Human Sperm Head Vacuoles Are Related to Nuclear-Envelope Invaginations
Abstract
:1. Introduction
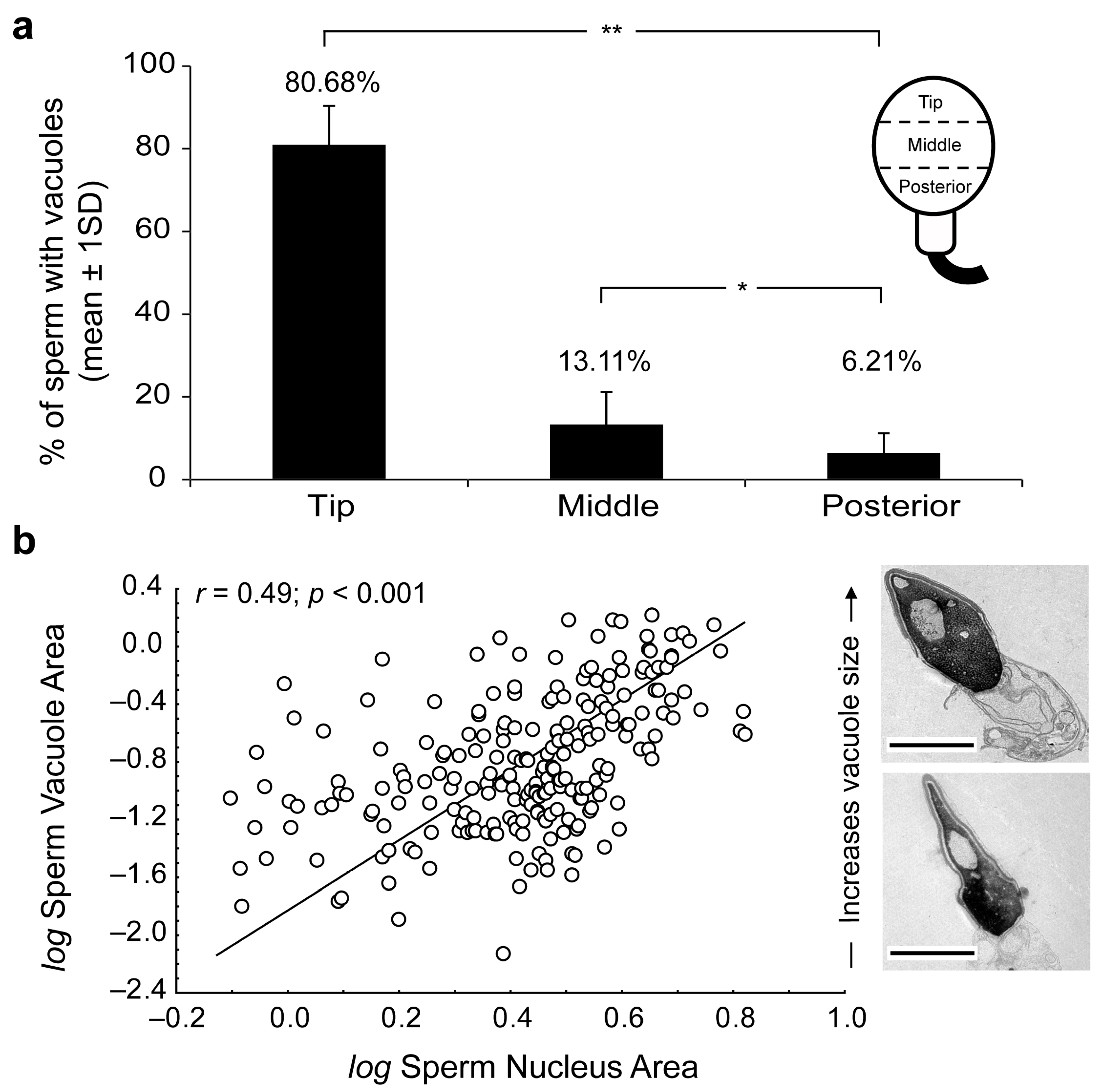
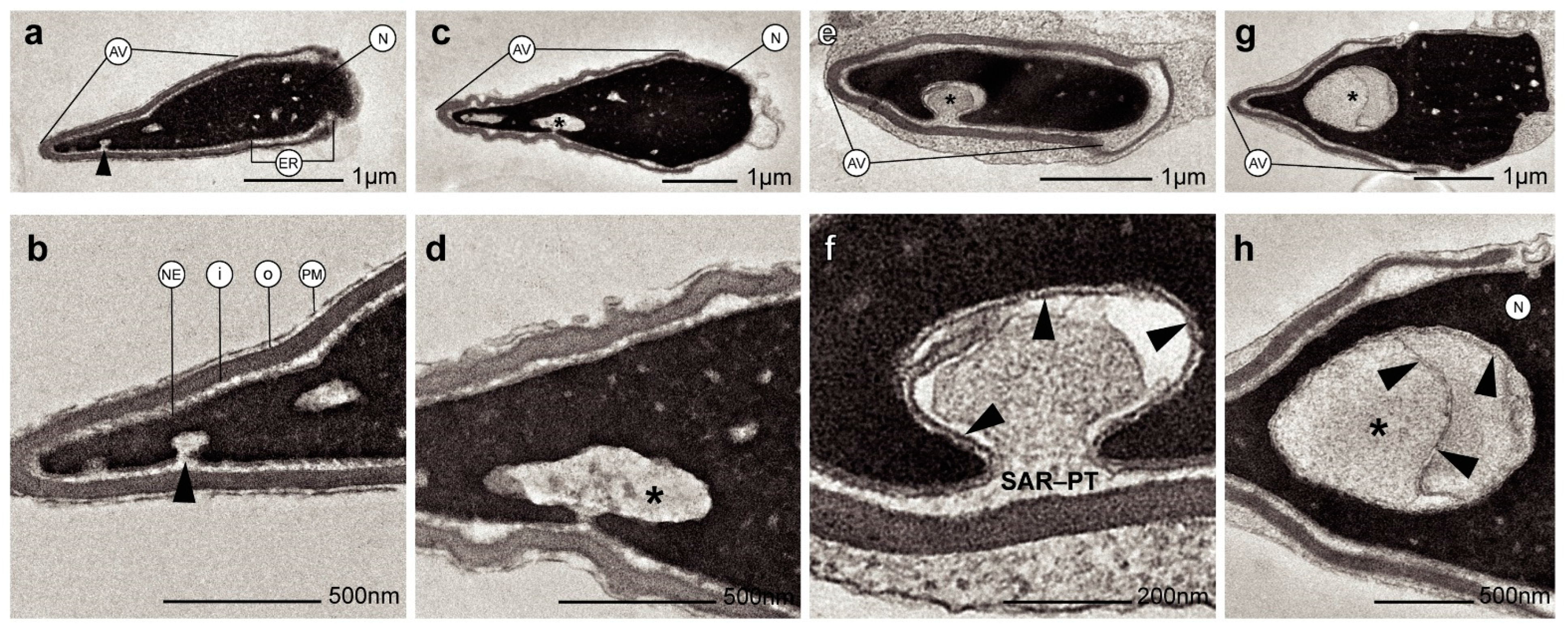
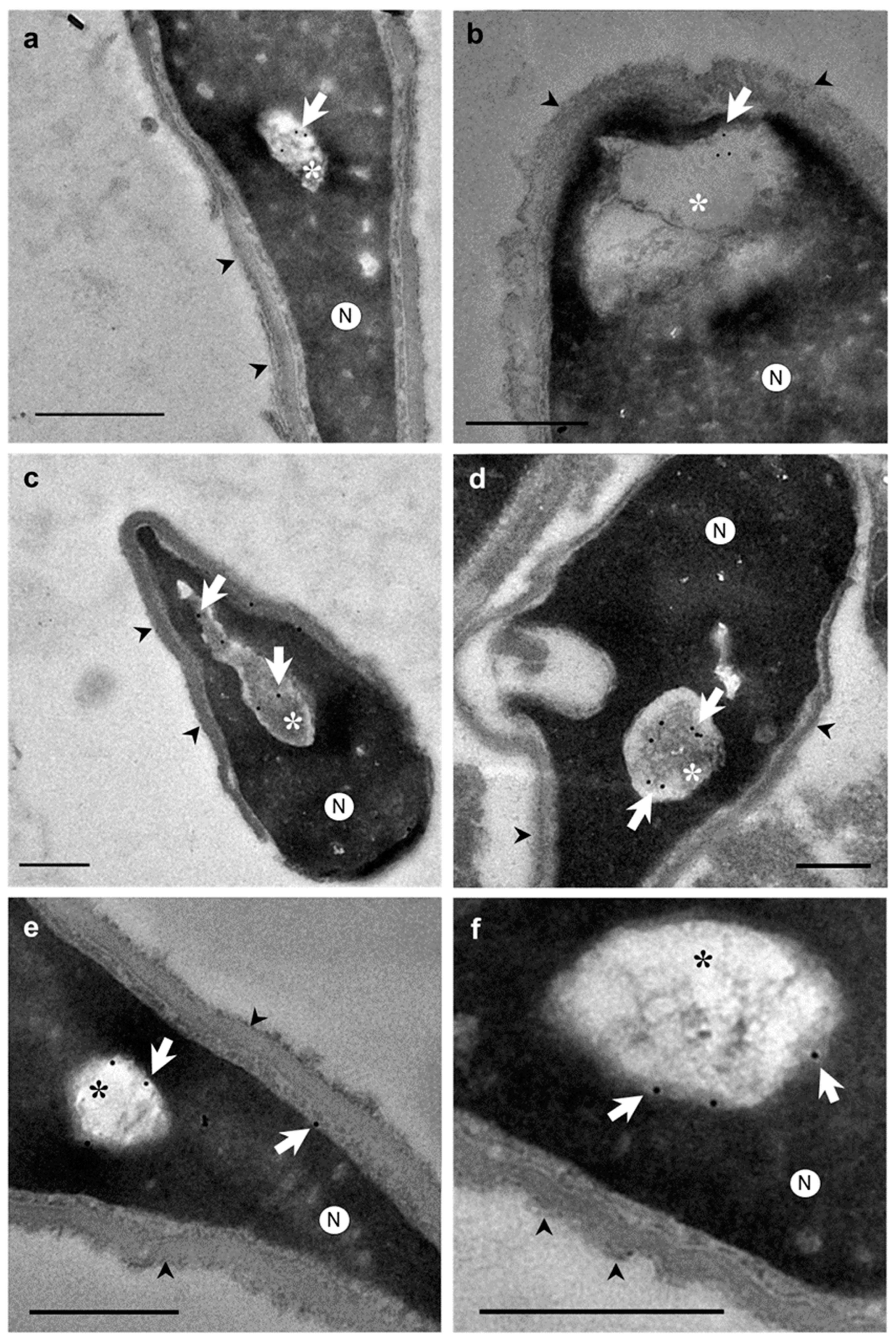
2. Results
3. Discussion
4. Materials and Methods
4.1. Semen Samples Preparation
4.2. Electron Microscopy
4.3. Immunocytochemical Study
4.4. Sperm Head Vacuole Count and Morphological Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and cycle of the seminiferous epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [PubMed]
- Oko, R.; Morales, C.R. Molecular and cellular biology of novel cytoskeletal proteins in spermatozoa. In Cellular and Molecular Regulation of Testicular Cells; Desjardins, C., Ed.; Springer: New York, NY, USA, 1996; pp. 135–165. [Google Scholar]
- Oko, R. Occurrence and formation of cytoskeletal proteins in mammalian spermatozoa. Andrologia 1998, 30, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Toshimori, K.; Eddy, E.M. The spermatozoon. In Knobil and Neill’s Physiology of Reproduction, 4th ed.; Plant, T., Zeleznik, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 99–148. [Google Scholar]
- Ito, C.; Akutsu, H.; Yao, R.; Kyono, K.; Suzuki-Toyota, F.; Toyama, Y.; Maekawa, M.; Noda, T.; Toshimori, K. Oocyte activation ability correlates with head flatness and presence of perinuclear theca substance in human and mouse sperm. Hum. Reprod. 2009, 24, 2588–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, C.; Yamatoya, K.; Yoshida, K.; Kyono, K.; Yao, R.; Noda, T.; Toshimori, K. Appearance of an oocyte activation-related substance during spermatogenesis in mice and humans. Hum. Reprod. 2013, 25, 2734–2744. [Google Scholar] [CrossRef] [Green Version]
- Oko, R.; Sutovsky, P. Biogenesis of sperm perinuclear theca and its role in sperm functional competence and fertilization. J. Reprod. Immunol. 2009, 83, 2–7. [Google Scholar] [CrossRef]
- Protopapas, N.; Hamilton, L.E.; Warkentin, R.; Xu, W.; Sutovsky, P.; Oko, R. The perforatorium and postacrosomal sheath of rat spermatozoa share common developmental origins and protein constituents. Biol. Reprod. 2019, 100, 1461–1472. [Google Scholar] [CrossRef] [Green Version]
