Common Genetic Variants of Response to Hepatitis B Vaccines Correlate with Risks of Chronic Infection of Hepatitis B Virus: A Community-Based Case-Control Study
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Study Subjects
2.2. Genotype Distributions and Genotypic Effects of the Tested SNPs
2.3. Association Analyses with Outcomes of HBV Infection
2.4. Genotypes of HBeAg-Positive Carriers
3. Discussion
4. Materials and Methods
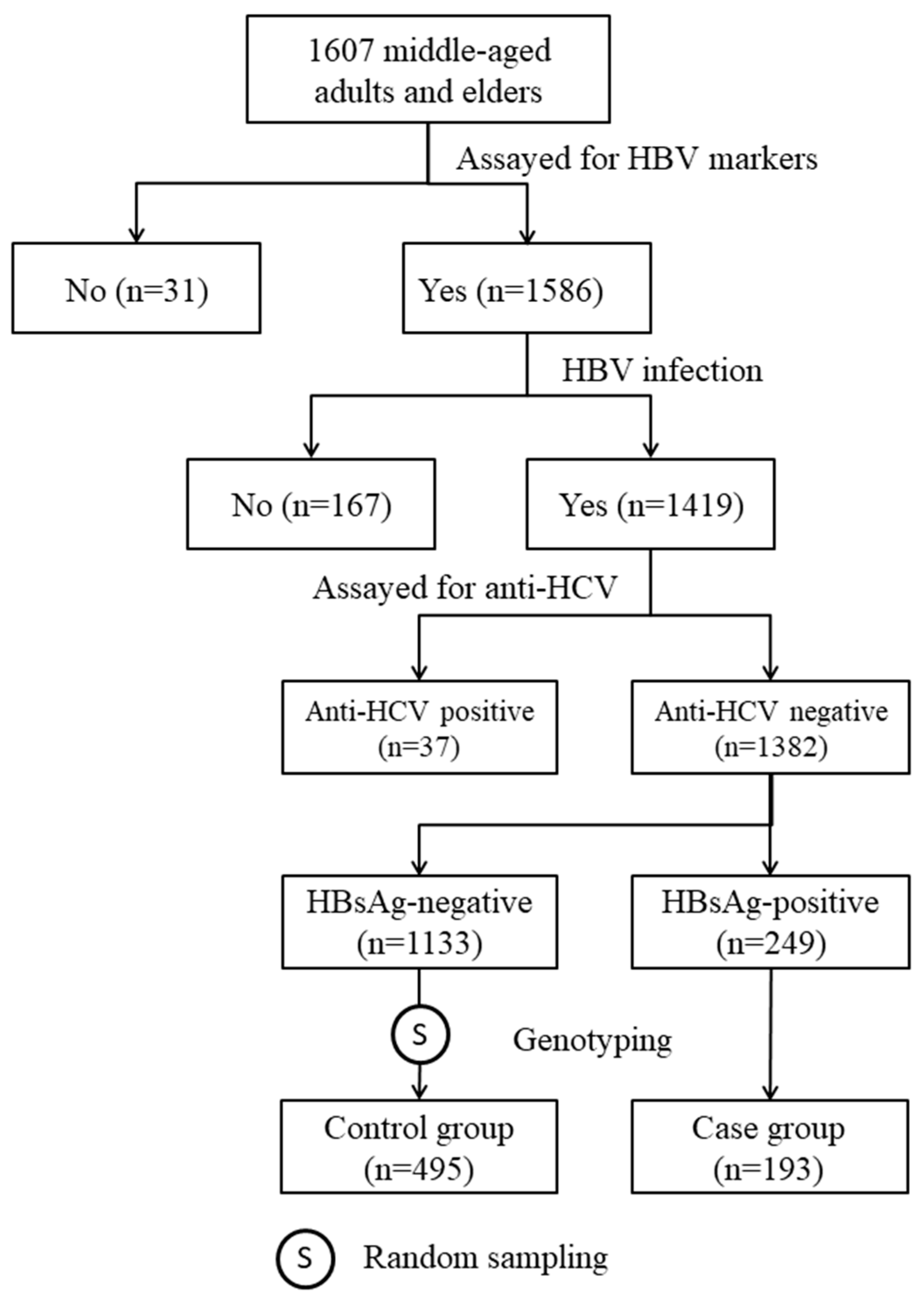
4.1. Study Subjects
4.2. Serologic Testing
4.3. SNP Selections
4.4. Genotyping
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, H.I.; Su, J.U.N.; Jen, C.L.; You, S.L.; Lu, S.N.; Huang, G.T.; Iloeje, U.H.; Reveal-HBV Study Group. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006, 295, 65–73. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, H.I.; Liu, J.; Batrla-Utermann, R.; Jen, C.L.; Iloeje, U.H.; Lu, S.N.; You, S.L.; Wang, L.Y.; Chen, C.J.; et al. Prediction models of long-term cirrhosis and hepatocellular carcinoma risk in chronic hepatitis B patients: Risk scores integrating host and virus profiles. Hepatology 2013, 58, 546–554. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Chen, H.L.; Chang, M.H.; Ni, Y.H.; Hsu, H.Y.; Lee, P.L.; Lee, C.Y.; Chen, D.S. Seroepidemiology of hepatitis B virus infection in children: Ten years of mass vaccination in Taiwan. JAMA 1996, 276, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.W.; Lin, H.H.; Wang, L.Y. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology 2013, 57, 37–45. [Google Scholar] [CrossRef]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lai, M.W.; Wu, T.C.; Wu, S.F.; Lee, C.M.; Yang, S.S.; Chu, H.C.; et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 2016, 151, 472–480. [Google Scholar] [CrossRef]
- Galles, N.C.; Liu, P.Y.; Updike, R.L.; Fullman, N.; Nguyen, J.; Rolfe, S.; Sbarra, A.N.; Schipp, M.F.; Marks, A.; Abady, G.G.; et al. Measuring routine childhood vaccination coverage in 204 countries and territories, 1980-2019: A systematic analysis for the Global Burden of Disease Study 2020, Release 1. Lancet 2021, 398, 503–521. [Google Scholar]
- Wu, T.W.; Chen, C.F.; Lai, S.K.; Lin, H.H.; Chu, C.C.; Wang, L.Y. SNP rs7770370 in HLA-DPB1 loci as a major genetic determinant of response to booster hepatitis B vaccination: Results of a genome-wide association study. J. Gastroenterol. Hepatol. 2015, 30, 891–899. [Google Scholar] [CrossRef]
