Dry Reforming of Methane over 5%Ni/Ce1-xTixO2 Catalysts Obtained via Synthesis in Supercritical Isopropanol
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Textural Properties of the Samples by N2 Adsorption
2.2. Characterization of the Structural Features of the Samples by XRD and Raman Spectroscopy
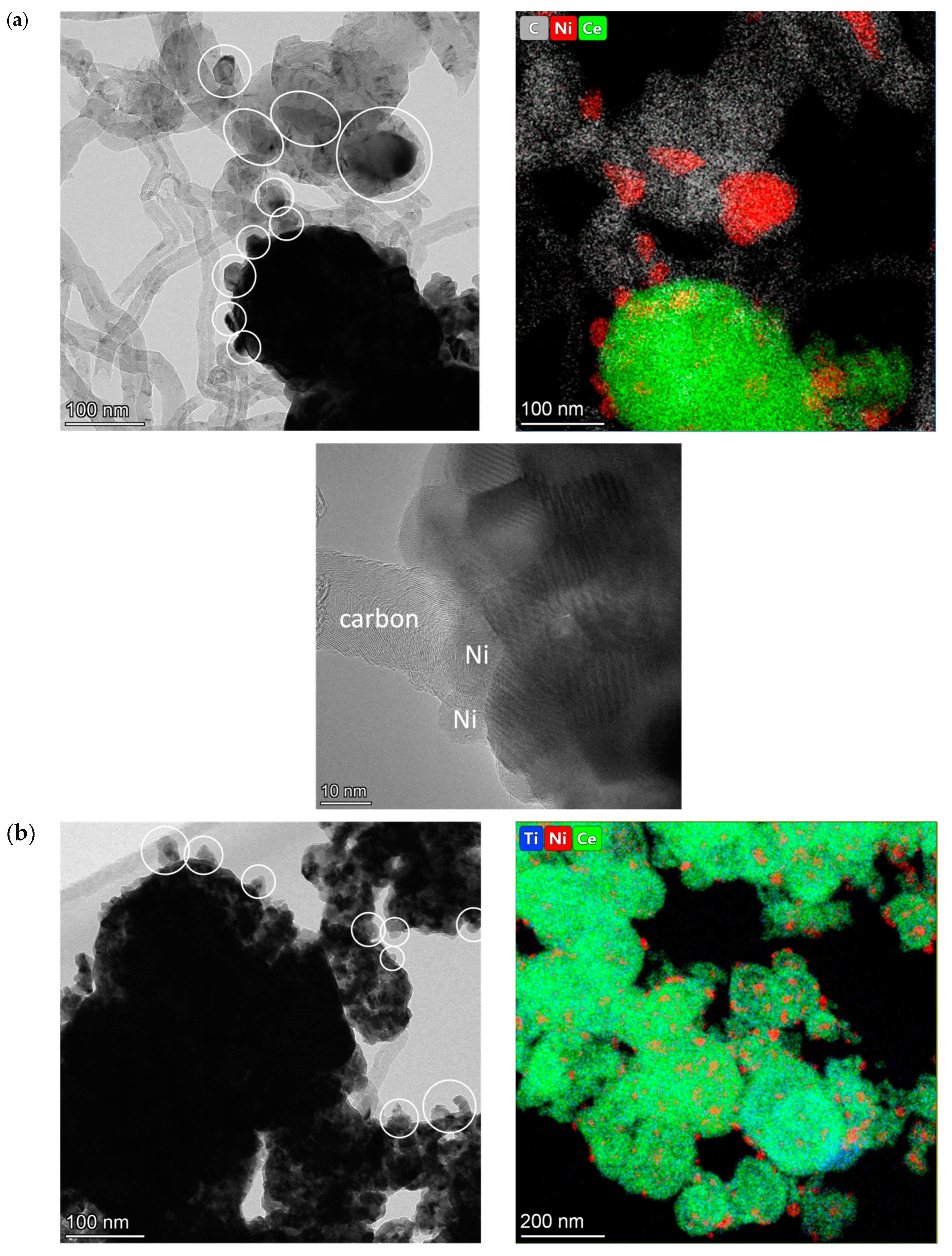
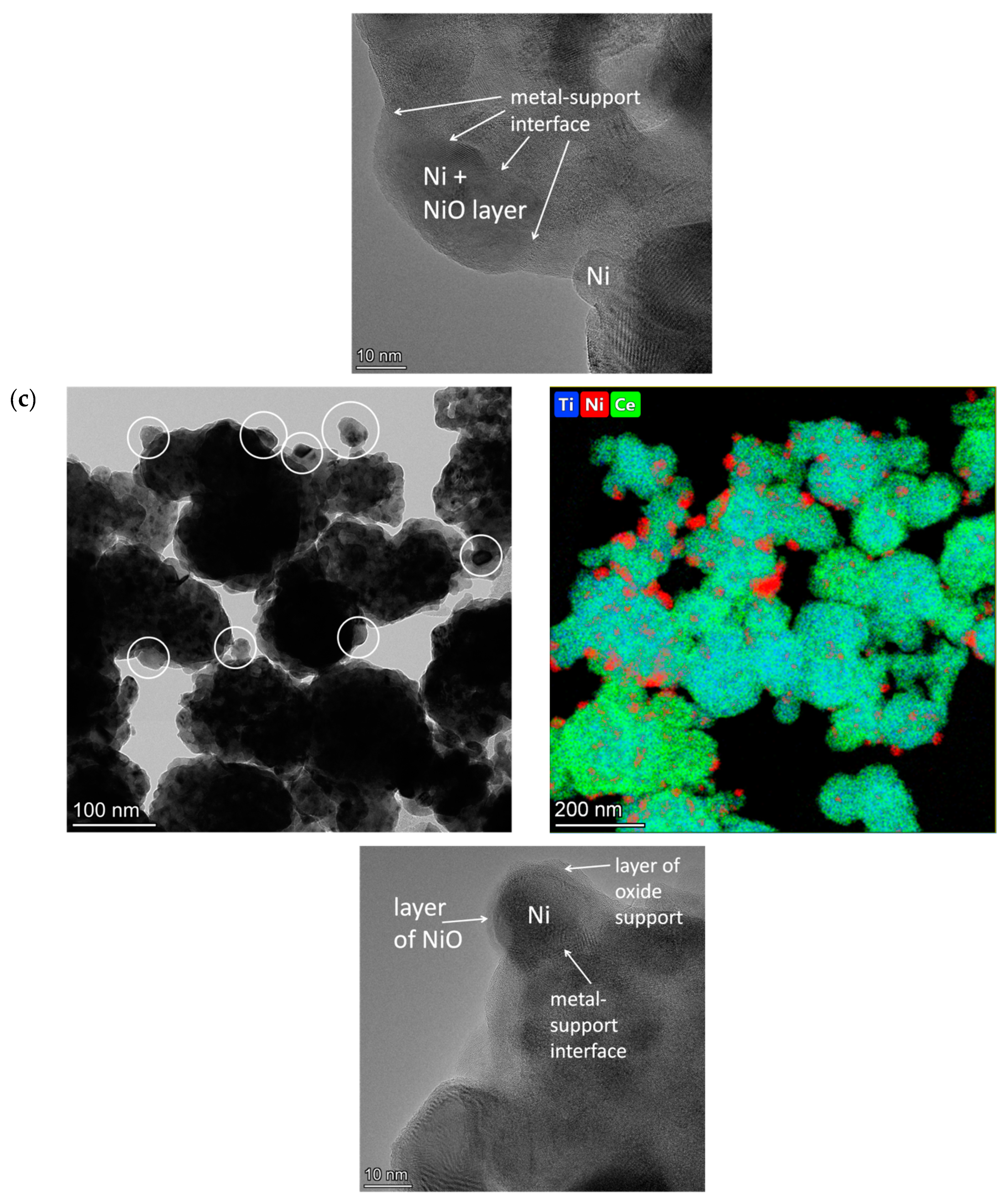
2.3. Characterization of the Morphology of Fresh Catalysts by HRTEM
2.4. Characterization of the Surface Sites of Catalysts by FTIR Spectroscopy of Adsorbed CO
2.5. Characterization of Sample Reducibility by TPR-H2
2.6. Characterization of the Oxygen Mobility of the Samples by Isotope Exchange with C18O2
2.7. Catalytic Studies in Dry Reforming of Methane
2.8. Characterization of Ni-CeTi0.45 and Ni-CeTi0.35 by XRD during In Situ Reduction by H2
3. Methods and Materials
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Catalytic Studies in Methane Dry Reforming
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usman, M.; Daud, W.M.A.W.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Esmaeilkhanian, A.; Bazli, L.; Eskandarinezhad, S.; Khaksar, S.; Shafiee, P.; Yusuf, M.; Abdullah, B.; Salahshour, P.; Sadeghi, F. A review on recent advances in dry reforming of methane over Ni- and Co-based nanocatalysts. Int. J. Hydrogen Energy 2022, 47, 42213–42233. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4-CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. CO2 reforming of CH4. Catal. Rev. 1999, 41, 1–42. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Inaba, M.; Tsunoda, T.; Hamakawa, S.; Suzuki, K.; Hayakawa, T. Dry reforming of methane over supported noble metals: A novel approach to preparing catalysts. Catal. Commun. 2003, 4, 493–498. [Google Scholar] [CrossRef]
- Hu, Y.H.; Ruckenstein, E. Catalytic Conversion of Methane to Synthesis Gas by Partial Oxidation and CO2 Reforming. Adv. Catal. 2004, 48, 297–345. [Google Scholar] [CrossRef]
- Marinho, A.L.A.; Toniolo, F.S.; Noronha, F.B.; Epron, F.; Duprez, D.; Bion, N. Highly active and stable Ni dispersed on mesoporous CeO2-Al2O3 catalysts for production of syngas by dry reforming of methane. Appl. Catal. B 2021, 281, 119459. [Google Scholar] [CrossRef]
- Radlik, M.; Adamowska-Teyssier, M.; Krzton, A.; Kozieł, K.; Krajewski, W.; Turek, W.; Da Costa, P. Dry reforming of methane over Ni/Ce0.62Zr0.38O2 catalysts: Effect of Ni loading on the catalytic activity and on H2/CO production. Comptes Rendus Chim. 2015, 18, 1242–1249. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Zavašnik, J.; Djinovi’c, P.; Efstathiou, A.M. Dry reforming of methane over Ni/Ce0.8Ti0.2O2-δ: The effect of Ni particle size on the carbon pathways studied by transient and isotopic techniques. Appl. Catal. B 2021, 296, 120321. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Promotion by poisoning. Stud. Surf. Sci. Catal. 1991, 68, 85–101. [Google Scholar] [CrossRef]