- Breitbart, H.; Cohen, G.; Rubinstein, S. Role of actin cytoskeleton in mammalian sperm capacitation and the acrosome reaction. Reproduction 2005, 129, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Dvoráková, K.; Moore, H.D.; Sebková, N.; Palecek, J. Cytoskeleton localization in the sperm head prior to fertilization. Reproduction 2005, 130, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Lécuyer, C.; Dacheux, J.L.; Hermand, E.; Mazeman, E.; Rousseaux, J.; Rousseaux-Prévost, R. Actin-binding properties and colocalization with actin during spermiogenesis of mammalian sperm calicin. Biol. Reprod. 2000, 63, 1801–1810. [Google Scholar] [CrossRef] [Green Version]
- Chyra-Jach, D.; Kaletka, Z.; Dobrakowski, M.; Machoń-Grecka, A.; Kasperczyk, S.; Birkner, E.; Kasperczyk, A. The Associations between Infertility and Antioxidants, Proinflammatory Cytokines, and Chemokines. Oxid. Med. Cell. Longev. 2018, 16, 8354747. [Google Scholar] [CrossRef] [Green Version]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the Actin Cytoskeleton During Mammalian Sperm Capacitation and Acrosome Reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, T.; Noda, Y.; Narimoto, K.; Shiotani, M.; Mori, T.; Matsuda, T.; Yoshida, O. Localization of CuZn-superoxide dismutase in the human male genital organs. Hum. Reprod. 1992, 7, 81–85. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, E.; Zhang, C.; Zhang, H. Study of the Changes of Acrosomal Enzyme, Nitric Oxide Synthase, and Superoxide Dismutase of Infertile Patients with Positive Antisperm Antibody in Seminal Plasma. Cell Biochem. Biophys. 2015, 73, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Boitrelle, F.; Ferfouri, F.; Petit, J.-M.; Segretain, D.; Tourain, C.; Bergere, M.; Bailly, M.; Vialard, F.; Albert, M.; Selva, J. Large human sperm vacuoles observed in motile spermatozoa under high magnification: Nuclear thumbprints linked to failure of chromatin condensation. Hum. Reprod. 2011, 26, 1650–1658. [Google Scholar] [CrossRef] [Green Version]
- Garolla, A.; Fortini, D.; Menegazzo, M.; De Toni, L.; Nicoletti, V.; Moretti, A.; Selice, R.; Engl, B.; Foresta, C. High-power microscopy for selecting spermatozoa for ICSI by physiological status. Reprod. Biomed. Online 2008, 17, 610–616. [Google Scholar] [CrossRef]
- Franco, J.G., Jr.; Baruffi, R.L.; Mauri, A.L.; Petersen, C.G.; Oliveira, J.B.; Vagnini, L. Significance of large nuclear vacuoles in human spermatozoa: Implications for ICSI. Reprod. Biomed. Online 2008, 17, 42–45. [Google Scholar] [CrossRef]
