin, R.; et al. Caveolae-Mediated Endocytosis as a Novel Mechanism of Resistance to Trastuzumab Emtansine (T-DM1). Mol. Cancer Ther. 2018, 17, 243–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldassarre, T.; Truesdell, P.; Craig, A.W. Endophilin A2 Promotes HER2 Internalization and Sensitivity to Trastuzumab-Based Therapy in HER2-Positive Breast Cancers. Breast Cancer Res. 2017, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Luci, C.; García-Alonso, S.; Díaz-Rodríguez, E.; Nadal-Serrano, M.; Arribas, J.; Ocaña, A.; Pandiella, A. Resistance to the Antibody-Drug Conjugate T-DM1 Is Based in a Reduction in Lysosomal Proteolytic Activity. Cancer Res. 2017, 77, 4639–4651. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, K.M.; Colby, A.H.; Zeng, J.; Las, G.; Feng, J.H.; Grinstaff, M.W.; Shirihai, O.S. Lysosome Acidification by Photoactivated Nanoparticles RestoRes. Autophagy under Lipotoxicity. J. Cell Biol. 2016, 214, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Hamblett, K.J.; Jacob, A.P.; Gurgel, J.L.; Tometsko, M.E.; Rock, B.M.; Patel, S.K.; Milburn, R.R.; Siu, S.; Ragan, S.P.; Rock, D.A.; et al. SLC46A3 Is Required to Transport Catabolites of Noncleavable Antibody Maytansine Conjugates from the Lysosome to the Cytoplasm. Cancer Res. 2015, 75, 5329–5340. [Google Scholar] [CrossRef] [Green Version]
- Mosele, M.F.; Lusque, A.; Dieras, V.; Deluche, E.; Ducoulombier, A.; Pistilli, B.; Bachelot, T.; Viret, F.; Levy, C.; Signolle, N.; et al. LBA1 Unraveling the Mechanism of Action and Resistance to Trastuzumab Deruxtecan (T-DXd): Biomarker Analyses from Patients from DAISY Trial. Ann. Oncol. 2022, 33, S123. [Google Scholar] [CrossRef]
- Shah, S.; Kim, Y.; Ostrovnaya, I.; Murali, R.; Schrader, K.A.; Lach, F.P.; Sarrel, K.; Rau-Murthy, R.; Hansen, N.; Zhang, L.; et al. Assessment of SLX4 Mutations in Hereditary Breast Cancers. PLoS ONE 2013, 8, e66961. [Google Scholar] [CrossRef]
- Leonard, G.D.; Fojo, T.; Bates, S.E. The Role of ABC Transporters in Clinical Practice. Oncologist 2003, 8, 411–424. [Google Scholar] [CrossRef]
- Yu, M.; Ocana, A.; Tannock, I.F. Reversal of ATP-Binding Cassette Drug Transporter Activity to Modulate Chemoresistance: Why Has It Failed to Provide Clinical Benefit? Cancer Metastasis Rev. 2013, 32, 211–227. [Google Scholar] [CrossRef]
- Kovtun, Y.V.; Audette, C.A.; Mayo, M.F.; Jones, G.E.; Doherty, H.; Maloney, E.K.; Erickson, H.K.; Sun, X.; Wilhelm, S.; Ab, O.; et al. Antibody-Maytansinoid Conjugates Designed to Bypass Multidrug Resistance. Cancer Res. 2010, 70, 2528–2537. [Google Scholar] [CrossRef] [Green Version]
- Cianfriglia, M. The Biology of MDR1-P-Glycoprotein (MDR1-Pgp) in Designing Functional Antibody Drug Conjugates (ADCs): The Experience of Gemtuzumab Ozogamicin. Ann. Ist. Super. Sanita 2013, 49, 150–168. [Google Scholar] [CrossRef]
- Lambert, J.M.; Chari, R.V.J. Ado-Trastuzumab Emtansine (T-DM1): An Antibody-Drug Conjugate (ADC) for HER2-Positive Breast Cancer. J. Med. Chem. 2014, 57, 6949–6964. [Google Scholar] [CrossRef]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-Angulo, A.M.; Stemke-Hale, K.; Hauptmann, M.; et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabbaghi, M.; Gil-Gómez, G.; Guardia, C.; Servitja, S.; Arpí, O.; García-Alonso, S.; Menendez, S.; Arumi-Uria, M.; Serrano, L.; Salido, M.; et al. Defective Cyclin B1 Induction in Trastuzumab-Emtansine (T-DM1) Acquired Resistance in HER2-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 7006–7019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baselga, J.; Lewis Phillips, G.D.; Verma, S.; Ro, J.; Huober, J.; Guardino, A.E.; Samant, M.K.; Olsen, S.; de Haas, S.L.; Pegram, M.D. Relationship between Tumor Biomarkers and Efficacy in EMILIA, a Phase III Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Ginther, C.; Kim, J.; Mosher, N.; Chung, S.; Slamon, D.; Vadgama, J.V. Expression of Wnt3 Activates Wnt/β-Catenin Pathway and Promotes EMT-like Phenotype in Trastuzumab-Resistant HER2-Overexpressing Breast Cancer Cells. Mol. Cancer Res. 2012, 10, 1597–1606. [Google Scholar] [CrossRef] [Green Version]
