Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway
Abstract
1. Introduction
2. Chemoprevention
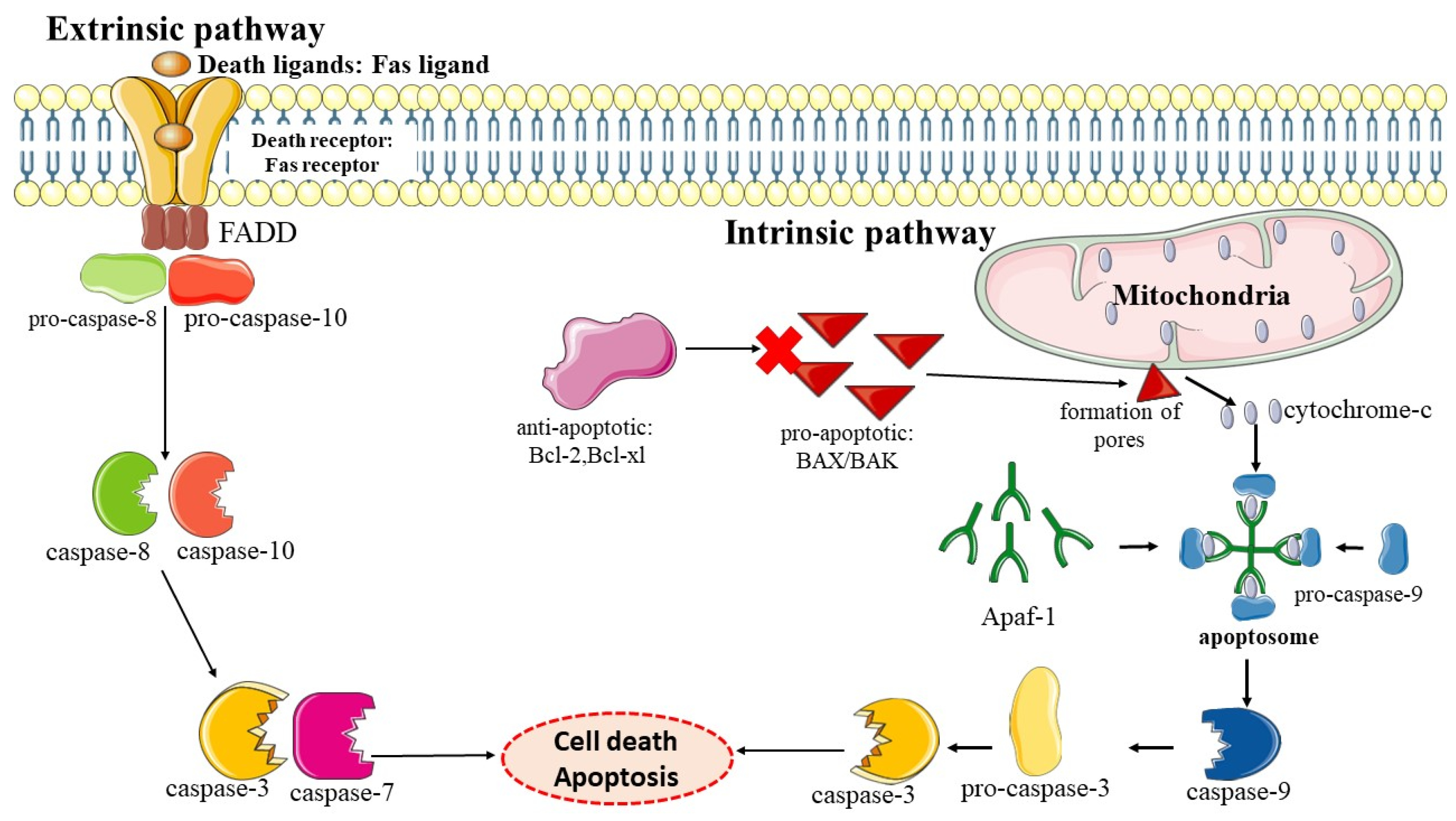
3. Apoptosis
Role of Apoptosis in Chemoprevention
4. Pterostilbene
4.1. Pterostilbene as a Chemoprevention Agent via Its Modulation on the Apoptosis Pathway
4.2. Pterostilbene as an Anti-Cancer Agent Acting via the Modulation of the Apoptosis Pathway
4.3. Other Molecular Mechanisms of Pterostilbene as an Anti-Cancer and Chemopreventive Agent
5. Future Prospects of Using Pterostilbene as a Chemopreventive Agent
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | aberrant crypt foci |
| AOM | azoxymethane |
| ALT | alanine transaminase |
| ALP | alkaline phosphatase |
| AST | aspartate aminotransferase |
| COX-2 | cyclooxygenase-2 |
| DNA | deoxyribonucleic acid |
| DISCs | death-induced-signalling complexes |
| iNOS | inducible nitric oxide synthase |
| LDH | lactate dehydrogenase |
| NTCU | N-nitroso-tris-chloroethylurea |
| ppm | Parts per million |
| ROS | Reactive oxygen species |
| SCC | squamous cell carcinoma |
References
- World Health Organization (WHO). Cancers. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 22 November 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, M.; Ji, M.; Fan, J.; Xie, J.; Wei, X.; Jiang, X.; Xu, J.; Chen, L.; Yin, R. Air Pollution, Genetic Factors, and the Risk of Lung Cancer: A Prospective Study in the UK Biobank. Am. J. Respir. Crit. Care Med. 2021, 204, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, P.; van Holm, N.; Verkasalo, P.K.; Iliadou, A.; Kaprio, J.; Koskenvuo, M.; Pukkala, E.; Skytthe, A.; Hemminki, K. Environmental and Heritable Factors in the Causation of Cancer—Analyses of Cohorts of Twins from Sweden, Denmark, and Finland. N. Engl. J. Med. 2000, 343, 78–85. [Google Scholar] [CrossRef]
- Hamilton, A.S.; Mack, T.M. Puberty and Genetic Susceptibility to Breast Cancer in a Case–Control Study in Twins. N. Engl. J. Med. 2003, 348, 2313–2322. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer Is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Weihe, P.; Spielmann, J.; Kielstein, H.; Henning-Klusmann, J.; Weihrauch-Blüher, S. Childhood Obesity and Cancer Risk in Adulthood. Curr. Obes. Rep. 2020, 9, 204–212. [Google Scholar] [CrossRef]
- Lee, S.; Woo, H.; Lee, J.; Oh, J.-H.; Kim, J.; Shin, A. Cigarette Smoking, Alcohol Consumption, and Risk of Colorectal Cancer in South Korea: A Case-Control Study. Alcohol 2019, 76, 15–21. [Google Scholar] [CrossRef]
- Okunade, K.S. Human Papillomavirus and Cervical Cancer. J. Obstet. Gynaecol. 2020, 40, 602–608. [Google Scholar] [CrossRef]
- Schultz, C.H.; Fairley, R.; Murphy, L.S.-L.; Doss, M. The Risk of Cancer from CT Scans and Other Sources of Low-Dose Radiation: A Critical Appraisal of Methodologic Quality. Prehosp. Disaster Med. 2020, 35, 3–16. [Google Scholar] [CrossRef]
- LoConte, N.K.; Gershenwald, J.E.; Thomson, C.A.; Crane, T.E.; Harmon, G.E.; Rechis, R. Lifestyle Modifications and Policy Implications for Primary and Secondary Cancer Prevention: Diet, Exercise, Sun Safety, and Alcohol Reduction. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Adeluola, A.A.; Amin, A.R.M.R. Chemoprevention: Achievements and Future Perspectives. Dhaka Univ. J. Pharm. Sci. 2022, 20, 359–372. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Sanna, V.; Ahmad, N.; Sechi, M.; Mukhtar, H. Resveratrol Nanoformulation for Cancer Prevention and Therapy. Ann. N. Y. Acad. Sci. 2015, 1348, 20–31. [Google Scholar] [CrossRef]
- Tsao, A.S.; Kim, E.S.; Hong, W.K. Chemoprevention of Cancer. CA Cancer J. Clin. 2004, 54, 150–180. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, R.M.; Lagiou, P.; ADAMI, H.; Trichopoulos, D. Prospects for Chemoprevention of Cancer. J. Intern. Med. 2002, 251, 286–300. [Google Scholar]