- Perdrix, A.; Travers, A.; Chelli, M.H.; Escalier, D.; Do Rego, J.L.; Milazzo, J.P.; Mousset Siméon, N.; Macé, B.; Rives, N. Assesment of acrosome and nuclear abnormalities in human spermatozoa with large vacuoles. Hum. Reprod. 2011, 26, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Perdrix, A.; Rives, N. Motile sperm organelle morphology examination (MSOME) and sperm head vacuoles: State of the art in 2013. Hum. Reprod. Update 2013, 19, 527–541. [Google Scholar] [CrossRef] [Green Version]
- Boitrelle, F.; Albert, M.; Petit, J.M.; Ferfouri, F.; Wainer, R.; Bergere, M.; Bailly, M.; Vialard, F.; Selva, J. Small human sperm vacuoles observed under magnification are pocket like-nuclear concavities linked to chromatin condensation failure. Reprod. Biomed. Online 2013, 27, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatimel, N.; Léandri, R.D.; Marino, L.; Esquerre-Lamare, C.; Parinaud, J. Sperm vacuoles cannot help to differentiate fertile men from infertile men with normal sperm parameter values. Hum. Reprod. 2014, 29, 2359–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R.D. Sperm morphology: Assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology 2017, 5, 845–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fawcett, D.W. The structure of the mammalian spermatozoon. Intern. Rev. Cytol. 1958, 7, 195–234. [Google Scholar]
- Schnall, M.D. Electron microscopic study of human spermatozoa. Fertil Steril 1952, 3, 62–81. [Google Scholar] [CrossRef]
- Schultz-Larsen, J. The morphology of the human sperm; electron microscopic investigations of the ultrastructure. Acta Path. Microbiol. Scand. Suppl. 1958, 44, 1–121. [Google Scholar]
- Perdrix, A.; Saïdi, R.; Ménard, J.F.; Gruel, E.; Milazzo, J.P.; Macé, B.; Rives, N. Relationship between conventional sperm parameters and motile sperm organelle morphology examination (MSOME). Int. J. Androl. 2012, 35, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Nagayoshi, M.; Tanaka, I.; Kusunoki, H. Human sperm head vacuoles are physiological structures formed during the sperm development and maturation process. Fertil. Steril. 2012, 98, 315–320. [Google Scholar] [CrossRef]