- Png, E.; Thalamuthu, A.; Ong, R.T.; Snippe, H.; Boland, G.J.; Seielstad, M. A genome-wide association study of hepatitis B vaccine response in an Indonesian population reveals multiple independent risk variants in the HLA region. Hum. Mol. Genet. 2011, 20, 3893–3898. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, L.; Zhang, W.; Wu, X.; Li, Y.; Yan, B.; Zhu, X.; Liu, X.; Yang, C.; Xu, J.; et al. A genome-wide association study identifies polymorphisms in the HLA-DR region associated with non-response to hepatitis B vaccination in Chinese Han populations. Hum. Mol. Genet. 2014, 23, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Sugiyama, M.; Sawai, H.; Nishina, S.; Sakai, A.; Ohashi, J.; Khor, S.S.; Kakisaka, K.; Tsuchiura, T.; Hino, K.; et al. Key HLA-DRB1-DQB1 haplotypes and role of the BTNL2 gene for response to a hepatitis B vaccine. Hepatology 2018, 68, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Roh, E.Y.; Park, B.; Lee, Y.; Shin, S.; Yoon, J.H.; Song, E.Y. GWAS identifying HLA-DPB1 gene variants associated with responsiveness to hepatitis B virus vaccination in Koreans: Independent association of HLA-DPB1*04:02 possessing rs1042169 G-rs9277355 C-rs9277356 A. J. Viral Hepat. 2019, 26, 1318–1329. [Google Scholar] [CrossRef]
- Wu, T.W.; Chan, H.L.; Hung, C.L.; Lu, I.J.; Wang, S.D.; Wang, S.W.; Wu, Y.J.; Wang, L.Y.; Yeh, H.I.; Wei, Y.H. Differential patterns of effects of age and sex on metabolic syndrome in Taiwan: Implication for the inadequate internal consistency of the current criteria. Diabetes Res. Clin. Pract. 2014, 105, 239–244. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Liu, Z.; Zou, G.; Li, J.; Lu, M. Host Genetic Determinants of Hepatitis B Virus Infection. Front. Genet. 2019, 10, 696. [Google Scholar] [CrossRef]
- Roh, E.Y.; Yoon, J.H.; In, J.W.; Lee, N.; Shin, S.; Song, E.Y. Association of HLA-DP variants with the responsiveness to hepatitis B virus vaccination in Korean infants. Vaccine 2016, 34, 2602–2607. [Google Scholar] [CrossRef]
- Yates, A.; Akanni, W.; Amode, M.R.; Barrell, D.; Billis, K.; Carvalho-Silva, D.; Cummins, C.; Clapham, P.; Fitzgerald, S.; Gil, L.; et al. Ensembl 2016. Nucleic Acids Res. 2016, 44, D710–D716. [Google Scholar] [CrossRef]