- Zhang, Q.; Akri, M.; Yang, Y.; Qiao, B. Atomically dispersed metals as potential coke-resistant catalysts for dry reforming of methane. Cell Rep. Phys. Sci. 2023, 4, 101310. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Damaskinos, C.M.; Djinović, P.; Pintar, A.; Efstathiou, A.M. Dry reforming of CH4 over NiCo/Ce0.75Zr0.25O2-δ: The effect of Co on the site activity and carbon pathways studied by transient techniques. Catal. Commun. 2021, 149, 106237. [Google Scholar] [CrossRef]
- Torimoto, M.; Sekine, Y. Effects of alloying for steam or dry reforming of methane: A review of recent studies. Catal. Sci. Technol. 2022, 12, 3387. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Djinovi, P.; Davlyatova, L.F.; Pintar, A.; Efstathiou, A.M. Origin and reactivity of active and inactive carbon formed during DRM over Ni/Ce0.38Zr0.62O2-δ studied by transient isotopic techniques. Catal. Today 2018, 299, 201–211. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sust. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Cai, X.; Hu, Y.H. Advances in catalytic conversion of methane and carbon dioxide to highly valuable products. Energy Sci. Eng. 2019, 7, 4–29. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, G.; Li, M.; Wu, Y.; Nie, H.; Li, D. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Rogers, J.L.; Mangarella, M.C.; D’Amico, A.D.; Gallagher, J.R.; Dutzer, M.R.; Stavitski, E.; Miller, J.T.; Sievers, C. Differences in the Nature of Active Sites for Methane Dry Reforming and Methane Steam Reforming over Nickel Aluminate Catalysts. ACS Catal. 2016, 6, 5873–5886. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Deo, G. Process and catalyst improvements for the dry reforming of methane. Chem. Eng. Sci. 2023, 276, 118767. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Peng, X.X.; Yao, L.; Deng, X.J.; Dong, H.Y.; Tong, D.M. Synthesis gas production from CO2 reforming of methane over Ni-Ce/SiO2 catalyst: The effect of calcination ambience. Int. J. Hydrogen Energy 2013, 38, 117–126. [Google Scholar] [CrossRef]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Yuan, B.; Zhu, T.; Han, Y.; Zhang, X.; Wang, M.; Li, C. Deactivation Mechanism and Anti-Deactivation Measures of Metal Catalyst in the Dry Reforming of Methane: A Review. Atmosphere 2023, 14, 770. [Google Scholar] [CrossRef]
- Ren, Y.; Ma, Y.-Y.; Mo, W.-L.; Guo, J.; Liu, Q.; Fan, X.; Zhang, S.-P. Research Progress of Carbon Deposition on Ni-Based Catalyst for CO2-CH4 Reforming. Catalysts 2023, 13, 647. [Google Scholar] [CrossRef]
- Sadovskaya, E.M.; Ivanova, Y.A.; Pinaeva, L.G.; Grasso, G.; Kuznetsova, T.G.; van Veen, A.; Sadykov, V.A.; Mirodatos, C. Kinetics of Oxygen Exchange over CeO2-ZrO2 Fluorite-Based Catalysts. J. Phys. Chem. A 2007, 111, 4498–4505. [Google Scholar] [CrossRef] [PubMed]