- Watanabe, S.; Tanaka, A.; Fujii, S.; Mizunuma, H.; Fukui, A.; Fukuhara, R.; Nakamura, R.; Yamada, K.; Tanaka, I.; Awata, S.; et al. An investigation of the potential effect of vacuoles in human sperm on DNA damage using a chromosome assay and the TUNEL assay. Hum. Reprod. 2011, 26, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Franco, J.G.; Mauri, A.L.; Petersen, C.G.; Massaro, F.C.; Silva, L.F.I.; Felipe, V.; Cavagna, M.; Pontes, A.; Baruffi, R.L.R.; Oliveira, J.B.A.; et al. Large nuclear vacuoles are indicative of abnormal chromatin packaging in human spermatozoa. Int. J. Androl. 2012, 35, 46–51. [Google Scholar] [CrossRef]
- Fekonja, N.; Strus, J.; Tusek Znidaric, T.; Knez, K.; Bokal, V.; Verdenid, I.; Virant-Klun, I. Clinical and structural features of sperm head vacuoles in men included in the in vitro fertilization programme. Biomed. Res. Int. 2014, 2014, 927841. [Google Scholar] [CrossRef]
- Yamanaka, M.; Tomita, K.; Hashimoto, S.; Matsumoto, H.; Satoh, M.; Kato, H.; Hosoi, Y.; Inoue, M.; Nakaoka, Y.; Morimoto, Y. Combination of density gradient centrifugation and swim-up methods effectively decreases morphologically abnormal sperms. J. Reprod. 2016, 62, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Kacem, O.; Sifer, C.; Barraud-Lange, V.; Ducot, B.; De Ziegler, D.; Poirot, C.; Wolf, J. Sperm nuclear vacuoles, as assessed by motile sperm organellar morphological examination, are mostly of acrosomal origin. Reprod. Biomed. Online 2010, 20, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montjean, D.; Belloc, S.; Benkhalifa, M.; Dalleac, A.; Menezo, Y. Sperm vacuoles are linked to capacitation and acrosomal status. Hum. Reprod. 2012, 27, 2927–2932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, B.; Yakin, K.; Alatas, C.; Oktem, O.; Isiklar, A.; Urman, B. Clinical outcome of intracytoplasmic injection of spermatozoa morphologically selected under high magnification: A prospective randomized study. Reprod. Biomed. Online 2011, 22, 472–476. [Google Scholar] [CrossRef] [Green Version]
- Bartoov, B.; Berkovitz, A.; Eltes, F.; Kogosowski, A.; Menezo, Y.; Barak, Y. Real-time fine morphology of motile human sperm cells is associated with IVF-ICSI outcome. J. Androl. 2002, 23, 1–8. [Google Scholar] [CrossRef]