- Sporn, M.B.; Dunlop, N.M.; Newton, D.L.; Smith, J.M. Prevention of Chemical Carcinogenesis by Vitamin A and Its Synthetic Analogs (Retinoids). Fed. Proc. 1976, 35, 1332–1338. [Google Scholar]
- Kelloff, G.J.; Hawk, E.T.; Karp, J.E.; Crowell, J.A.; Boone, C.W.; Steele, V.E.; Lubet, R.A.; Sigman, C.C. Progress in Clinical Chemoprevention. Semin. Oncol. 1997, 24, 241–252. [Google Scholar]
- Aggarwal, B.B.; Takada, Y.; van Oommen, O. From Chemoprevention to Chemotherapy: Common Targets and Common Goals. Expert Opin. Investig. Drugs 2004, 13, 1327–1338. [Google Scholar] [CrossRef]
- Mehta, R.G. Current Paradigms of Cancer Chemoprevention. Turk. J. Biol. 2014, 38, 839–847. [Google Scholar]
- Cabrespine-Faugeras, A.; Bayet-Robert, M.; Bay, J.-O.; Chollet, P.; Barthomeuf, C. Possible Benefits of Curcumin Regimen in Combination with Taxane Chemotherapy for Hormone-Refractory Prostate Cancer Treatment. Nutr. Cancer 2010, 62, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Steward, W.P.; Brown, K. Cancer Chemoprevention: A Rapidly Evolving Field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Shukla, Y.; Pal, S.K. Dietary Cancer Chemoprevention: An Overview. Int. J. Hum. Genet. 2004, 4, 265–276. [Google Scholar] [CrossRef]
- Manson, M.M.; Gescher, A.; Hudson, E.A.; Plummer, S.M.; Squires, M.S.; Prigent, S.A. Blocking and Suppressing Mechanisms of Chemoprevention by Dietary Constituents. Toxicol. Lett. 2000, 112, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-Y.; Hail, N., Jr.; Lotan, R. Apoptosis as a Novel Target for Cancer Chemoprevention. J. Natl. Cancer Inst. 2004, 96, 662–672. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Weedon, D.; Searle, J.; Kerr, J.F. Apoptosis. Its Nature and Implications for Dermatopathology. Am. J. Dermatopathol. 1979, 1, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Kumat, V.; Abbas, A.K.; Robbins, S.L.; Cotran, R.S. Robbins and Cotran Pathological Basis of Diasease, 8th ed.; Saunders Elsevier: Philadelphia, PA, USA, 2010. [Google Scholar]
- Thornberry, N.A. Caspases: Key Mediators of Apoptosis. Chem. Biol. 1998, 5, R97–R103. [Google Scholar] [CrossRef]
- Boatright, K.M.; Salvesen, G.S. Mechanisms of Caspase Activation. Curr. Opin. Cell Biol. 2003, 15, 725–731. [Google Scholar] [CrossRef]
- Boatright, K.M.; Renatus, M.; Scott, F.L.; Sperandio, S.; Shin, H.; Pedersen, I.M.; Ricci, J.-E.; Edris, W.A.; Sutherlin, D.P.; Green, D.R. A Unified Model for Apical Caspase Activation. Mol. Cell 2003, 11, 529–541. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, R.; Gandhi, V. Targeting the Apoptosis Pathway in Hematologic Malignancies. Leuk. Lymphoma 2014, 55, 1980–1992. [Google Scholar] [CrossRef]
- Stennicke, H.R.; Renatus, M.; Meldal, M.; Salvesen, G.S. Internally Quenched Fluorescent Peptide Substrates Disclose the Subsite Preferences of Human Caspases 1, 3, 6, 7 and 8. Biochem. J. 2000, 350, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A. Identification and Inhibition of the ICE/CED-3 Protease Necessary for Mammalian Apoptosis. Nature 1995, 376, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Thornberry, N.A.; Rano, T.A.; Peterson, E.P.; Rasper, D.M.; Timkey, T.; Garcia-Calvo, M.; Houtzager, V.M.; Nordstrom, P.A.; Roy, S.; Vaillancourt, J.P. A Combinatorial Approach Defines Specificities of Members of the Caspase Family and Granzyme B: Functional Relationships Established for Key Mediators of Apoptosis. J. Biol. Chem. 1997, 272, 17907–17911. [Google Scholar] [CrossRef]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian Caspases: Structure, Activation, Substrates, and Functions during Apoptosis. Annu. Rev. Biochem. 1999, 68, 383–424. [Google Scholar] [CrossRef]