- Damaskinos, C.M.; Vasiliades, M.A.; Efstathiou, A.M. The effect of Ti4+ dopant in the 5 wt% Ni/Ce1-xTixO2-δ catalyst on the carbon pathways of dry reforming of methane studied by various transient and iso-topic techniques. Appl. Catal. A 2019, 579, 116–129. [Google Scholar] [CrossRef]
- Leitenburg, C.; Trovarelli, A.; Kaspar, J. A Temperature-Programmed and Transient Kinetic Study of CO2 Activation and Methanation over CeO2 Supported Noble Metals. J. Catal. 1997, 166, 98–107. [Google Scholar] [CrossRef]
- Luo, M.; Chen, J.; Chen, L.; Lu, J.; Feng, Z.; Li, C. Structure and Redox Properties of CexTi1-xO2 Solid Solution. Chem. Mater. 2001, 13, 197–202. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; Chen, X.; Rui, N.; Betancourt, L.E.; Lin, L.; Xu, W.; Sun, C.; Abeykoon, A.M.M.; Rodriguez, J.A.; et al. Effects of Zr Doping into Ceria for the Dry Reforming of Methane over Ni/CeZrO2 Catalysts: In Situ Studies with XRD, XAFS, and APXPS. ACS Catal. 2020, 10, 3274–3284. [Google Scholar] [CrossRef]
- Makri, M.M.; Vasiliades, M.A.; Petallidou, K.C.; Efstathiou, A.M. Effect of support composition on the origin and reactivity of carbon formed during dry reforming of methane over 5wt%Ni/Ce1−xMxO2−I (M = Zr4+, Pr3+) catalysts. Catal. Today 2016, 259, 150–164. [Google Scholar] [CrossRef]
- Menegazzo, F.; Pizzolitto, C.; Ghedini, E.; Michele, A.D.; Cruciani, G.; Signoretto, M. Development of La Doped Ni/CeO2 for CH4/CO2 Reforming. J. Carbon Res. 2018, 4, 60. [Google Scholar] [CrossRef]
- Nagaoka, K.; Okamura, M.; Akia, K. Titania supported ruthenium as a coking resistant catalyst for high pressure dry reforming of methane. Catal. Commun. 2001, 2, 255–260. [Google Scholar] [CrossRef]
- Bradford, M.C.J.; Vannice, M.A. The role of metal-support interactions in CO2 reforming of CH4. Catal. Today 1999, 50, 87–96. [Google Scholar] [CrossRef]
- Dutta, G.; Waghmare, U.V.; Baidya, T.; Hegde, M.S.; Priolkar, K.R.; Sarode, P.R. Origin of Enhanced Reducibility/Oxygen Storage Capacity of Ce1-xTixO2 Compared to CeO2 or TiO2. Chem. Mater. 2006, 18, 3249–3256. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.M.; Won, J.M.; Yang, H.J.; Hong, S.C. Effect of Ce/Ti ratio on the catalytic activity and stability of Ni/CeO2–TiO2 catalyst for dry reforming of methane. Chem. Eng. J. 2015, 280, 433–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wen, X.; Liu, Y. Partial oxidation of methane over Ni/Ce-Ti-O catalysts. Chem. Eng. J. 2006, 121, 115–123. [Google Scholar] [CrossRef]
- Ye, J.L.; Wang, Y.Q.; Liu, Y.; Wang, H. Steam reforming of ethanol over Ni/CexTi1-xO2 catalysts. Int. J. Hydrogen Energy 2008, 33, 6602–6611. [Google Scholar] [CrossRef]
- Simonov, M.; Bespalko, Y.; Smal, E.; Valeev, K.; Fedorova, V.; Krieger, T.; Sadykov, V. Nickel-Containing Ceria-Zirconia Doped with Ti and Nb. Effect of Support Composition and Preparation Method on Catalytic Activity in Methane Dry Reforming. Nanomaterials 2020, 10, 1281. [Google Scholar] [CrossRef]