- Berkovitz, A.; Eltes, F.; Yaari, S.; Katz, N.; Barr, I.; Fishman, A.; Bartoov, B. The morphological normalcy of the sperm nucleus and pregnancy rate of intracytoplasmic injection with morphologically selected sperm. Hum. Reprod. 2005, 20, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Berkovitz, A.; Eltes, F.; Ellenbogen, A.; Peer, S.; Feldberg, D.; Bartoov, B. Does the presence of nuclear vacuoles in human sperm selected for ICSI affect pregnancy outcome? Hum. Reprod. 2006, 21, 1787–1790. [Google Scholar] [CrossRef] [Green Version]
- Vanderzwalmen, P.; Hiemer, A.; Rubner, P.; Bach, M.; Neyer, A.; Stecher, A.; Uher, P.; Zintz, M.; Lejeune, B.; Vanderzwalmen, S.; et al. Blastocyst development after sperm selection at high magnification is associated with size and number of nuclear vacuoles. Reprod. Biomed. Online 2008, 17, 617–627. [Google Scholar] [CrossRef]
- Utsuno, H.; Miyamoto, T.; Oka, K.; Shiozawa, T. Morphological alterations in protamine-deficient spermatozoa. Hum. Reprod. 2014, 29, 2374–2381. [Google Scholar] [CrossRef] [Green Version]
- Skowronek, F.; Casanova, G.; Alciaturi, J.; Capurro, A.; Cantu, L.; Montes, J.M.; Sapiro, R. DNA sperm damage correlates with nuclear ultrastructural sperm defects in teratozoospermic men. Andrologia 2012, 44, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, A.; Boni, R.; Leo, R.; Nacchia, G.; Liguori, F.; Casale, S.; Bonassisa, P.; Tosti, E. Vacuoles in sperm head are not associated with head morphology, DNA damage and reproductive success. Reprod. Biomed. Online 2015, 32, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Monqaut, A.L.; Zavaleta, C.; López, G.; Lafuente, R.; Brassesco, M. Use of high-magnification microscopy for the assessment of sperm recovered after two different sperm processing methods. Fertil. Steril. 2011, 95, 277–280. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction; WHO: Singapore, 1980. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction; WHO: Cambridge, UK, 1987. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction; WHO: Cambridge, UK, 1992. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Semen-Cervical Mucus Interaction; WHO: Cambridge, UK, 1999. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneve, Switzerland, 2010. [Google Scholar]
- Bartoov, B.; Berkovitz, A.; Eltes, F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N. Engl. J. Med. 2001, 345, 1067–1068. [Google Scholar] [CrossRef]
- Kovac, J.R.; Smith, R.P.; Cajipe, M.; Lamb, D.J.; Lipshultz, L.I. Men with a complete absence of normal sperm morphology exhibit high rates of success without assisted reproduction. Asian J. Androl. 2017, 19, 39–42. [Google Scholar] [PubMed]