- Walter, R.B.; Raden, B.W.; Cronk, M.R.; Bernstein, I.D.; Appelbaum, F.R.; Banker, D.E. The Peripheral Benzodiazepine Receptor Ligand PK11195 Overcomes Different Resistance Mechanisms to Sensitize AML Cells to Gemtuzumab Ozogamicin. Blood 2004, 103, 4276–4284. [Google Scholar] [CrossRef]
- Moore, J.; Seiter, K.; Kolitz, J.; Stock, W.; Giles, F.; Kalaycio, M.; Zenk, D.; Marcucci, G. A Phase II Study of Bcl-2 Antisense (Oblimersen Sodium) Combined with Gemtuzumab Ozogamicin in Older Patients with Acute Myeloid Leukemia in First Relapse. Leuk. Res. 2006, 30, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Dornan, D.; Bennett, F.; Chen, Y.; Dennis, M.; Eaton, D.; Elkins, K.; French, D.; Go, M.A.T.; Jack, A.; Junutula, J.R.; et al. Therapeutic Potential of an Anti-CD79b Antibody-Drug Conjugate, Anti-CD79b-vc-MMAE, for the Treatment of Non-Hodgkin Lymphoma. Blood 2009, 114, 2721–2729. [Google Scholar] [CrossRef] [Green Version]
- Ab, O.; Whiteman, K.R.; Bartle, L.M.; Sun, X.; Singh, R.; Tavares, D.; LaBelle, A.; Payne, G.; Lutz, R.J.; Pinkas, J.; et al. IMGN853, a Folate Receptor-α (FRα)-Targeting Antibody-Drug Conjugate, Exhibits Potent Targeted Antitumor Activity against FRα-Expressing Tumors. Mol. Cancer Ther. 2015, 14, 1605–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab Ravtansine: A Novel Mesothelin-Targeting Antibody-Drug Conjugate CuRes. Tumors with Heterogeneous Target Expression Favored by Bystander Effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Emmerton, K.K.; Jonas, M.; Zhang, X.; Miyamoto, J.B.; Setter, J.R.; Nicholas, N.D.; Okeley, N.M.; Lyon, R.P.; Benjamin, D.R.; et al. Intracellular Released Payload Influences Potency and Bystander-Killing Effects of Antibody-Drug Conjugates in Preclinical Models. Cancer Res. 2016, 76, 2710–2719. [Google Scholar] [CrossRef] [Green Version]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.M.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N.; et al. A Biparatopic HER2-Targeting Antibody-Drug Conjugate Induces Tumor Regression in Primary Models Refractory to or Ineligible for HER2-Targeted Therapy. Cancer Cell 2016, 29, 117–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreev, J.; Thambi, N.; Perez Bay, A.E.; Delfino, F.; Martin, J.; Kelly, M.P.; Kirshner, J.R.; Rafique, A.; Kunz, A.; Nittoli, T.; et al. Bispecific Antibodies and Antibody-Drug Conjugates (ADCs) Bridging HER2 and Prolactin Receptor Improve Efficacy of HER2 ADCs. Mol. Cancer Ther. 2017, 16, 681–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, H.; Yan, M.; Tong, Z.; Wu, X.; Ryu, M.-H.; Kim, J.H.; Park, J.; Zhong, Y.; Han, W.; Liu, C.; et al. Abstract CT175: Safety, Tolerability, Pharmacokinetics, and Antitumor Activity of SHR-A1811 in HER2-Expressing/Mutated Advanced Solid Tumors: A Global Phase 1, Multi-Center, First-in-Human Study. Cancer Res. 2023, 83, CT175. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Gao, S.; Li, W.; Chen, Y.; Meng, Y.; Liu, C.; Jin, W.; Wu, J.; Wang, Y.; et al. Phase I Study of A166, an Antibody-drug Conjugate in Advanced HER2-Expressing Solid Tumours. NPJ Breast Cancer 2023, 9, 28. [Google Scholar] [CrossRef]
- Gerber, H.-P.; Sapra, P.; Loganzo, F.; May, C. Combining Antibody-Drug Conjugates and Immune-Mediated Cancer Therapy: What to Expect? Biochem. Pharmacol. 2016, 102, 1–6. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab Emtansine plus Atezolizumab versus Trastuzumab Emtansine plus Placebo in Previously Treated, HER2-Positive Advanced Breast Cancer (KATE2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Loi, S.; Schneeweiss, A.; Song, E.; Harries, M.; Laurentiis, M.D.; Li, Y.; Wiese, C.; Poppe, R.; Emens, L.A. 329TiP KATE3: A Phase III Study of Trastuzumab Emtansine (T-DM1) in Combination with Atezolizumab or Placebo in Patients with Previously Treated HER2-Positive and PD-L1–Positive Locally Advanced or Metastatic Breast Cancer. Ann. Oncol. 2021, 32, S509. [Google Scholar] [CrossRef]