- Van Fiandalo, M.; Kyprianou, N. Caspase Control: Protagonists of Cancer Cell Apoptosis. Exp. Oncol. 2012, 34, 165. [Google Scholar]
- Thompson, C.B. Apoptosis in the Pathogenesis and Treatment of Disease. Science 1995, 267, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yankner, B.A. Apoptosis in the Nervous System. Nature 2000, 407, 802–809. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Apoptosis in Cancer: From Pathogenesis to Treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- O’Brien, M.A.; Kirby, R. Apoptosis: A Review of Pro-apoptotic and Anti-apoptotic Pathways and Dysregulation in Disease. J. Vet. Emerg. Crit. Care 2008, 18, 572–585. [Google Scholar] [CrossRef]
- Danial, N.N.; Korsmeyer, S.J. Cell Death: Critical Control Points. Cell 2004, 116, 205–219. [Google Scholar] [CrossRef]
- Itoh, N.; Nagata, S. A Novel Protein Domain Required for Apoptosis. Mutational Analysis of Human Fas Antigen. J. Biol. Chem. 1993, 268, 10932–10937. [Google Scholar] [CrossRef] [PubMed]
- Trauth, B.C.; Klas, C.; Peters, A.M.J.; Matzku, S.; Möller, P.; Falk, W.; Debatin, K.-M.; Krammer, P.H. Monoclonal Antibody-Mediated Tumor Regression by Induction of Apoptosis. Science 1989, 245, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Kischkel, F.C.; Hellbardt, S.; Behrmann, I.; Germer, M.; Pawlita, M.; Krammer, P.H.; Peter, M.E. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated Proteins Form a Death-inducing Signaling Complex (DISC) with the Receptor. EMBO J. 1995, 14, 5579–5588. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K. Death Effecter Domain for the Assembly of Death-Inducing Signaling Complex. Apoptosis 2015, 20, 235–239. [Google Scholar] [CrossRef]
- Özören, N.; El-Deiry, W.S. Defining Characteristics of Types I and II Apoptotic Cells in Response to TRAIL. Neoplasia 2002, 4, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 Interacting Protein, Mediates Cytochrome c Release from Mitochondria in Response to Activation of Cell Surface Death Receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Xu, C.; Yuan, J. Cleavage of BID by Caspase 8 Mediates the Mitochondrial Damage in the Fas Pathway of Apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 Family: Regulators of the Cellular Life-or-Death Switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 Protein Family: Opposing Activities That Mediate Cell Death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Adams, J.M.; Cory, S. The Bcl-2 Protein Family: Arbiters of Cell Survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rafiuddin-Shah, M.; Tu, H.-C.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.-D.; Cheng, E.H.-Y. Hierarchical Regulation of Mitochondrion-Dependent Apoptosis by BCL-2 Subfamilies. Nat. Cell Biol. 2006, 8, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Willis, S.N.; Wei, A.; Smith, B.J.; Fletcher, J.I.; Hinds, M.G.; Colman, P.M.; Day, C.L.; Adams, J.M.; Huang, D.C.S. Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function. Mol. Cell 2005, 17, 393–403. [Google Scholar] [CrossRef]
- Ren, D.; Tu, H.-C.; Kim, H.; Wang, G.X.; Bean, G.R.; Takeuchi, O.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.-D.; Cheng, E.H.-Y. BID, BIM, and PUMA Are Essential for Activation of the BAX-and BAK-Dependent Cell Death Program. Science 2010, 330, 1390–1393. [Google Scholar] [CrossRef]
- Vogel, S.; Raulf, N.; Bregenhorn, S.; Biniossek, M.L.; Maurer, U.; Czabotar, P.; Borner, C. Cytosolic Bax: Does It Require Binding Proteins to Keep Its pro-Apoptotic Activity in Check? J. Biol. Chem. 2012, 287, 9112–9127. [Google Scholar] [CrossRef]