- Arapova, M.; Smal, E.; Bespalko, Y.; Fedorova, V.; Valeev, K.; Cherepanova, S.; Ischenko, A.; Sadykov, V.; Simonov, M. Ethanol dry reforming over Ni supported on modified ceria-zirconia catalysts: The effect of Ti and Nb dopants. Int. J. Hydrogen Energy 2021, 46, 39236–39250. [Google Scholar] [CrossRef]
- Aymonier, C.; Loppinet-Serani, A.; Reveron, H.; Garrabos, Y.; Cansell, F. Review of supercritical fluids in inorganic materials science. J. Supercrit. Fluids 2006, 38, 242–251. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Lu, F.; Jiang, B.; Wang, J.; Huang, Z.; Liao, Z.; Yang, Y.; Zheng, J. Promotional effect of Ti doping on the ketonization of acetic acid over a CeO2 catalyst. RSC Adv. 2017, 7, 22017–22026. [Google Scholar] [CrossRef]
- McBride, J.R.; Hass, K.C.; Poindexter, B.D.; Weber, W.H. Raman and X-ray studies of Ce1-xRExO2-y, where RE = La, Pr, Nd, Eu, Gd, and Tb. J. Appl. Phys. 1994, 76, 2435–2441. [Google Scholar] [CrossRef]
- Kosacki, I.; Suzuki, T.; Anderson, H.U.; Colomban, P. Raman scattering and lattice defects in nano-crystalline CeO2 thin films. Solid State Ion. 2002, 149, 99–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, D.; Wei, S.; Zhang, Y. Determination of active site densities and mechanisms for soot combustion with O2 on Fe-doped CeO2 mixed oxides. J. Catal. 2010, 276, 16–23. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, Q.; Jiang, Z. Nanosized CuO–ZrxCe1−xOy aerogel catalysts prepared by ethanol supercritical drying for catalytic deep oxidation of benzene. Powder Technol. 2009, 194, 109–114. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Yuan, Q.; Feng, W.; Xu, J.; Sun, L.; Song, W.; Yan, C. Sustainable and Facile Route to Nearly Monodisperse Spherical Aggregates of CeO2 Nanocrystals with Ionic Liquids and Their Catalytic Activities for CO Oxidation. J. Phys. Chem. C 2008, 112, 18405–18411. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Zhang, M.-S.; Yin, Z.; Chen, Q.; Xijun, W.; Xiaoli, J. Raman scattering by nanophase titanium dioxide. Ferroelectrics 1995, 168, 131–137. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, W.; Du, Z.; Huang, Y. Structural transformation in nanophase titanium dioxide. Mod. Phys. Lett. B 1999, 13, 167–174. [Google Scholar] [CrossRef]
- Romero-Nunez, A.; Dıaz, G. High oxygen storage capacity and enhanced catalytic performance of NiO/NixCe1-xO2 nanorods: Synergy between Ni-doping and 1D morphology. RSC Adv. 2015, 5, 54571. [Google Scholar] [CrossRef]
- Busca, G.; Ramis, G.; Amores, J.M.G.; Escribano, V.S.; Piaggio, P. FT Raman and FTIR studies of titanias and metatitanate powders. J. Chem. Soc. Faraday Trans. 1994, 90, 3181–3190. [Google Scholar] [CrossRef]