- Schwarz, L.J.; Hutchinson, K.E.; Rexer, B.N.; Estrada, M.V.; Gonzalez Ericsson, P.I.; Sanders, M.E.; Dugger, T.C.; Formisano, L.; Guerrero-Zotano, A.; Red-Brewer, M.; et al. An ERBB1-3 Neutralizing Antibody Mixture with High Activity Against Drug-Resistant HER2+ Breast Cancers with ERBB Ligand Overexpression. J. Natl. Cancer Inst. 2017, 109, djx065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown University. BrUOG 413: A Phase II Study of Adjuvant Trastuzumab Deruxtecan (Enhertu) + Nivolumab for Patients Who Are Disease Free After Completion of Trimodality Treatment for HER-2- Positive Cancers of the Esophagus and Gastroesophageal Junction; Brown University: Providence, RI, USA, 2023. [Google Scholar]
- Seagen Inc. A Single Arm, Open Label Phase 2 Study of Tucatinib in Combination with Trastuzumab Deruxtecan in Subjects with Previously Treated Unresectable Locally-Advanced or Metastatic HER2+ Breast Cancer; Seagen Inc.: Bothell, WA, USA, 2023. [Google Scholar]
- Md Anderson Cancer Center. Phase 1b Study of EZH1/2 Inhibitor Valemetostat in Combination with Trastuzumab Deruxtecan in Subjects with HER2 Low/Ultra-Low/Null Metastatic Breast Cancer; Md Anderson Cancer Center: League City, TX, USA, 2023. [Google Scholar]
- Middle District of Florida. TRUDI: A Phase II Study of Neoadjuvant Trastuzumab Deruxtecan and Durvalumab for Stage III, HER2-Expressing Inflammatory Breast Cancer; Middle District of Florida: Tampa, FL, USA, 2023.
- National Cancer Institute (NCI). Phase I Trial of DS-8201a (Trastuzumab Deruxtecan) in Combination with Neratinib in Solid Tumors with HER2 Alterations; National Cancer Institute (NCI): Bethesda, MD, USA, 2023.
- National Cancer Institute (NCI). Phase 1/1B Study of DS-8201a in Combination with ATR Inhibition (AZD6738) in Advanced Solid Tumors with HER2 Expression (DASH Trial); National Cancer Institute (NCI): Bethesda, MD, USA, 2023.
- AstraZeneca. A Phase 1b/2 Multicentre, Open-Label, Modular, Dose-Finding and Dose-Expansion Study to Explore the Safety, Tolerability, and Anti-Tumour Activity of Trastuzumab Deruxtecan (T-DXd) in Combination with Other Anti-Cancer Agents in Patients with HER2-Positive Metastatic Breast Cancer (DESTINY-Breast07); AstraZeneca: Cambridge, UK, 2023. [Google Scholar]
- Daiichi Sankyo, Inc. A Phase 1b, Multicenter, Two-Part, Open-Label Study of Trastuzumab Deruxtecan (DS-8201a), an Anti-Human Epidermal Growth Factor Receptor-2 (HER2)-Antibody Drug Conjugate (ADC), in Combination with Pembrolizumab, an Anti-PD-1 Antibody, for Subjects with Locally Advanced/Metastatic Breast Or Non-Small Cell Lung Cancer (NSCLC); Daiichi Sankyo, Inc.: Tokyo, Japan, 2023. [Google Scholar]
- AstraZeneca. A Phase Ib Multicenter, Open-Label Dose-Escalation Study to Evaluate the Safety and Tolerability of Trastuzumab Deruxtecan (T-DXd) and Durvalumab in Combination with Cisplatin, Carboplatin or Pemetrexed in First-Line Treatment of Patients with Advanced or Metastatic Non-Squamous Non-Small Cell Lung Cancer (NSCLC) and Human Epidermal Growth Factor Receptor 2 Overexpression (HER2+) (DESTINY-Lung03); AstraZeneca: Cambridge, UK, 2023. [Google Scholar]
- AstraZeneca. A Phase 1b/2 Multicenter, Open-Label, Dose-Escalation and Dose-Expansion Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Immunogenicity, and Antitumor Activity of Trastuzumab Deruxtecan (T-DXd) Monotherapy and Combinations in Adult Participants with HER2 Overexpressing Gastric Cancer (DESTINY-Gastric03); AstraZeneca: Cambridge, UK, 2023. [Google Scholar]
- National Cancer Institute (NCI). A Phase I Study of DS-8201a in Combination with Olaparib in HER2-Expressing Malignancies; National Cancer Institute (NCI): Bethesda, MD, USA, 2023.