- Johnstone, R.W. Histone-Deacetylase Inhibitors: Novel Drugs for the Treatment of Cancer. Nat. Rev. Drug Discov. 2002, 1, 287–299. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Letai, A. Control of Mitochondrial Apoptosis by the Bcl-2 Family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef]
- Riedl, S.J.; Salvesen, G.S. The Apoptosome: Signalling Platform of Cell Death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef]
- Peart, M.J.; Tainton, K.M.; Ruefli, A.A.; Dear, A.E.; Sedelies, K.A.; O’Reilly, L.A.; Waterhouse, N.J.; Trapani, J.A.; Johnstone, R.W. Novel Mechanisms of Apoptosis Induced by Histone Deacetylase Inhibitors. Cancer Res. 2003, 63, 4460–4471. [Google Scholar]
- Shi, Y. Mechanisms of Caspase Activation and Inhibition during Apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Woo, M.; Hakem, R.; Soengas, M.S.; Duncan, G.S.; Shahinian, A.; Kägi, D.; Hakem, A.; McCurrach, M.; Khoo, W.; Kaufman, S.A. Essential Contribution of Caspase 3/CPP32 to Apoptosis and Its Associated Nuclear Changes. Genes Dev. 1998, 12, 806–819. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Ekert, P.G.; Pakusch, M.; Silke, J.; Connolly, L.M.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Vaux, D.L. Identification of DIABLO, a Mammalian Protein That Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell 2000, 102, 43–53. [Google Scholar] [CrossRef]
- Du, C.; Fang, M.; Li, Y.; Li, L.; Wang, X. Smac, a Mitochondrial Protein That Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell 2000, 102, 33–42. [Google Scholar] [CrossRef]
- Cavalcante, G.C.; Schaan, A.P.; Cabral, G.F.; Santana-da-Silva, M.N.; Pinto, P.; Vidal, A.F.; Ribeiro-dos-Santos, Â. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019, 20, 4133. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, Cell Cycle and Apoptosis in Cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell Cycle and Apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Chipuk, J.E.; Green, D.R. Dissecting P53-Dependent Apoptosis. Cell Death Differ. 2006, 13, 994–1002. [Google Scholar] [CrossRef]
- Norbury, C.J.; Zhivotovsky, B. DNA Damage-Induced Apoptosis. Oncogene 2004, 23, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Toshiya, K.; Testuya, T.; Akira, H.; Takuji, T. Cancer Chemoprevention through the Induction of Apoptosis by Natural Compounds. J. Biophys. Chem. 2012, 3, 156–173. [Google Scholar]
- Kurosaka, K.; Takahashi, M.; Watanabe, N.; Kobayashi, Y. Silent Cleanup of Very Early Apoptotic Cells by Macrophages. J. Immunol. 2003, 171, 4672–4679. [Google Scholar] [CrossRef] [PubMed]
- Kuno, T.; Yamada, Y.; Hirose, Y.; Katayama, M.; Sakata, K.; Hara, A.; Saji, S.; Mori, H. Induction of Apoptosis by Sulindac in Azoxymethane-induced Possible Colonic Premalignant Lesions in Rats. Jpn. J. Cancer Res. 2002, 93, 242–246. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Carcinogenesis and Apoptosis: Paradigms and Paradoxes. Carcinogenesis 2006, 27, 1939–1945. [Google Scholar] [CrossRef]
- Reed, J.C. Dysregulation of Apoptosis in Cancer. J. Clin. Oncol. 1999, 17, 2941. [Google Scholar] [CrossRef] [PubMed]
- Fernald, K.; Kurokawa, M. Evading Apoptosis in Cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Parsons, M.J.; McCormick, L.; Janke, L.; Howard, A.; Bouchier-Hayes, L.; Green, D.R. Genetic Deletion of Caspase-2 Accelerates MMTV/c-Neu-Driven Mammary Carcinogenesis in Mice. Cell Death Differ. 2013, 20, 1174–1182. [Google Scholar] [CrossRef]
- Bean, G.R.; Ganesan, Y.T.; Dong, Y.; Takeda, S.; Liu, H.; Chan, P.M.; Huang, Y.; Chodosh, L.A.; Zambetti, G.P.; Hsieh, J.J.-D. PUMA and BIM Are Required for Oncogene Inactivation–Induced Apoptosis. Sci. Signal 2013, 6, ra20. [Google Scholar] [CrossRef] [PubMed]