- Kamatani, Y.; Wattanapokayakit, S.; Ochi, H.; Kawaguchi, T.; Takahashi, A.; Hosono, N.; Kubo, M.; Tsunoda, T.; Kamatani, N.; Kumada, H.; et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat. Genet. 2009, 41, 591–595. [Google Scholar] [CrossRef]
- Mbarek, H.; Ochi, H.; Urabe, Y.; Kumar, V.; Kubo, M.; Hosono, N.; Takahashi, A.; Kamatani, Y.; Miki, D.; Abe, H.; et al. A genome-wide association study of chronic hepatitis B identified novel risk locus in a Japanese population. Hum. Mol. Genet. 2011, 20, 3884–3892. [Google Scholar]
- Hu, L.; Zhai, X.; Liu, J.; Chu, M.; Pan, S.; Jiang, J.; Zhang, Y.; Wang, H.; Chen, J.; Shen, H.; et al. Genetic variants in human leukocyte antigen/DP-DQ influence both hepatitis B virus clearance and hepatocellular carcinoma development. Hepatology 2012, 55, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Sawai, H.; Matsuura, K.; Sugiyama, M.; Ahn, S.H.; Park, J.Y.; Hige, S.; Kang, J.-H.; Suzuki, K.; Kurosaki, M.; et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS ONE 2012, 7, e39175. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-W.; Fann, C.S.-J.; Su, W.-H.; Wang, Y.C.; Weng, C.C.; Yu, C.-J.; Hsu, C.-L.; Hsieh, A.-R.; Chien, R.-N.; Chu, C.-M.; et al. A genome-wide association study on chronic HBV infection and its clinical progression in male Han-Taiwanese. PLoS ONE 2014, 9, e99724. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Cheng, Y.-j.; Cheng, M.-l.; Yao, Y.-m.; Zhang, Q.; Zhao, X.-k.; Liu, H.-j.; Hu, Y.-x.; Mu, M.; Wang, B.; et al. Quantitative assessment of common genetic variations in HLA-DP with hepatitis B virus infection, clearance and hepatocellular carcinoma development. Sci. Rep. 2015, 5, 14933. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Liao, S.F.; Khor, S.S.; Lin, Y.J.; Chen, H.Y.; Chang, Y.H.; Huang, Y.H.; Lu, S.N.; Lee, H.W.; Ko, W.Y.; et al. Large-scale genome-wide association study identifies HLA class II variants associated with chronic HBV infection: A study from Taiwan Biobank. Aliment. Pharmacol. Ther. 2020, 52, 682–691. [Google Scholar] [CrossRef]
- Hamilton, E.; Yang, L.; Mentzer, A.J.; Guo, Y.; Chen, Y.; Lv, J.; Fletcher, R.; Wright, N.; Lin, K.; Walters, R.; et al. Conventional and genetic risk factors for chronic Hepatitis B virus infection in a community-based study of 0.5 million Chinese adults. Sci. Rep. 2022, 12, 12075. [Google Scholar] [CrossRef]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef]