- AstraZeneca. An Open-Label, Multi-Drug, Biomarker-Directed, Multi-Centre Phase II Umbrella Study in Patients with Non-Small Cell Lung Cancer, Who Progressed on an Anti-PD-1/PD-L1 Containing Therapy (HUDSON); AstraZeneca: Cambridge, UK, 2023. [Google Scholar]
- Md Anderson Cancer Center. A Phase II Study of Tucatinib and Ado-Trastuzumab Emtansine (T-DM1) in Patients with HER2-Positive Metastatic Solid Tumors and Metastases to Brain (TUCATEMEB); Md Anderson Cancer Center: League City, TX, USA, 2023. [Google Scholar]
- Seagen Inc. Randomized, Double-Blind, Phase 3 Study of Tucatinib or Placebo in Combination with Ado-Trastuzumab Emtansine (T-DM1) for Subjects with Unresectable Locally-Advanced or Metastatic HER2+ Breast Cancer (HER2CLIMB-02); Seagen Inc.: Bothell, WA, USA, 2023. [Google Scholar]
- Hoffmann-La Roche. A Phase III, Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Adjuvant Atezolizumab or Placebo and Trastuzumab Emtansine for HER2-Positive Breast Cancer at High Risk of Recurrence Following Preoperative Therapy; Hoffmann-La Roche: Basel, Switzerland, 2023. [Google Scholar]
- Alliance for Clinical Trials in Oncology. The CompassHER2 Trials (Comprehensive Use of Pathologic Response Assessment to Optimize Therapy in HER2-Positive Breast Cancer) CompassHER2 Residual Disease (RD), a Double-Blinded, Phase III Randomized Trial of T-DM1 Compared with T-DM1 and Tucatinib; Alliance for Clinical Trials in Oncology: Boston, MA, USA, 2023. [Google Scholar]
- Tianjin Medical University Cancer Institute and Hospital. A Prospective, Multicenter, Phase II Clinical Study of Trastuzumab and Pyrotinib Maleate in Patients with HER2-Positive Metastatic Breast Cancer Who Had Progressed on TKI Therapy; Tianjin Medical University Cancer Institute and Hospital: Tianjin, China, 2022. [Google Scholar]
- Sharma, P. Phase I Trial Of Alpelisib Plus Sacituzumab Govitecan in Patients with Metastatic or Locally Recurrent HER2-Negative Breast Cancer; University of Kansas Medical Center: Kansas City, KS, USA, 2023. [Google Scholar]
- MD, B.A.M. Sacituzumab Govitecan Plus Enfortumab Vedotin for Metastatic Urothelial Carcinoma Progressing on Platinum-Based Chemotherapy and PD1/L1 Inhibitors: The Double Antibody Drug Conjugate (DAD) Phase I Trial; Dana Farber Cancer Institute: Boston, MA, USA, 2023. [Google Scholar]
- Mittendorf, E.A. A Single Arm Phase 2 Trial of Atezolizumab with Sacituzumab Govitecan to Prevent Recurrence in Triple Negative Breast Cancer (ASPRIA); Dana Farber Cancer Institute: Boston, MA, USA, 2022. [Google Scholar]
- Garrido-Castro, A.C. Saci-IO TNBC: Randomized Phase II Study of Sacituzumab Govitecan with or without Pembrolizumab in PD-L1-Negative Metastatic Triple Negative Breast Cancer (TNBC); Dana Farber Cancer Institute: Boston, MA, USA, 2022. [Google Scholar]
- Garrido-Castro, A.C. Saci-IO HR+: Randomized Phase II Study of Sacituzumab Govitecan with or without Pembrolizumab in Hormone Receptor-Positive (HR+)/HER2- Metastatic Breast Cancer (MBC); Dana Farber Cancer Institute: Boston, MA, USA, 2022. [Google Scholar]
- Bardia, A. Phase 1b/2 Study to Evaluate Antibody-Drug Conjugate Sacituzumab Govitecan in Combination with PARP Inhibitor Talazoparib in Patients with Metastatic Breast Cancer; Massachusetts General Hospital: Boston, MA, USA, 2023. [Google Scholar]
- National Cancer Institute (NCI). A Phase I/II Study of Sacituzumab Govitecan Plus Berzosertib in Small Cell Lung Cancer, Extra-Pulmonary Small Cell Neuroendocrine Cancer and Homologous Recombination-Deficient Cancers Resistant to PARP Inhibitors; National Cancer Institute (NCI): Bethesda, MD, USA, 2023.