- Taraphdar, A.K.; Roy, M.; Bhattacharya, R.K. Natural Products as Inducers of Apoptosis: Implication for Cancer Therapy and Prevention. Curr. Sci. 2001, 80, 1387–1396. [Google Scholar]
- Kawamori, T.; Lubet, R.; Steele, V.E.; Kelloff, G.J.; Kaskey, R.B.; van Rao, C.; Reddy, B.S. Chemopreventive Effect of Curcumin, a Naturally Occurring Anti-Inflammatory Agent, during the Promotion/Progression Stages of Colon Cancer. Cancer Res. 1999, 59, 597–601. [Google Scholar]
- Pan, J.; Zhang, Q.; Liu, Q.; Komas, S.M.; Kalyanaraman, B.; Lubet, R.A.; Wang, Y.; You, M. Honokiol Inhibits Lung Tumorigenesis through Inhibition of Mitochondrial Function. Cancer Prev. Res. 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Brazeau, D.; Amin, A.R. Perspectives on Natural Compounds in Chemoprevention and Treatment of Cancer: An Update with New Promising Compounds. Eur. J. Cancer 2021, 149, 165–183. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J. Pterostilbene: Mechanisms of Its Action as Oncostatic Agent in Cell Models and In Vivo Studies. Pharmacol. Res. 2019, 145, 104265. [Google Scholar] [CrossRef]
- Lin, W.-S.; Leland, J.V.; Ho, C.-T.; Pan, M.-H. Occurrence, Bioavailability, Anti-Inflammatory, and Anticancer Effects of Pterostilbene. J. Agric. Food Chem. 2020, 68, 12788–12799. [Google Scholar] [CrossRef] [PubMed]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef] [PubMed]
- Riviere, C.; Pawlus, A.D.; Merillon, J.-M. Natural Stilbenoids: Distribution in the Plant Kingdom and Chemotaxonomic Interest in Vitaceae. Nat. Prod. Rep. 2012, 29, 1317–1333. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, J.; Gao, X.; Amponsem, E.; Kang, L.; Hu, L.; Zhang, B.; Chang, Y. Simultaneous Determination of Stilbenes, Phenolic Acids, Flavonoids and Anthraquinones in Radix Polygoni Multiflori by LC–MS/MS. J. Pharm. Biomed. Anal. 2012, 62, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.Y. Applications and Mechanistic Studies of Direct Ionization Mass Spectrometry. Ph.D. Thesis, The Hong Kong Polytechnic University, Kowloon, China, August 2018. [Google Scholar]
- Spath, E.; Schlager, J. On the Constituents of ’Red Sandalwood’ [Pterocarpus Santalinus]. 2: The Constitution of Pterostilbene. Ber. Deutsch. Chem. Gesellsch. 1940, 73, 881–884. [Google Scholar]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P.A. Indian Herbs and Herbal Drugs Used for the Treatment of Diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the Intestinal Functionality, Morphology and Microbiome Following Intra-Amniotic Administration (Gallus gallus) of Grape (Vitis vinifera) Stilbenes (Resveratrol and Pterostilbene). Nutrients 2021, 13, 3247. [Google Scholar] [CrossRef]
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-Dose Pterostilbene, but Not Resveratrol, Is a Potent Neuromodulator in Aging and Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 2062–2071. [Google Scholar] [CrossRef]
- Kiselev, K.V.; Ogneva, Z.V.; Suprun, A.R.; Grigorchuk, V.P.; Dubrovina, A.S. Action of Ultraviolet-C Radiation and p-Coumaric Acid on Stilbene Accumulation and Expression of Stilbene Biosynthesis-Related Genes in the Grapevine Vitis Amurensis Rupr. Acta Physiol. Plant 2019, 41, 28. [Google Scholar] [CrossRef]
- Xin, H.; Li, Q.; Zhou, H.; Chai, F.; Wang, Z.; Fang, L.; Duan, W.; Fan, P.; Liang, Z.; Li, S. Comparative Metabolomics Analysis of Dormancy Buds during Cold Accumulation between Cold-Sensitive Grapevine (Vitis vinifera) and Cold-Hardy Grapevine (Vitis amurensis). Available online: https://ssrn.com/abstract=4150365 (accessed on 9 April 2022).