- Baraton, M.I.; Busca, G.; Prieto, M.C.; Ricchiardi, G.; Escribano, V.S. On the Vibrational Spectra and Structure of FeCrO3 and of the Ilmenite-Type Compounds CoTiO3 and NiTiO3. J. Solid State Chem. 1994, 112, 9–14. [Google Scholar] [CrossRef]
- Bespalko, Y.; Smal, E.; Simonov, M.; Valeev, K.; Fedorova, V.; Krieger, T.; Cherepanova, S.; Ishchenko, A.; Rogov, V.; Sadykov, V. Novel Ni/Ce(Ti)ZrO2 Catalysts for Methane Dry Reforming Pre-pared in Supercritical Alcohol Media. Energies 2020, 13, 3365. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Kuznetsova, T.G.; Alikina, G.M.; Frolova, Y.V.; Lukashevich, A.I.; Potapova, Y.V.; Muzykantov, V.S.; Rogov, V.A.; Kriventsov, V.V.; Kochubei, D.I.; et al. Ceria-based fluorite-like oxide solid solutions as catalysts of methane selective oxidation into syngas by the lattice oxygen: Synthesis, characterization and performance. Catal. Today 2004, 93–95, 45–53. [Google Scholar] [CrossRef]
- Kuznetsova, T.G.; Sadykov, V.A.; Moroz, E.M.; Trukhan, S.N.; Paukshtis, E.A.; Kolomiichuk, V.N.; Burgina, E.B.; Zaikovskii, V.I.; Fedotov, M.A.; Lunin, V.V.; et al. Preparation of Ce-Zr-O composites by a polymerized complex method. Stud. Surf. Sci. Catal. 2002, 143, 659–667. [Google Scholar] [CrossRef]
- Romero-Galarza, A.; Dahlberg, K.A.; Chen, X.; Schwank, J.W. Crystalline structure refinements and properties of Ni/TiO2 and Ni/TiO2-Ce catalysts and application to catalytic reaction of “CO + NO”. Appl. Catal. A 2014, 478, 21–29. [Google Scholar] [CrossRef]
- Busca, G.; Saussey, H.; Saur, O.; Lavalley, J.-C.; Lorenzelli, V. FT-IR characterization of the surface acidity of different titanium dioxide anatase preparations. Appl. Catal. 1985, 14, 245. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Klissurski, D.G. Surface Chemistry of Titania (Anatase) and Titania-supported Catalysts. Chem. Soc. Rev. 1996, 25, 61–69. [Google Scholar] [CrossRef]
- Manoilova, O.V.; Dakka, J.; Sheldon, R.A.; Tsyganenko, A.A. Infrared study of Ti-containing zeolites using CO as a probe molecule. Stud. Surf. Sci. Catal. 1995, 94, 163–170. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Kuznetsova, T.G.; Alikina, G.M.; Frolova, Y.V.; Lukashevich, A.I.; Muzykantov, V.S.; Rogov, V.A.; Batuev, L.C.; Kriventsov, V.V.; Kochubei, D.I.; et al. Ceria-based fluorite-like oxide solid solutions promoted by precious metals as catalysts of methane transformation into syngas. In New Topics in Catalysis Research; McReynolds, D.K., Ed.; Nova Science Publishers: New York, NY, USA, 2007; pp. 97–197. [Google Scholar]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of oxide surfaces and zeolites by carbon monoxide as an IR probe molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar] [CrossRef]
- Bernal, S.; Calvino, J.J.; Cauqui, M.A.; Gatica, J.M.; López Cartes, C.; Pérez Omil, J.A.; Pintado, J.M. Some contributions of electron microscopy to the characterization of the strong metal–support interaction effect. Catal. Today 2003, 77, 385–406. [Google Scholar] [CrossRef]