- Merck Sharp & Dohme LLC. An Open-Label, Multicenter, Phase 3 Randomized, Active-Comparator-Controlled Clinical Study of Pembrolizumab (MK-3475) in Combination with Sacituzumab Govitecan Versus MK-3475 Monotherapy as First-Line Treatment in Participants with PD L1 TPS Greater Than or Equal to 50% Metastatic Non-Small Cell Lung Cancer (KEYNOTE D46/EVOKE-03); Merck Sharp & Dohme LLC: Rahway, NJ, USA, 2023. [Google Scholar]
- H. Lee Moffitt Cancer Center and Research Institute. Phase I/II Study of Ipilimumab Plus Nivolumab (IPI-NIVO) Combined with Sacituzumab Govitecan (SG)as First-Line Treatment for Cisplatin-Ineligible Metastatic Urothelial Carcinoma; H. Lee Moffitt Cancer Center and Research Institute: Tampa, FL, USA, 2023. [Google Scholar]
- Gilead Sciences. A Randomized, Open-Label, Phase 3 Study of Adjuvant Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician’s Choice in Patients with Triple Negative Breast Cancer Who Have Residual Invasive Disease after Surgery and Neoadjuvant Therapy; Gilead Sciences: Foster City, CA, USA, 2023. [Google Scholar]
- Gilead Sciences. A Phase II Open-Label Study of Sacituzumab Govitecan in Unresectable Locally Advanced/Metastatic Urothelial Cancer; Gilead Sciences: Foster City, CA, USA, 2023. [Google Scholar]
- Guerrieri, C. An Open Label, Single-Arm, Phase 2 Study of Perioperative Pembrolizumab Plus Sacituzumab Govitecan for Patients with Muscle-Invasive Bladder Cancer Who Cannot Receive or Refuse Cisplatin-Based Chemotherapy; Fondazione San Raffaele: Milan, Italy, 2023. [Google Scholar]
- Gilead Sciences. A Randomized, Open-Label, Phase 3 Study of Sacituzumab Govitecan and Pembrolizumab Versus Treatment of Physician’s Choice and Pembrolizumab in Patients with Previously Untreated, Locally Advanced Inoperable or Metastatic Triple-Negative Breast Cancer, Whose Tumors Express PD-L1; Gilead Sciences: Foster City, CA, USA, 2023. [Google Scholar]
- MD, H.R. Innovative Combination Immunotherapy for Metastatic Triple Negative Breast Cancer (TNBC): A Multicenter, Multi-Arm Translational Breast Cancer Research Consortium Study; University of California: San Francisco, CA, USA, 2022. [Google Scholar]
- Memorial Sloan Kettering Cancer Center. Enfortumab Vedotin in Combination with Pembrolizumab for Locally Advanced and/or Node Positive Urothelial Carcinoma Prior to Surgery (EV-ECLIPSE); Memorial Sloan Kettering Cancer Center: Chennai, India, 2023. [Google Scholar]
- National Cancer Institute (NCI). Phase Ib Trial of Erdafitinib Combined with Enfortumab Vedotin Following Platinum and PD1/L1 Inhibitors for Metastatic Urothelial Carcinoma with FGFR2/3 Genetic Alterations; National Cancer Institute (NCI): Bethesda, MD, USA, 2023.
- ALX Oncology Inc. A Phase 1, Open-Label, Multicenter, Safety, Pharmacokinetic, Pharmacodynamic Study of ALX148 in Combination with Enfortumab Vedotin and/or Other Anticancer Therapies in Subjects with Urothelial Carcinoma (ASPEN-07); ALX Oncology Inc.: San Francisco, CA, USA, 2023. [Google Scholar]
- Jonsson Comprehensive Cancer Center. Neoadjuvant Combination Pembrolizumab/Enfortumab Vedotin with Adjuvant Pembrolizumab Prior to and After Radical Nephroureterectomy for High-Risk Upper Tract Urothelial Carcinoma; Jonsson Comprehensive Cancer Center: Los Angeles, CA, USA, 2023. [Google Scholar]
- Merck Sharp & Dohme LLC. A Randomized Phase 3 Study Evaluating Cystectomy with Perioperative Pembrolizumab and Cystectomy with Perioperative Enfortumab Vedotin and Pembrolizumab Versus Cystectomy Alone in Participants Who Are Cisplatin-Ineligible or Decline Cisplatin with Muscle-Invasive Bladder Cancer (KEYNOTE-905/EV-303); Merck Sharp & Dohme LLC: Rahway, NJ, USA, 2023. [Google Scholar]