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological Actions and Molecular Effects of Resveratrol, Pterostilbene, and 3′-Hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A Stress-Inducible Resveratrol O-Methyltransferase Involved in the Biosynthesis of Pterostilbene in Grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. Benefits of Resveratrol and Pterostilbene to Crops and Their Potential Nutraceutical Value to Mammals. Agriculture 2022, 12, 368. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, C.W.; Tan, Y.H.; Foo, J.P.Y.; Wong, S.K.; Chan, H.T. Resveratrol and Pterostilbene: A Comparative Overview of Their Chemistry, Biosynthesis, Plant Sources and Pharmacological Properties. J. Appl. Pharm. Sci. 2019, 9, 124–129. [Google Scholar]
- Kim, H.; Seo, K.-H.; Yokoyama, W. Chemistry of Pterostilbene and Its Metabolic Effects. J. Agric. Food Chem. 2020, 68, 12836–12841. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, Oral Bioavailability, and Metabolic Profile of Resveratrol and Its Dimethylether Analog, Pterostilbene, in Rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Jeandet, P.; Sobarzo-Sánchez, E.; Silva, A.S.; Clément, C.; Nabavi, S.F.; Battino, M.; Rasekhian, M.; Belwal, T.; Habtemariam, S.; Koffas, M. Whole-Cell Biocatalytic, Enzymatic and Green Chemistry Methods for the Production of Resveratrol and Its Derivatives. Biotechnol. Adv. 2020, 39, 107461. [Google Scholar] [CrossRef] [PubMed]
- Riche, D.M.; McEwen, C.L.; Riche, K.D.; Sherman, J.J.; Wofford, M.R.; Deschamp, D.; Griswold, M. Analysis of Safety from a Human Clinical Trial with Pterostilbene. J. Toxicol. 2013, 2013. [Google Scholar] [CrossRef]
- Ruiz, M.J.; Fernández, M.; Pico, Y.; Manes, J.; Asensi, M.; Carda, C.; Asensio, G.; Estrela, J.M. Dietary Administration of High Doses of Pterostilbene and Quercetin to Mice Is Not Toxic. J. Agric. Food Chem. 2009, 57, 3180–3186. [Google Scholar] [CrossRef]
- Jin, J.; Shan, Y.; Zhang, L.; Wu, Z.; Wu, S.; Sun, M.; Bao, W. Pterostilbene Ameliorates Fumonisin B1-Induced Cytotoxic Effect by Interfering in the Activation of JAK/STAT Pathway. Antioxidants 2022, 11, 2360. [Google Scholar] [CrossRef]
- Monceaux, K.; Gressette, M.; Karoui, A.; Pires Da Silva, J.; Piquereau, J.; Ventura-Clapier, R.; Garnier, A.; Mericskay, M.; Lemaire, C. Ferulic Acid, Pterostilbene, and Tyrosol Protect the Heart from ER-Stress-Induced Injury by Activating SIRT1-Dependent Deacetylation of EIF2α. Int. J. Mol. Sci. 2022, 23, 6628. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Wang, G.; Niu, J.; Peng, M.; Liu, Y. Pterostilbene Attenuates the Proliferation and Differentiation of TNF-α-treated Human Periodontal Ligament Stem Cells. Exp. Ther. Med. 2022, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Harun, Z.; Ghazali, A.R. Potential Chemoprevention Activity of Pterostilbene by Enhancing the Detoxifying Enzymes in the HT-29 Cell Line. Asian Pac. J. Cancer Prev. 2012, 13, 6403–6407. [Google Scholar] [CrossRef]
- Liu, J.; Fan, C.; Yu, L.; Yang, Y.; Jiang, S.; Ma, Z.; Hu, W.; Li, T.; Yang, Z.; Tian, T. Pterostilbene Exerts an Anti-Inflammatory Effect via Regulating Endoplasmic Reticulum Stress in Endothelial Cells. Cytokine 2016, 77, 88–97. [Google Scholar] [CrossRef]
- Nagapan, T.S.; Lim, W.N.; Ghazali, A.R.; Basri, D.F. Pterostilbene Supplementation Inhibits Early Inflammatory Response and Oxidative Stress in UVB-Induced BALB/C Mice. Sains Malays. 2021, 50, 1407–1414. [Google Scholar] [CrossRef]
- Sourani, Z.; Rezaei Dezaki, Z.; Rahimnejad, T.; Pourgheysari, B. A Novel Combination of Pterostilbene and Dexamethasone Enhances Anti-Proliferation and Apoptosis Induction Properties in Lymphoblastic Leukemia Cell Line. Middle East J. Cancer 2020, 11, 469–475. [Google Scholar]
- Chiou, Y.-S.; Tsai, M.-L.; Wang, Y.-J.; Cheng, A.-C.; Lai, W.-M.; Badmaev, V.; Ho, C.-T.; Pan, M.-H. Pterostilbene Inhibits Colorectal Aberrant Crypt Foci (ACF) and Colon Carcinogenesis via Suppression of Multiple Signal Transduction Pathways in Azoxymethane-Treated Mice. J. Agric. Food Chem. 2010, 58, 8833–8841. [Google Scholar] [CrossRef]
- Chen, R.-J.; Tsai, S.-J.; Ho, C.-T.; Pan, M.-H.; Ho, Y.-S.; Wu, C.-H.; Wang, Y.-J. Chemopreventive Effects of Pterostilbene on Urethane-Induced Lung Carcinogenesis in Mice via the Inhibition of EGFR-Mediated Pathways and the Induction of Apoptosis and Autophagy. J. Agric. Food Chem. 2012, 60, 11533–11541. [Google Scholar] [CrossRef]
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary Pterostilbene Is a Novel MTA1-Targeted Chemopreventive and Therapeutic Agent in Prostate Cancer. Oncotarget 2016, 7, 18469. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Tan, K.; Wang, H.; Zhang, X. Pterostilbene Inhibits Hepatocellular Carcinoma through P53/SOD2/ROS-Mediated Mitochondrial Apoptosis. Oncol. Rep. 2016, 36, 3233–3240. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How Does P53 Induce Apoptosis and How Does This Relate to P53-Mediated Tumour Suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef]