- Bernal, S.; Botana, F.J.; Calvino, J.J.; Lopez, C.; Perez-Omil, J.A.; Rodriguez-Izquierdo, J.M. High-resolution electron microscopy investigation of metal-support interactions in Rh/TiO2. J. Chem. Soc. Faraday Trans. 1996, 92, 2799–2809. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Pd/CeO2–TiO2 catalyst for CO oxidation at low temperature: A TPR study with H2 and CO as reducing agents. J. Catal. 2004, 225, 267–277. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Wu, F.; Zhang, L.; Liu, X. Dispersion–precipitation synthesis of nanorod Mn3O4 with high reducibility and the catalytic complete oxidation of air pollutants. Catal. Commun. 2013, 31, 52–56. [Google Scholar] [CrossRef]
- Shan, W.; Luo, M.; Ying, P.; Shen, W.; Li, C. Reduction property and catalytic activity of Ce1-XNiXO2 mixed oxide catalysts for CH4 oxidation. Appl. Catal. A 2003, 246, 1–9. [Google Scholar] [CrossRef]
- Montoya, J.A.; Romero-Pascual, E.; Gimon, C.; Del Angel, P.; Monzón, A. Methane reforming with CO2 over Ni/ZrO2-CeO2 catalysts prepared by sol-gel. Catal. Today 2000, 63, 71–85. [Google Scholar] [CrossRef]
- Smal, E.; Bespalko, Y.; Arapova, M.; Fedorova, V.; Valeev, K.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Glazneva, T.; Sadykov, V.; et al. Car-bon Formation during Methane Dry Reforming over Ni-Containing Ceria-Zirconia Catalysts. Nanomaterials 2022, 12, 3676. [Google Scholar] [CrossRef]
- Frusteri, F.; Freni, S.; Chiodo, V.; Donato, S.; Bonura, G.; Cavallaro, S. Steam and auto-thermal reforming of bio-ethanol over MgO and CeO2 Ni-supported catalysts. Int. J. Hydrogen Energy 2006, 31, 2193–2199. [Google Scholar] [CrossRef]
- Li, M.; van Veen, A.C. Tuning the catalytic performance of Ni-catalysed dry reforming of methane and carbon deposition via Ni-CeO2-x interaction. Appl. Catal. B 2018, 237, 641–648. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Romano, C.; Boaro, M.; Di Bartolomeo, E.; Kesavan, J.K.; Kumar, S.S.; Selvakumar, K. Dry reforming of methane over Ni supported on doped CeO2: New insight on the role of dopants for CO2 activation. J. CO2 Util. 2019, 30, 63–78. [Google Scholar] [CrossRef]
- Taufiq-Yap, Y.H.; Sudarno; Rashid, U.; Zainal, Z. CeO2–SiO2 supported nickel catalysts for dry re-forming of methane toward syngas production. Appl. Catal. A 2013, 468, 359–369. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Daza, L. Long-term stability test of Ni-based catalyst in carbon dioxide reforming of methane. Appl. Catal. A 2014, 474, 107–113. [Google Scholar] [CrossRef]