- Bilen, M. A Phase I/Ib Open Label, Single-Arm Study of Cabozantinib in Combination with Enfortumab Vedotin (EV) in the Treatment of Locally Advanced or Metastatic Urothelial Cancer; Emory University Hospital/Winship Cancer Institute: Atlanta, GA, USA, 2022. [Google Scholar]
- AstraZeneca. A Phase III Randomized, Open-Label, Multicenter Study to Determine the Efficacy and Safety of Durvalumab in Combination with Tremelimumab and Enfortumab Vedotin or Durvalumab in Combination with Enfortumab Vedotin for Perioperative Treatment in Patients Ineligible for Cisplatin or Who Refuse Cisplatin Undergoing Radical Cystectomy for Muscle Invasive Bladder Cancer (VOLGA); AstraZeneca: Cambridge, UK, 2023. [Google Scholar]
- Astellas Pharma Global Development, Inc. An Open-Label, Randomized, Controlled Phase 3 Study of Enfortumab Vedotin in Combination with Pembrolizumab Versus Chemotherapy Alone in Previously Untreated Locally Advanced or Metastatic Urothelial Cancer; Astellas Pharma Global Development, Inc.: Northbrook, IL, USA, 2023. [Google Scholar]
- Merck Sharp & Dohme LLC. A Phase 3, Randomized, Open-Label Study to Evaluate Perioperative Enfortumab Vedotin Plus Pembrolizumab (MK-3475) Versus Neoadjuvant Gemcitabine and Cisplatin in Cisplatin-Eligible Participants with Muscle-Invasive Bladder Cancer (KEYNOTE-B15/EV-304); Merck Sharp & Dohme LLC: Rahway, NJ, USA, 2023. [Google Scholar]

| Trial | Drugs | Phase | No. of pts. | Population | Primary Endpoint | Grade ≥ 3 AEs |
|---|---|---|---|---|---|---|
| EMILIA (NCT008291666) | T-DM1 vs. lapatinib + capecitabine | III | 991 | HER2+ MBC previously treated with taxanes + T | mPFS: 9.6 vs. 6.4 m (p < 0.001) mOS: 30.9 vs. 25.1 m (p < 0.001) | 41% vs. 57% T-DM1: Thrombocytopenia (13%) Elevated ASAT (4%) Anemia (3%) |
| DESTINY-Breast02 (NCT03523585) [22] | T-DXd vs. T + capecitabine or lapatinib + capecitabine | III | 608 | HER2+ MBC previously treated with T-DM1 | mPFS: 17.8 vs. 6.9 m (p < 0.0001) | 53% vs. 44% ILD: 10% |
| DESTINY-Breast 03 (NCT03529110) [23] | T-DXd vs. T-DM1 | III | 524 | HER2+ MBC previously treated with T and taxane | mPFS: not reached vs. 6.8 m 12-mPFS rate (76 vs. 34%) (p < 0.0001) | 52% vs. 48% T-DXd: Neutropenia (19%) Thrombocytopenia (7%) ILD (1%) |
| DESTINY-Gastric01 (NCT03329690) [24] | T-DXd vs. physician of choice chemotherapy | II | 187 | HER2+ gastric cancer receiving at least 2 previous lines including T | ORR: 51% vs. 14% (p < 0.001) | Neutropenia (51%) Anemia (38%) Leucopenia (21%) ILD (2%) |
| DESTINY-Lung01 (NCT03505710) [25] | T-DXd | II | 91 | Metastatic HER2-mutant NSCLC refractory to standard treatment | ORR: 55% (mPFS: 8.2 m; mOS: 17.8 m) | Neutropenia (19%) Anemia (10%) Nausea (9%) |
| ASCENT (NCT02574455) [26] | SG vs. single-agent CT | III | 468 | metastatic TNBC refractory to two prior lines | mPFS: 5.6 vs. 1.7 (p < 0.001) | Neutropenia (51%) Leukopenia (10%) Diarrhea (10%) Anemia (8%) |
| TROPiCS-02 (NCT03901339) [27] | SG vs. single-agent CT | III | 543 | HR+/HER2- mBC prior taxane, ET, CDK4/6 inhibitor and 2-4 prior CTs | mPFS: 5.5 vs. 4.0 m (p < 0.001) | Neutropenia (51%) Diarrhea (10%) |
| TROPHY-U-01 (NCT03547973) [28] | SG | II | 113 | mUC progressed after platinum-base CT and ICIs | ORR: 27% | Neutropenia (35%) Leukopenia (18%) Anemia (14%) Diarrhea (10%) Febrile neutropenia (10%) |
| EV-301 (NCT03474107) [29] | EV vs. docetaxel or paclitaxel of vinflunine | III | 608 | mUC progressed after platinum-base CT and ICIs | mOS: 12.9 vs. 9.0 m (p = 0.001) | Maculopapular rash (7%) Fatigue (6%) Neutropenia (5%) |