- Surien, O.; Ghazali, A.R.; Masre, S.F. Chemopreventive Effects of Pterostilbene through P53 and Cell Cycle in Mouse Lung of Squamous Cell Carcinoma Model. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Tan, K.; Chen, P.; Li, S.; Ke, T.; Lin, S.; Yang, C. Pterostilbene Inhibits Lung Squamous Cell Carcinoma Growth in Vitro and in Vivo by Inducing S Phase Arrest and Apoptosis. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Elsherbini, A.M.; Sheweita, S.A.; Sultan, A.S. Pterostilbene as a Phytochemical Compound Induces Signaling Pathways Involved in the Apoptosis and Death of Mutant P53-Breast Cancer Cell Lines. Nutr. Cancer 2021, 73, 1976–1984. [Google Scholar] [CrossRef]
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses Both Cancer Cells and Cancer Stem-like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Z.; Xu, W.; Wang, Q.; Zhang, C.; Ding, Y.; Nie, W.; Lai, J.; Chen, Y.; Huang, H. Pterostilbene Promotes Mitochondrial Apoptosis and Inhibits Proliferation in Glioma Cells. Sci. Rep. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wawszczyk, J.; Jesse, K.; Kapral, M. Pterostilbene-Mediated Inhibition of Cell Proliferation and Cell Death Induction in Amelanotic and Melanotic Melanoma. Int. J. Mol. Sci. 2023, 24, 1115. [Google Scholar] [CrossRef] [PubMed]
- González-Sarrías, A.; Espín-Aguilar, J.C.; Romero-Reyes, S.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study. Int. J. Mol. Sci. 2022, 23, 15102. [Google Scholar] [CrossRef]
- Wawszczyk, J.; Jesse, K.; Smolik, S.; Kapral, M. Mechanism of Pterostilbene-Induced Cell Death in HT-29 Colon Cancer Cells. Molecules 2022, 27, 369. [Google Scholar] [CrossRef]
- Chen, R.-J.; Lyu, Y.-J.; Chen, Y.-Y.; Lee, Y.-C.; Pan, M.-H.; Ho, Y.-S.; Wang, Y.-J. Chloroquine Potentiates the Anticancer Effect of Pterostilbene on Pancreatic Cancer by Inhibiting Autophagy and Downregulating the RAGE/STAT3 Pathway. Molecules 2021, 26, 6741. [Google Scholar] [CrossRef]
- Lim, S.M.; Mohamad Hanif, E.A.; Chin, S.-F. Is Targeting Autophagy Mechanism in Cancer a Good Approach? The Possible Double-Edge Sword Effect. Cell Biosci. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Hojo, Y.; Kishi, S.; Mori, S.; Fujiwara-Tani, R.; Sasaki, T.; Fujii, K.; Nishiguchi, Y.; Nakashima, C.; Luo, Y.; Shinohara, H. Sunitinib and Pterostilbene Combination Treatment Exerts Antitumor Effects in Gastric Cancer via Suppression of PDZD8. Int. J. Mol. Sci. 2022, 23, 4002. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, T.; Chen, X.; Cheng, J.; Wang, L. Pterostilbene Regulates Cell Proliferation and Apoptosis in Non-small-cell Lung Cancer via Targeting COX-2. Biotechnol. Appl. Biochem. 2023, 70, 106–119. [Google Scholar] [CrossRef]
- Kawakami, S.; Tsuma-Kaneko, M.; Sawanobori, M.; Uno, T.; Nakamura, Y.; Matsuzawa, H.; Suzuki, R.; Onizuka, M.; Yahata, T.; Naka, K. Pterostilbene Downregulates BCR/ABL and Induces Apoptosis of T315I-Mutated BCR/ABL-Positive Leukemic Cells. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.L.; Lai, C.; Chen, W.; Ho, C.; Pan, M. Pterostilbene, a Natural Analogue of Resveratrol, Potently Inhibits 7,12-Dimethylbenz[a]Anthracene (DMBA)/12-O-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Mouse Skin Carcinogenesis. Food Funct. 2012, 3, 1185–1194. [Google Scholar] [CrossRef]
- New, M.; Keith, R. Early Detection and Chemoprevention of Lung Cancer. F1000Research 2018, 7, 61. [Google Scholar] [CrossRef]
- Katona, B.W.; Weiss, J.M. Chemoprevention of Colorectal Cancer. Gastroenterology 2020, 158, 368–388. [Google Scholar] [CrossRef]
- Surien, O.; Ghazali, A.R.; Masre, S.F. Lung Cancers and the Roles of Natural Compounds as Potential Chemotherapeutic and Chemopreventive Agents. Biomed. Pharmacol. J. 2019, 12, 85–98. [Google Scholar] [CrossRef]
- Ubago-Guisado, E.; Rodríguez-Barranco, M.; Ching-López, A.; Petrova, D.; Molina-Montes, E.; Amiano, P.; Barricarte-Gurrea, A.; Chirlaque, M.-D.; Agudo, A.; Sánchez, M.-J. Evidence Update on the Relationship between Diet and the Most Common Cancers from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: A Systematic Review. Nutrients 2021, 13, 3582. [Google Scholar] [CrossRef]
- Farvid, M.S.; Barnett, J.B.; Spence, N.D. Fruit and Vegetable Consumption and Incident Breast Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Br. J. Cancer 2021, 125, 284–298. [Google Scholar] [CrossRef]