- Schmiedel, B.J.; Singh, D.; Madrigal, A.; Valdovino-Gonzalez, A.G.; White, B.M.; Zapardiel-Gonzalo, J.; Ha, B.; Altay, G.; Greenbaum, J.A.; McVicker, G.; et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell 2018, 175, 1701–1715. [Google Scholar] [CrossRef]
- Wang, L.Y.; Chen, C.F.; Wu, T.W.; Lai, S.K.; Chu, C.C.; Lin, H.H. Response to hepatitis B vaccination is co-determined by HLA-DPA1 and -DPB1. Vaccine 2019, 37, 6435–6440. [Google Scholar] [CrossRef]
- Kao, J.H. Molecular epidemiology of hepatitis B virus. Korean J. Intern. Med. 2011, 26, 255–261. [Google Scholar] [CrossRef] [PubMed]
| HBV Carriers (n = 193) | Non-Carrier Controls (n = 495) | p-Value | |||
|---|---|---|---|---|---|
| HBV markers | n | (%) | n | (%) | |
| HBsAg+ | 193 | (100.0) | 0 | (0.0) | - |
| HBsAb+ | 20 | (10.4) | 484 | (97.8) | <0.0001 |
| HBcAb+ | 191 | (99.0) | 470 | (95.0) | 0.015 |
| HBeAg+ | 8 | (4.3) | ND | - | |
| HBeAb+ | 163 | (85.3) | ND | - | |
| Male sex | 93 | (48.2) | 247 | (49.9) | 0.69 |
| Cigarette smoking | 39 | (20.2) | 83 | (16.7) | 0.39 |
| Alcohol drinking | 18 | (9.3) | 33 | (6.6) | 0.30 |
| Schooling >12 years | 67 | (34.7) | 138 | (28.0) | 0.085 |
| Mean | (SD) | Mean | SD | ||
| Age (years) | 50.1 | (8.0) | 56.4 | (9.1) | <0.0001 |
| AST (IU/L) | 26.4 | (10.1) | 24.9 | (12.9) | 0.098 |
| ALT (IU/L) | 27.1 | (17.3) | 24.1 | (14.4) | 0.030 |
| Typed SNP | Position (GRCh38) | Candidate SNP | Allele A/B 1 | HBV Carriers (n = 193) | Non-Carrier Controls (n = 495) | p-Value 2 | ||
|---|---|---|---|---|---|---|---|---|
| A (%) | AA/AB/BB | A (%) | AA/AB/BB | |||||
| rs4947302 | 6:31106351 | rs12527394 | T/C | 23.1 | 4/81/108 | 21.9 | 23/170/302 | 0.076 |
| rs9268176 | 6:32306302 | rs9268202 | T/C | 17.9 | 9/51/133 | 20.5 | 18/166/311 | 0.18 |
| rs3135363 | 6:32421871 | rs3135363 | G/A | 39.6 | 33/90/70 | 38.6 | 72/237/185 | 0.71 |
| rs3129846 | 6:32428698 | rs2395179 | G/A | 21.0 | 7/67/119 | 21.1 | 20/168/307 | 0.96 |
| rs9268831 | 6:32459971 | rs9268831 | C/T | 40.7 | 30/97/66 | 36.3 | 57/244/195 | 0.27 |
| rs34039593 | 6:32602534 | rs34039593 | G/T | 10.6 | 4/33/156 | 15.9 | 8/141/346 | 0.0084 |
| rs614348 | 6:32606100 | rs477515 | C/T | 11.7 | 4/37/152 | 18.6 | 12/159/322 | 0.0025 |
| rs6457620 | 6:32696222 | rs7745040 | G/C | 39.1 | 31/90/72 | 39.6 | 83/224/186 | 0.95 |
| rs1015166 | 6:32830954 | rs1015166 | T/C | 30.1 | 19/78/96 | 27.2 | 33/202/260 | 0.36 |