| EV-103 (NCT04223856) [30] | EV + pembro | Ib/II | 45 | mUC first-line cisplatin-ineligible pts | ORR 73% including 16% CR | Increased lipase (18%) Maculopapular rash (11%) Fatigue (11%) |
| Mechanism of Resistance | Overcoming Resistance | |
|---|---|---|
| Antigen-related resistance |
|
|
| Payload-related resistance |
|
|
| Altered internalization |
| |
| Impaired lysosomal function |
|
|
| Overexpression of drug-efflux pumps |
|
|
| Activation of signaling pathways |
|
|
| Study | Drugs | Phase | No. of pts. | Population | Primary Endpoints |
|---|---|---|---|---|---|
| NCT05480384 (BrUOG 413) [102] | T-DXd + nivolumab | II | 25 | Adjuvant treatment after trimodality treatment in HER2+ esophagus and GEJ | Safety |
| NCT04539938 (HER2CLIMB-04) [103] | T-DXd + tucatinib | II | 70 | HER2+ MBC after progression on taxane + T | ORR |
| NCT05633979 [104] | T-DXd + valemetostat | Ib | 37 | Low-HER2 /ultra-low/null MBC | Safety, MTD, ORR, RDE |
| NCT05795101 (TRUDI) [105] | T-DXd + durvalumab | II | 63 | First-line HER2+/low inflammatory breast cancer | pCR |
| NCT05372614 [106] | T-DXd + neratinib | I | 18 | Metastatic HER2-altered cancers | DLTs TEAEs |
| NCT04704661 (DASH) [107] | T-DXd + AZD6738 | I | 15 | Advanced solid tumors with HER2 expression | Safety, RP2D |
| NCT04538742 (DB-07) [108] | T-DXd + durvalumab or pertuzumab or paclitaxel or tucatinib | I/II | 245 | First-line HER2+ advanced and/or MBC | AEs, SAEs |
| NCT04042701 [109] | T-DXd + pembrolizumab | I | 115 | MBC or NSCLC | DLTs, ORR |
| NCT04686305 (DESTINY-Lung03) [110] | T-DXd + durvalumab + pemetrexed or platinum | Ib | 136 | First-line HER2+ advanced or metastatic NSCLC | AEs SAEs |
| NCT04379596 (DESTINY-Gastric03) [111] | T-DXd alone or + durvalumab or pembro and CT | Ib/II | 351 | HER2+ previously treated or untreated gastric and GEJ or esophageal cancer patients | AEs, SAEs, ORR |
| NCT04585958 [112] | T-DXd + olaparib | I | 55 | Metastatic HER2-expressing cancers | MTD, RP2D, AEs |
| NCT03334617 (HUDSON) [113] | Cohort T-DXd + durvalumab | II | 570 | NSCLC progressed on anti-PD-1/PD-L1 containing therapy | ORR |
| Study | Drugs | Phase | No. of pts. | Population | Primary Endpoints |
|---|---|---|---|---|---|
| NCT05673928 (TUCATEMEB) [114] | T-DM1 + tucatinib | II | 30 | HER2+ metastatic solid tumors and brain metastases | Intracranial antitumor activity |
| NCT03975647 (HER2CLIMB-02) [115] | T-DM1 + tucatinib or placebo | III | 565 | Metastatic HER2+ BC with history of prior taxane and T | PFS |
| NCT04873362 (Astefania) [116] | T-DM1 + atezolizumab or placebo | III | 1700 | Adjuvant treatment for HER2+ BC with high risk of recurrence | IDFS |
| NCT04457596 (CompassHER2 RD) [117] | T-DM1 + tucatinib or placebo | III | 1031 | HER2+ BC with residual disease after neoadjuvant HER2-directed therapy | IDFS |
| NCT05560308 [118] | T-DM1 + pyrotinib maleate | II | 50 | HER2+ MBC progressed on TKI therapy | ORR |
| NCT05143229 (ASSET) [119] | SG + aleplisib | I | 18 | Metastatic HER2- BC | RP2D |
| NCT04724018 (DAD) [120] | SG + EV | I | 24 | Metastatic UC progressiong on platinum-based CT and PD-1/PD-L1 inhibitors | MTD, DLTs |
| NCT04434040 (ASPRIA) [121] | SG + atezolizumab | II | 40 | Residual invasive disease in TNBC following neoadjuvant CT | Undetectable ctDNA |
| NCT04468061 (Saci-IO TNBC) [122] | SG with or without pembrolizumab | II | 110 | First-line PD-L1 negative metastatic TNBC | PFS |
| NCT04448886 (Saci-IO HR+) [123] | SG with or withour pembrolizumab | II | 110 | Metastatic HR+/HER2- BC | PFS |
| NCT04039230 [124] | SG + talazoparib | I/II | 75 | Metastatic TNBC | DLT |