- Fedorova, V.; Bespalko, Y.; Arapova, M.; Smal, E.; Valeev, K.; Prosvirin, I.; Sadykov, V.; Parkhomenko, K.; Roger, A.-C.; Simonov, M. Ethanol Dry Reforming over Bimetallic Ni-Containing Catalysts Based on Ceria-Zirconia for Hydrogen Production. ChemCatChem 2023, 15, e01491. [Google Scholar] [CrossRef]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the Acid–Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Fedorova, V.; Simonov, M.; Valeev, K.; Bespalko, Y.; Smal, E.; Eremeev, N.; Sadovskaya, E.; Krieg-er, T.; Ishchenko, A.; Sadykov, V. Kinetic regularities of methane dry reforming reaction on nickel-containing modified ceria–zirconia. Energies 2021, 14, 2973. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Uvarov, N.F. Methods of isotopic relaxations for estimation of oxygen diffusion coefficients in solid electrolytes and materials with mixed ionic-electronic conductivity. Russ. J. Electrochem. 2015, 51, 458–467. [Google Scholar] [CrossRef]

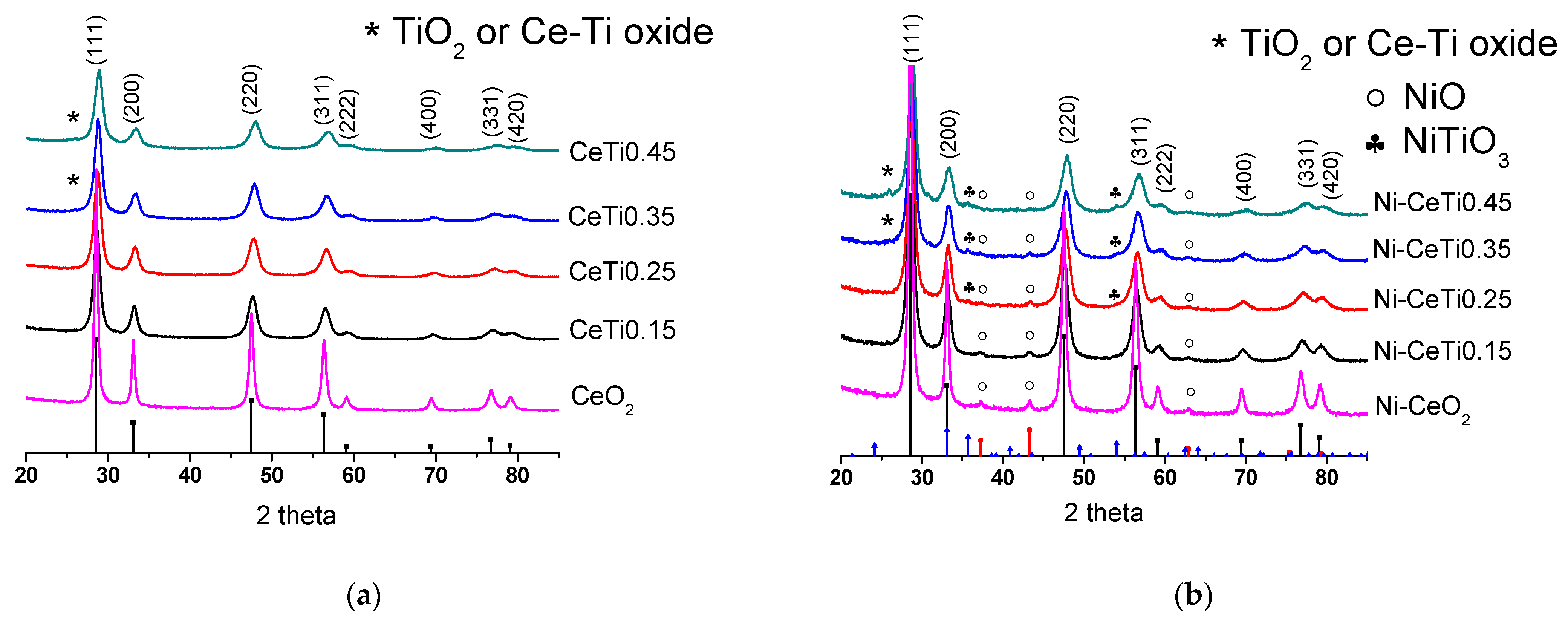




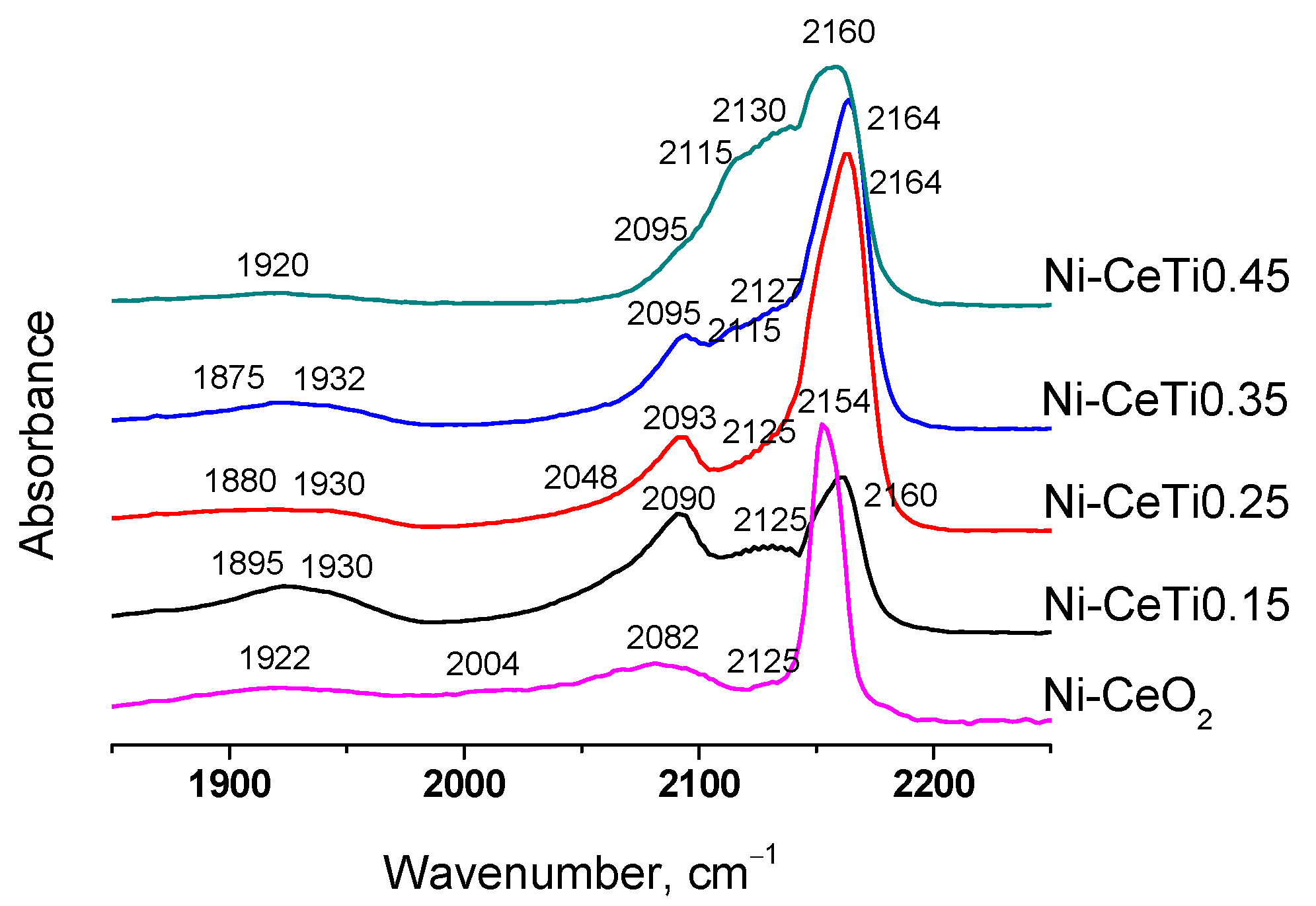

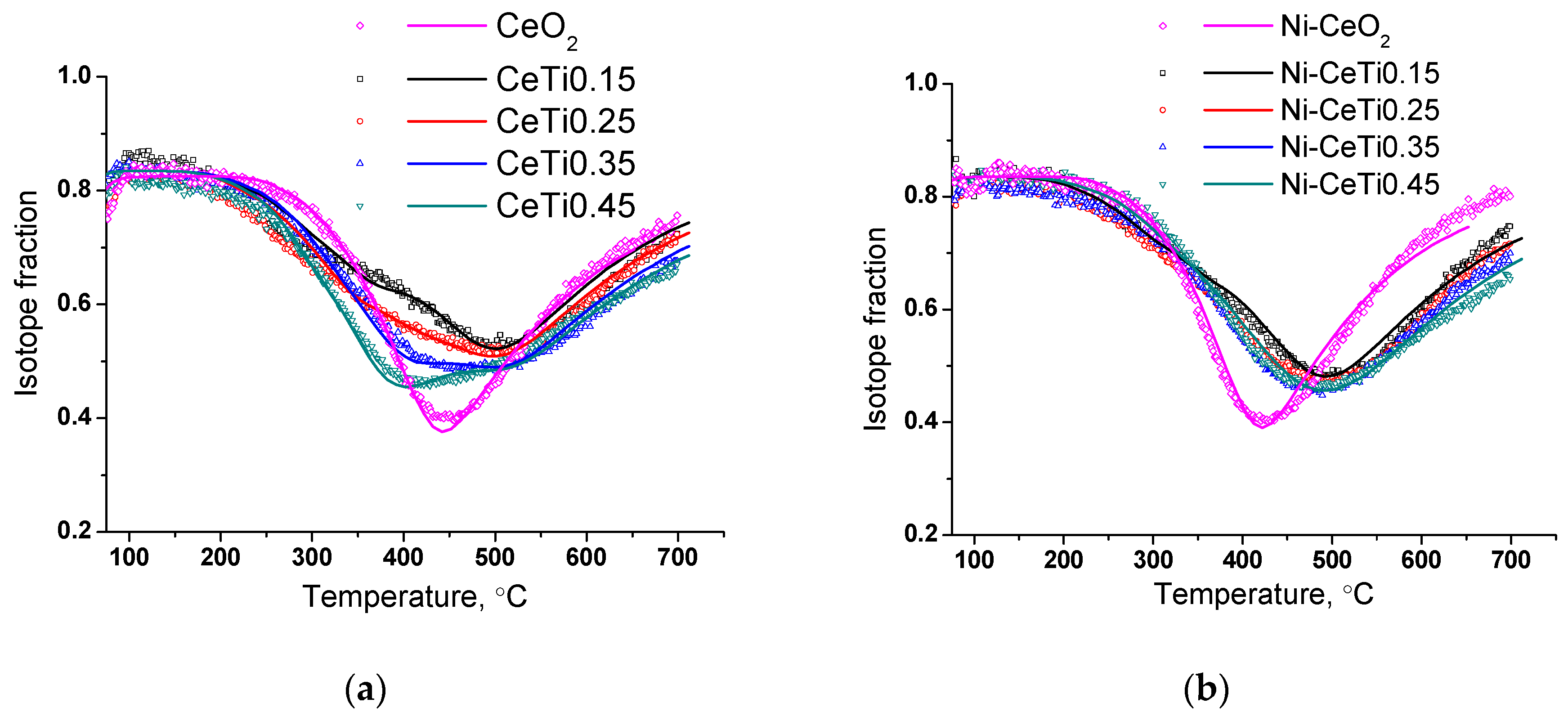
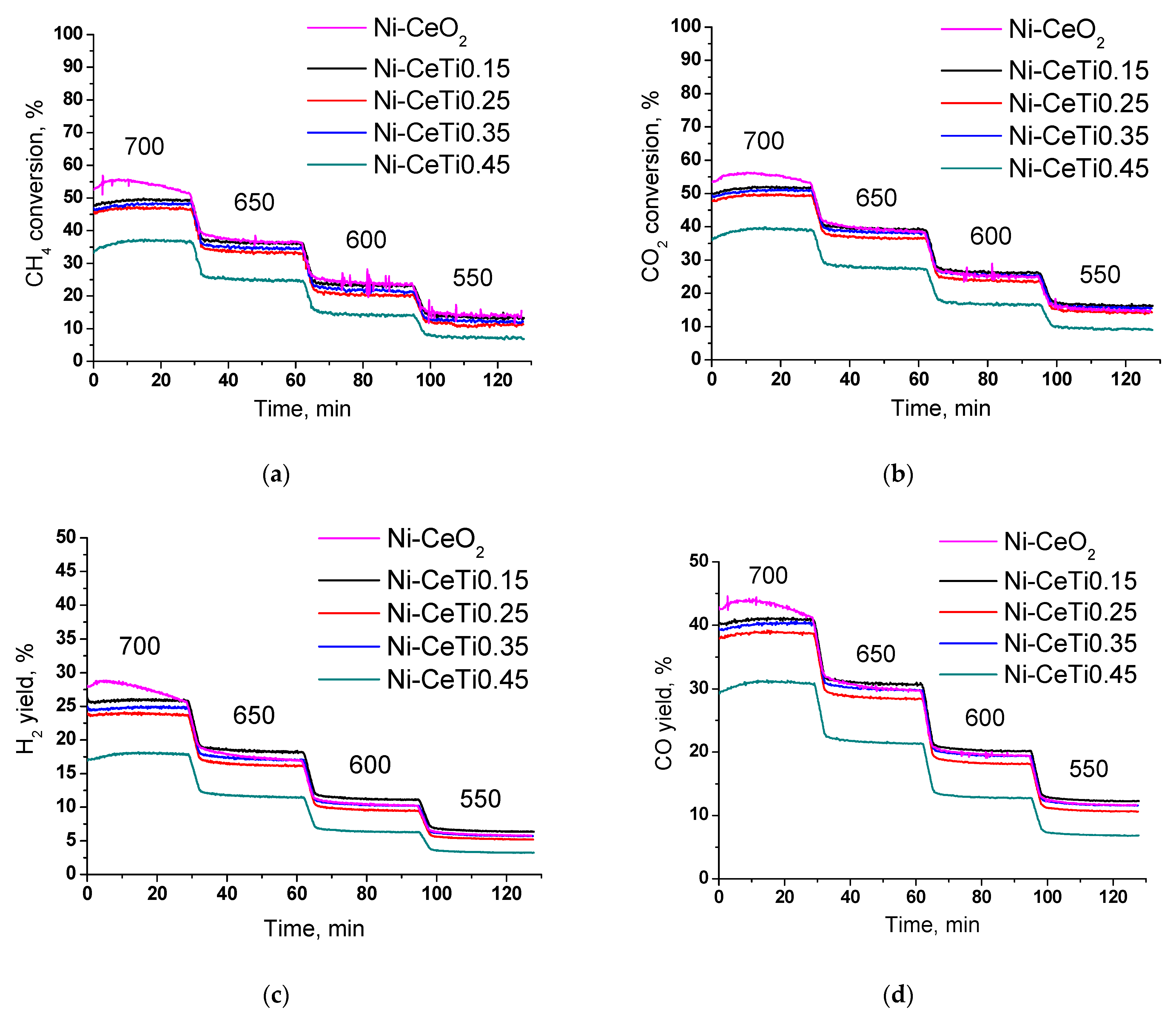



| № | Abbreviation | Composition | SBET *, m2/g | Vpore *, cm3/g | a, Å | d, nm |
|---|---|---|---|---|---|---|
| Supports | ||||||
| 1 | CeO2 | CeO2 | 35 | 0.085 | 5.411(2) | 29 |
| 2 | CeTi0.15 | Ce0.85Ti0.15O2 | 26 | 0.157 | 5.400(1) | 12.6(5) |
| 3 | CeTi0.25 | Ce0.75Ti0.25O2 | 25 | 0.179 | 5.396(1) | 12.2(4) |
| 4 | CeTi0.35 | Ce0.65Ti0.35O2 | 18 | 0.146 | 5.394(1) | 11.9(1) |
| 5 | CeTi0.45 | Ce0.55Ti0.45O2 | 17 | 0.174 | 5.388(2) | 10.8(2) |
| Catalysts | ||||||
| 6 | Ni-CeO2 | 5%Ni/CeO2 | 25 | 0.052 | 5.410(1) | 29 |
| 7 | Ni-CeTi0.15 | 5%Ni/Ce0.85Ti0.15O2 | 18 | 0.164 | 5.402(1) | 14.5(2) |
| 8 | Ni-CeTi0.25 | 5%Ni/Ce0.75Ti0.25O2 | 17 | 0.184 | 5.398(1) | 13.5(1) |
| 9 | Ni-CeTi0.35 | 5%Ni/Ce0.65Ti0.35O2 | 13 | 0.163 | 5.394(2) | 13.0(4) |
| 10 | Ni-CeTi0.45 | 5%Ni/Ce0.55Ti0.45O2 | 12 | 0.181 | 5.391(2) | 12.1(5) |
| Sample | νCO, cm−1 | Sites Amount, μmol/g | Bridging CO Amount, μmol/g | On-Top CO Amount, μmol/g | Total Amount, μmol/g |
|---|---|---|---|---|---|
| Ni-CeO2 | 1922 | 9 | 9 | 13 | 22 |
| 2004 | 1 | ||||
| 2082 | 12 | ||||
| Ni-CeTi0.15 | 1895 | 7 | 10 | 16 | 26 |
| 1930 | 3 | ||||
| 2090 | 16 | ||||
| Ni-CeTi0.25 | 1880 | 4 | 6 | 9 | 15 |
| 1930 | 2 | ||||
| 2048 | 2 | ||||
| 2093 | 7 | ||||
| Ni-CeTi0.35 | 1875 | 2 | 5 | 11 | 16 |
| 1932 | 3 | ||||
| 2095 | 11 | ||||
| Ni-CeTi0.45 | 1920 | 2 | 2 | 4 | 6 |
| 2095 | 4 |
| № | Sample | H2 Consumption, mmol H2/gcat | Ni Reducibility, % | |
|---|---|---|---|---|
| Oxide | Ni/Oxide | |||
| 1 | CeO2 | 0.82 | 1.5 | 85 |
| 2 | CeTi0.15 | 1.28 | 1.96 | 88 |
| 3 | CeTi0.25 | 1.5 | 2.12 | 82 |
| 4 | CeTi0.35 | 1.68 | 2.28 | 80 |
| 5 | CeTi0.45 | 1.77 | 2.38 | 82 |
| Sample | D*fast|600 °C [cm2/s] | θfast [%] | D*slow|600 °C [cm2/s] | θslow [%] | D*average|600 °C [cm2/s] |
|---|---|---|---|---|---|
| Supports | |||||
| CeO2 | - | - | 2.2 × 10−13 | 100 | 2.2 × 10−13 |
| CeTi0.15 | ≈3 × 10−12 | 10 | 2.2 × 10−13 | 90 | 5 × 10−13 |
| CeTi0.25 | 15 | 85 | 6.4 × 10−13 | ||