| rs3097662 | 6:33053000 | rs9277176 | C/T | 11.9 | 2/42/149 | 13.7 | 8/119/368 | 0.68 |
| rs3830066 | 6:33069410 | rs3830066 | C/G | 21.8 | 9/66/118 | 27.9 | 35/205/254 | 0.062 |
| rs7770370 | 6:33081144 | rs7770370 | A/G | 32.1 | 13/98/82 | 40.2 | 79/238/178 | 0.0050 |
| rs9277535 | 6:33087084 | rs9277356 | A/G | 26.9 | 8/88/97 | 33.2 | 55/217/223 | 0.016 |
| Age-Sex-Adjusted | Multivariable | |||
|---|---|---|---|---|
| Protective Genotype | OR | (95% CI) | OR 1 | (95% CI) |
| rs34039593 TG | 0.51 ** | (0.33–0.79) | - | |
| rs614348 TC | 0.49 ** | (0.32–0.75) | 0.50 ** | (0.33–0.77) |
| rs7770370 AA | 0.33 ** | (0.18–0.63) | 0.35 ** | (0.19–0.68) |
| rs9277535 AA | 0.32 ** | (0.14–0.70) | - | |
| HBV Carriers (n = 193) | Non-Carrier Controls (n = 495) | Multivariable | ||||
|---|---|---|---|---|---|---|
| Variable | n | (%) | n | (%) | OR 1 | (95% CI) |
| 4-locus GPS 2 | ||||||
| Mean (SD) | 0.47 | (0.88) | 0.88 | (1.15) | ||
| GPS | ||||||
| 0 | 146 | (75.7) | 284 | (57.4) | 1.00 | |
| 1–2 | 44 | (22.8) | 179 | (36.2) | 0.45 *** | (0.30–0.67) |
| 3–4 | 3 | (1.6) | 32 | (6.5) | 0.17 ** | (0.05–0.58) |
| Per 1.0 GPS | 0.65 *** | (0.54–0.79) | ||||
| 2-locus GPS 3 | ||||||
| Mean (SD) | 0.26 | (0.47) | 0.48 | (0.62) | ||
| GPS | ||||||
| 0 | 146 | (75.7) | 289 | (58.4) | 1.00 | |
| 1 | 44 | (22.8) | 172 | (34.8) | 0.47 ** | (0.32–0.71) |
| 2 | 3 | (1.6) | 34 | (6.9) | 0.16 ** | (0.05–0.54) |
| Per 1.0 GPS | 0.45 *** | (0.32–0.63) | ||||
| No. | Age | Sex | HBsAb | HBcAb | HBeAb | AST | ALT | rs34039593 | rs614348 | rs7770370 | rs9277535 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 112 | 44.3 | M | - | + | - | 27 | 39 | TT | TT | AG | AG |
| 255 | 69.6 | F | + | + | - | 36 | 39 | TT | TT | AG | AG |
| 261 | 69.1 | F | - | + | - | 31 | 23 | TT | TT | GG | GG |
| 398 | 67.6 | M | - | + | - | 50 | 46 | TT | TT | AG | AG |
| 418 | 53.7 | F | - | + | - | 23 | 22 | TT | TT | GG | GG |
| 569 | 42.8 | F | - | + | - | 23 | 27 | TT | TT | AG | AG |
| 626 | 45.2 | F | - | + | - | 18 | 19 | TT | TT | GG | GG |
| 629 | 46.1 | F | - | + | - | 19 | 17 | TT | TT | AA | AA |
| Candidate SNP | Position (GRCh38) | Tested SNP | Position (GRCh38) | LD (r2) | Type of Variant | Mapped Gene |
|---|---|---|---|---|---|---|
| rs12527394 | 6:31087241 | rs4947302 | 6:31106351 | 0.97 | intergenic | C6orf15, RNU6-1133P |