- Komiya, A.; Watanabe, A.; Kawauchi, Y.; Fuse, H. Sperm with large nuclear vacuoles and semen quality in the evaluation of male infertility. Syst. Biol. Reprod. Med. 2013, 59, 13–20. [Google Scholar] [CrossRef]
- Moretti, E.; Sutera, G.; Collodel, G. The importance of transmission electron microscopy analysis of spermatozoa: Diagnostic applications and basic research. Syst. Biol. Reprod. Med. 2016, 62, 171–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, N.V.; Medina, H.; Colasante, C.; Osuna, A. Ultrastructural investigation of human sperm using atomic force microscopy. Arch. Androl. 2000, 44, 51–57. [Google Scholar]
- Visco, V.; Raffa, S.; Elia, J.; Delfino, M.; Imbrogno, N.; Torrisi, M.R.; Mazzilli, F. Morphological sperm defects analysed by light microscopy and transmission electron microscopy and their correlation with sperm motility. Int. J. Urol. 2010, 17, 259–266. [Google Scholar] [CrossRef]
- Oliveira, J.B.; Petersen, C.G.; Massaro, F.C.; Baruffi, R.L.; Mauri, A.L.; Silva, L.F.; Ricci, J.; Franco, J.G., Jr. Motile sperm organelle morphology examination (MSOME): Intervariation study of normal sperm and sperm with large nuclear vacuoles. Reprod. Biol. Endocrinol. 2010, 8, 56. [Google Scholar] [CrossRef] [Green Version]
- Bedford, J.M. Observations on the fine structure of spermatozoa of the bush baby (Galago senegalensis), the African green monkey (Cercopithecus aethiops) and man. Am. J. Anat. 1967, 121, 443–459. [Google Scholar] [CrossRef]
- Zamboni, L.; Zemjanis, R.; Stefanini, M. The fine structure of monkey and human spermatozoa. Anat. Rec. 1971, 169, 129–154. [Google Scholar] [CrossRef]
- Jiménez-Movilla, M.; Avilés, M.; Gómez-Torres, M.J.; Fernández-Colom, P.J.; Castells, M.T.; de Juan, J.; Romeu, A.; Ballesta, J. Carbohydrate analysis of the zona pellucida and cortical granules of human oocytes by means of ultrastructural cytochemistry. Hum. Reprod. 2004, 19, 1842–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flaherty, S.P.; Winfrey, V.P.; Olson, G.E. Localization of actin in human, bull, rabbit, and hamster sperm by immunoelectron microscopy. Anat. Rec. 1988, 221, 599–610. [Google Scholar] [CrossRef]