- Guo, X.; Shao, X.; Li, J.; Li, S.; Li, K.; Li, D. Fruit and Vegetable Intake and Liver Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. Food Funct. 2019, 10, 4478–4485. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Jahan, S.; Singh, R.; Saxena, J.; Ashraf, S.A.; Khan, A.; Choudhary, R.K.; Balakrishnan, S.; Badraoui, R.; Bardakci, F. Plants in Anticancer Drug Discovery: From Molecular Mechanism to Chemoprevention. Biomed. Res. Int. 2022, 2022, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-KB in Development and Progression of Human Cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-KB) Signaling in Cancer Development and Immune Diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary Intake of Pterostilbene, a Constituent of Blueberries, Inhibits the β-Catenin/P65 Downstream Signaling Pathway and Colon Carcinogenesis in Rats. Carcinogenesis 2010, 31, 1272–1278. [Google Scholar] [CrossRef]
- Bhakkiyalakshmi, E.; Sireesh, D.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Anti-Hyperlipidemic and Anti-Peroxidative Role of Pterostilbene via Nrf2 Signaling in Experimental Diabetes. Eur. J. Pharmacol. 2016, 777, 9–16. [Google Scholar] [CrossRef]
- Ghazali, A.R.; Mahindran, E.; Ramalingam, A.; Chee, L.; Zainalabidin, S. Protective Effects of Pterostilbene Against Cardiac Oxidative Stressand Dysfunctionin Nicotine-Induced Cardiac Injury Rat Model. Biomed. Pharmacol. J. 2021, 14, 623–633. [Google Scholar] [CrossRef]
- Lee, W.X.; Basri, D.F.; Ghazali, A.R. Bactericidal Effect of Pterostilbene Alone and in Combination with Gentamicin against Human Pathogenic Bacteria. Molecules 2017, 22, 463. [Google Scholar] [CrossRef]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Nagarajan, S.; Mohandas, S.; Ganesan, K.; Xu, B.; Ramkumar, K.M. New Insights into Dietary Pterostilbene: Sources, Metabolism, and Health Promotion Effects. Molecules 2022, 27, 6316. [Google Scholar] [CrossRef] [PubMed]


| Type of Cancer | Dose or Concentration and Route of Administration | Outcomes | Effects on Apoptosis |
|---|---|---|---|
| Azoxymethane (AOM) induced aberrant crypt foci (ACF) and colorectal cancer | Concentration: 50 and 200 ppm (dietary) | A diet containing pterostilbene significantly reduced the number of ACF in the colon after 6 weeks of AOM exposure. A diet containing pterostilbene significantly reduced the number of adenomas in an AOM-induced colon carcinogenesis mouse model (23 weeks). | Upregulation of pro-apoptotic proteins Fas, Fas L, Bax, and Bid. Upregulation of apoptotic caspases: cleaved caspase-9, -8, and -3 [112] |
| Urethane-induced lung adenoma mouse model | Dose: 50 and 250 mg/kg (intraperitoneal injection) | Pterostilbene significantly reduced the tumour multiplicity, volume, and burden. | Upregulation of cleaved caspase-3 [113] |
| Phosphatase and tensin homolog (Pten) loss in transgenic mouse model of prostate cancer | Concentration: 10 mg/kg diet (dietary) | Mice with a diet containing pterostilbene showed smaller-sized prostate glands and a reduction in the formation of premalignant lesions of prostatic intraepithelial neoplasia (PIN). | Upregulation of pro-apoptotic p27 and cleaved caspase-3 [114] |
| Diethylnitrosamine (DEN)- and carbon tetrachloride (CCl4)-induced hepatocellular carcinoma mouse model | Dose: 100 and 200 mg/kg (intraperitoneal injection) | Pterostilbene inhibited tumour growth as the number and the maximum size of tumours was significantly reduced. Pterostilbene also protects the liver from injury by reducing liver enzymes (AST, ALT, LDH, and ALP) | Increase in the percentage of apoptosis cells [115] |
| NTCU-induced lung SCC mouse model. | Dose: 10 and 50 mg/kg (intraperitoneal injection) | Pterostilbene reduced cell proliferation and induced cell cycle arrest to inhibit the carcinogenesis of lung cancer. | Upregulation of cleaved caspase-3 [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surien, O.; Masre, S.F.; Basri, D.F.; Ghazali, A.R. Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway. Int. J. Mol. Sci. 2023, 24, 9707. https://doi.org/10.3390/ijms24119707
Surien O, Masre SF, Basri DF, Ghazali AR. Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway. International Journal of Molecular Sciences. 2023; 24(11):9707. https://doi.org/10.3390/ijms24119707
Chicago/Turabian StyleSurien, Omchit, Siti Fathiah Masre, Dayang Fredalina Basri, and Ahmad Rohi Ghazali. 2023. "Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway" International Journal of Molecular Sciences 24, no. 11: 9707. https://doi.org/10.3390/ijms24119707
APA StyleSurien, O., Masre, S. F., Basri, D. F., & Ghazali, A. R. (2023). Potential Chemopreventive Role of Pterostilbene in Its Modulation of the Apoptosis Pathway. International Journal of Molecular Sciences, 24(11), 9707. https://doi.org/10.3390/ijms24119707