| rs9268202 | 6:32311563 | rs9268176 | 6:32306302 | 1.00 | intronic | TSBP1, TSBP1-AS1 |
| rs3135363 | 6:32421871 | rs3135363 | 6:32421871 | 1.00 | intergenic | BTNL2, TSBP1-AS1, HLA-DRA |
| rs2395179 | 6:32439525 | rs3129846 | 6:32428698 | 0.97 | intergenic | BTNL2, TSBP1-AS1, HLA-DRA |
| rs9268831 | 6:32459971 | rs9268831 | 6:32459971 | 1.00 | NCTEV | HLA-DRB9 |
| rs477515 | 6:32601914 | rs614348 | 6:32606100 | 0.96 | intergenic | HLA-DQA1, HLA-DRB1 |
| rs34039593 | 6:32602534 | rs34039593 | 6:32602534 | 1.00 | intergenic | HLA-DQA1, HLA-DRB1 |
| rs7745040 | 6:32696555 | rs6457620 | 6:32696222 | 0.93 | intergenic | HLA-DQB1, MTCO3P1 |
| rs1015166 | 6:32830954 | rs1015166 | 6:32830954 | 1.00 | intronic | TAP2 |
| rs9277176 | 6:33055373 | rs3097662 | 6:33053000 | 0.97 | regulatory | HLA-DPA1 |
| rs3830066 | 6:33069410 | rs3830066 | 6:33069410 | 1.00 | NCTEV | HLA-DPA1 |
| rs9277356 | 6:33080917 | rs9277535 | 6:33087084 | 0.94 | 3′-UTR | HLA-DPB1 |
| rs7770370 | 6:33081144 | rs7770370 | 6:33081144 | 1.00 | NCTEV | HLA-DPA1, HLA-DPB1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.-W.; Chou, C.-L.; Chen, C.-F.; Wang, L.-Y. Common Genetic Variants of Response to Hepatitis B Vaccines Correlate with Risks of Chronic Infection of Hepatitis B Virus: A Community-Based Case-Control Study. Int. J. Mol. Sci. 2023, 24, 9741. https://doi.org/10.3390/ijms24119741
Wu T-W, Chou C-L, Chen C-F, Wang L-Y. Common Genetic Variants of Response to Hepatitis B Vaccines Correlate with Risks of Chronic Infection of Hepatitis B Virus: A Community-Based Case-Control Study. International Journal of Molecular Sciences. 2023; 24(11):9741. https://doi.org/10.3390/ijms24119741
Chicago/Turabian StyleWu, Tzu-Wei, Chao-Liang Chou, Chuen-Fei Chen, and Li-Yu Wang. 2023. "Common Genetic Variants of Response to Hepatitis B Vaccines Correlate with Risks of Chronic Infection of Hepatitis B Virus: A Community-Based Case-Control Study" International Journal of Molecular Sciences 24, no. 11: 9741. https://doi.org/10.3390/ijms24119741
APA StyleWu, T.-W., Chou, C.-L., Chen, C.-F., & Wang, L.-Y. (2023). Common Genetic Variants of Response to Hepatitis B Vaccines Correlate with Risks of Chronic Infection of Hepatitis B Virus: A Community-Based Case-Control Study. International Journal of Molecular Sciences, 24(11), 9741. https://doi.org/10.3390/ijms24119741