- Senn, A.; Germond, M.; De Grandi, P. Immunofluorescence study of actin, acrosin, dynein, tubulin and hyaluronidase and their impact on in-vitro fertilization. Hum. Reprod. 1992, 7, 841–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paranko, J.; Salonen, I. Calicin in human sperm fertilizing zona-free hamster eggs in vitro. Reprod. Fertil. Dev. 1995, 7, 97–105. [Google Scholar] [CrossRef] [PubMed]
| Sample | Sperm Cells (n) | Vacuolated Sperm (%) | Vacuoles/Cell (n) | Relative Vacuole Area (RVA) a |
|---|---|---|---|---|
| 1 | 101 | 42.574 | 1.615 | 9.659 |
| 2 | 96 | 64.583 | 1.455 | 9.204 |
| 3 | 124 | 43.548 | 1.238 | 10.081 |
| 4 | 98 | 51.020 | 1.391 | 7.365 |
| 5 | 170 | 24.118 | 1.167 | 1.144 |
| 6 | 106 | 58.491 | 1.455 | 9.310 |
| 7 | 83 | 48.193 | 1.391 | 7.826 |
| 8 | 75 | 30.667 | 1.294 | 10.185 |
| 9 | 167 | 16.766 | 1.409 | 11.379 |
| 10 | 134 | 38.060 | 1.150 | 8.893 |
| 11 | 100 | 65.000 | 1.235 | 9.160 |
| 12 | 106 | 48.113 | 1.500 | 6.540 |
| 13 | 109 | 34.862 | 1.100 | 11.039 |
| 14 | 106 | 45.283 | 1.118 | 10.119 |
| 15 | 106 | 70.755 | 1.200 | 11.370 |
| 16 | 104 | 62.500 | 1.667 | 11.550 |
| 17 | 123 | 66.667 | 1.391 | 6.359 |
| Mean | 47.718 | 1.340 | 9.658 | |
| SD | 15.678 | 0.170 | 1.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Torres, M.J.; Luna-Romero, J.; Fernández-Colom, P.J.; Aizpurua, J.; Avilés, M.; Romero, A. Human Sperm Head Vacuoles Are Related to Nuclear-Envelope Invaginations. Int. J. Mol. Sci. 2023, 24, 10027. https://doi.org/10.3390/ijms241210027
Gómez-Torres MJ, Luna-Romero J, Fernández-Colom PJ, Aizpurua J, Avilés M, Romero A. Human Sperm Head Vacuoles Are Related to Nuclear-Envelope Invaginations. International Journal of Molecular Sciences. 2023; 24(12):10027. https://doi.org/10.3390/ijms241210027
Chicago/Turabian StyleGómez-Torres, María José, Javier Luna-Romero, Pedro José Fernández-Colom, Jon Aizpurua, Manuel Avilés, and Alejandro Romero. 2023. "Human Sperm Head Vacuoles Are Related to Nuclear-Envelope Invaginations" International Journal of Molecular Sciences 24, no. 12: 10027. https://doi.org/10.3390/ijms241210027
APA StyleGómez-Torres, M. J., Luna-Romero, J., Fernández-Colom, P. J., Aizpurua, J., Avilés, M., & Romero, A. (2023). Human Sperm Head Vacuoles Are Related to Nuclear-Envelope Invaginations. International Journal of Molecular Sciences, 24(12), 10027. https://doi.org/10.3390/ijms241210027