| NCT04826341 [125] | SG + berzosertib | I/II | 85 | SCLC and HRD cancers resistant to PARP inhibitors | ORR, MTD |
| NCT05609968 (MK-3475-D46) [126] | SG + pembrolizumab vs. pembrolizumab | III | 614 | First-line metastatic NSCLC with PD-L1 TPS ≥ 50% | PFS, OS |
| NCT04863885 [127] | SG + nivo + ipi | I/II | 46 | First line for cisplatin-ineligible mUC | MTD, ORR |
| NCT05633654 (ASCENT-05) [128] | SG + pembrolizumab vs. capecitabine | III | 1514 | TNBC with residual invasive disease after surgery and neoadjuvant therapy | IDFS |
| NCT03547973 (TROPHY U-01) [129] | SG alone or + pembrolizumab or cisplatin + avelumab or cisplatin + zimberelimab | II | 643 | Unresectable mUC | ORR, PFS |
| NCT05535218 (SURE-02) [130] | SG + pembrolizumab | II | 48 | High-risk localized bladder cancer | cPR |
| NCT05382286 (ASCENT-04) [131] | SG + pembrolizumab vs. TPO | III | 440 | First-line metastatic TNBC whose tumors express PD-L1 | PFS |
| NCT05186974 (EVOKE-02) [126] | SG + pembro + platinum-based | II | 224 | First-line metastatic NSCLC without genomic alterations | ORR, DLTs |
| NCT03971409 (InCITe) [132] | SG + immunotherapy | II | 150 | Metastatic TNBC | ORR |
| Study | Drugs | Phase | No. of pts. | Population | Primary Endpoints |
|---|---|---|---|---|---|
| NCT05239624 (EV-ECLIPSE) [133] | EV + pembrolizumab | II | 23 | LA- or node-positive UC before surgery | pCR rate |
| NCT04963153 [134] | EV + erdafitinib | Ib | 30 | Metastatic UC progressed on platinul and PD1/L1 inhibitors with FGFR2/3 alterations | AEs; RP2D; MTD |
| NCT05524545 (ASPEN-07) [135] | EV + evorpacept (ALX148) | I | 30 | Mettastatic UC progressed on platinul and PD1/L1 inhibitors | DLTs; AEs |
| NCT05775471 [136] | Pembrolizumab + EV followed by pembrolizumab | II | 21 | Neoadjuvant before radical nephroureterectomy for high-risk UTUC followed by adjuvant pembrolizumab | ORR; RFS |
| NCT03924895 (KEYNOTE-905/EV-303) [137] | Pembrolizumab alone or pembrolizumab + EV or nothing | III | 857 | Perioperative treatment before cystectomy in cisplatin-ineligible or cisplatin-declining patients with MIBC | EFS |
| NCT04878029 [138] | EV +cabozantinib | Ib | 32 | Metastatic UC after progression on platine and ICIs | RP2D |
| NCT04960709 (VOLGA) [139] | Durva + treme + EV vs. durva + EV | III | 830 | MIBC ineligible for cisplatin or who refused cisplatin | pCR rate; EFS; AEs |
| NCT04223856 (EV-302) [140] | EV + pembrolizumab vs. chemotherapy | III | 990 | Previously untreated LA or metastatic UC | PFS; OS |
| NCT04700124 (KEYNOTE-B15/EV-304) [141] | Perioperative EV + pembrolizumab vs. neoadjuvant chemotherapy | III | 784 | Cisplatin-eligible MIBC | EFS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoury, R.; Saleh, K.; Khalife, N.; Saleh, M.; Chahine, C.; Ibrahim, R.; Lecesne, A. Mechanisms of Resistance to Antibody-Drug Conjugates. Int. J. Mol. Sci. 2023, 24, 9674. https://doi.org/10.3390/ijms24119674
Khoury R, Saleh K, Khalife N, Saleh M, Chahine C, Ibrahim R, Lecesne A. Mechanisms of Resistance to Antibody-Drug Conjugates. International Journal of Molecular Sciences. 2023; 24(11):9674. https://doi.org/10.3390/ijms24119674
Chicago/Turabian StyleKhoury, Rita, Khalil Saleh, Nadine Khalife, Mohamad Saleh, Claude Chahine, Rebecca Ibrahim, and Axel Lecesne. 2023. "Mechanisms of Resistance to Antibody-Drug Conjugates" International Journal of Molecular Sciences 24, no. 11: 9674. https://doi.org/10.3390/ijms24119674
APA StyleKhoury, R., Saleh, K., Khalife, N., Saleh, M., Chahine, C., Ibrahim, R., & Lecesne, A. (2023). Mechanisms of Resistance to Antibody-Drug Conjugates. International Journal of Molecular Sciences, 24(11), 9674. https://doi.org/10.3390/ijms24119674