| CeTi0.35 | 23 | 77 | 8.6 × 10−13 | ||
| CeTi0.45 | 30 | 70 | 11 × 10−13 | ||
| Catalysts | |||||
| Ni-CeO2 | - | - | 6.6 × 10−13 | 100 | 6.6 × 10−13 |
| Ni-CeTi0.15 | ≈3 × 10−12 | 3 | 4.1 × 10−13 | 97 | 4.9 × 10−13 |
| Ni-CeTi0.25 | 3 | 3.3 × 10−13 | 97 | 4.1 × 10−13 | |
| Ni-CeTi0.35 | 3 | 97 | 4.1 × 10−13 | ||
| Ni-CeTi0.45 | 5 | 2.2 × 10−13 | 95 | 3.6 × 10−13 | |
| Sample | mgC/gcat | Reaction Conditions | Reference |
|---|---|---|---|
| Ni-CeO2 | 321 | 15% CH4 + 15% CO2 + N2, 700 °C, 8 h | This work |
| Ni-CeTi0.45 | 66.1 | ||
| 5%Ni/ | 1314 (25 h) | 15% CH4 + 15% CO2 + N2, 700 °C, 25 and 3 h | [71] * |
| Ce0.75Ti0.05Nb0.05Zr0.15O2 | 998 (3 h) | ||
| 5% Ni/CeO2 | 241 | 40% CH4 + 40% CO2 + N2, 800 °C, 50 h | [74] * |
| 5% Ni/Ce0.85La0.15O2 | 20.4 | ||
| 5% Ni/CeO2 | 80.4 | 20% CH4 + 20% CO2 + He, 750 °C, 12 h | [26] |
| 5% Ni/Ce0.8Ti0.2O2 | 0.3 | ||
| 5% Ni/Ce0.5Ti0.5O2 | 66.4 | ||
| 5% Ni/CeO2 | 8.9 | 50%CH4 + 50%CO2, 700 °C, 24 h | [29] |
| 5% Ni/75%CeO2-25%ZrO2 | 83 | ||
| 8% Ni/ZrO2 | 370 | 55% CH4 + 35% CO2 + Ar, 750 °C, 10 h | [76] * |
| 8% Ni/8.1% La2O3-ZrO2 | 661 | ||
| 8% Ni/18.8% CeO2-ZrO2 | 695 | ||
| La-NiMgAlO | 690 (750 °C) | CH4:CO2 = 1:1, 650, 700 and 750 °C, 300 h | [77] * |
| 540 (700 °C) | |||
| 420 (650 °C) | |||
| 5% Ni/SiO2 | 1857 | 50% CH4 + 50% CO2, 800 °C, 10 h | [75] * |
| 5% Ni/CeO2 | 667 | ||
| 5% Ni/30%CeO2–SiO2 | 220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smal, E.; Bespalko, Y.; Arapova, M.; Fedorova, V.; Valeev, K.; Eremeev, N.; Sadovskaya, E.; Krieger, T.; Glazneva, T.; Sadykov, V.; et al. Dry Reforming of Methane over 5%Ni/Ce1-xTixO2 Catalysts Obtained via Synthesis in Supercritical Isopropanol. Int. J. Mol. Sci. 2023, 24, 9680. https://doi.org/10.3390/ijms24119680
Smal E, Bespalko Y, Arapova M, Fedorova V, Valeev K, Eremeev N, Sadovskaya E, Krieger T, Glazneva T, Sadykov V, et al. Dry Reforming of Methane over 5%Ni/Ce1-xTixO2 Catalysts Obtained via Synthesis in Supercritical Isopropanol. International Journal of Molecular Sciences. 2023; 24(11):9680. https://doi.org/10.3390/ijms24119680
Chicago/Turabian StyleSmal, Ekaterina, Yulia Bespalko, Marina Arapova, Valeria Fedorova, Konstantin Valeev, Nikita Eremeev, Ekaterina Sadovskaya, Tamara Krieger, Tatiana Glazneva, Vladislav Sadykov, and et al. 2023. "Dry Reforming of Methane over 5%Ni/Ce1-xTixO2 Catalysts Obtained via Synthesis in Supercritical Isopropanol" International Journal of Molecular Sciences 24, no. 11: 9680. https://doi.org/10.3390/ijms24119680
APA StyleSmal, E., Bespalko, Y., Arapova, M., Fedorova, V., Valeev, K., Eremeev, N., Sadovskaya, E., Krieger, T., Glazneva, T., Sadykov, V., & Simonov, M. (2023). Dry Reforming of Methane over 5%Ni/Ce1-xTixO2 Catalysts Obtained via Synthesis in Supercritical Isopropanol. International Journal of Molecular Sciences, 24(11), 9680. https://doi.org/10.3390/ijms24119680








