Tomato Biodiversity and Drought Tolerance: A Multilevel Review
Abstract
:1. Introduction
2. Gene-Based Resistance to Drought
3. Protective Role of Proteins against Drought
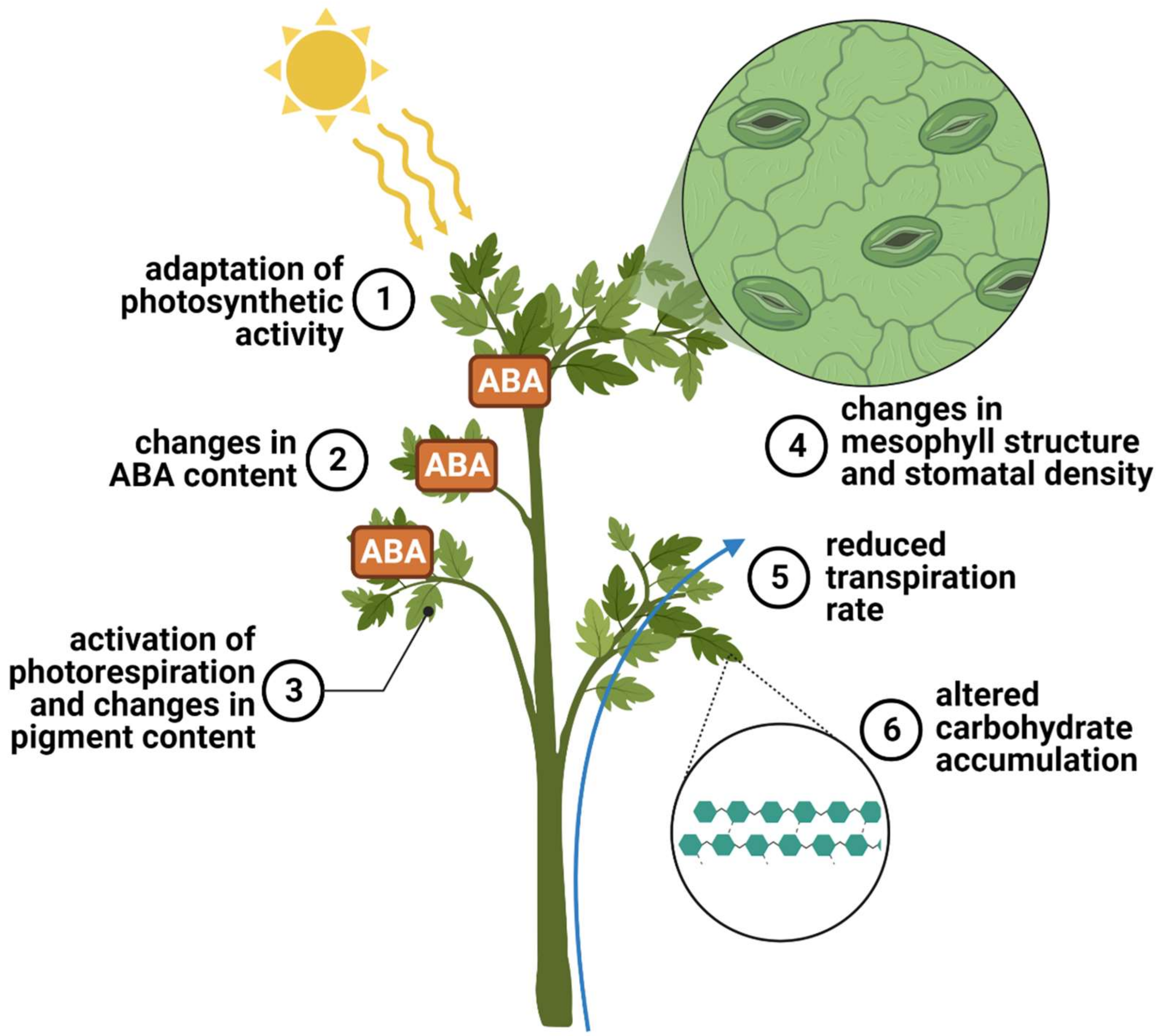
4. Diverse Physiological Responses to Drought
5. Drought Fortifies Tomato Fruit Quality
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change, I. Global Warming of 1.5 °C. Available online: https://www.ipcc.ch/sr15/ (accessed on 27 March 2023).
- D’Odorico, P.; Chiarelli, D.D.; Rosa, L.; Bini, A.; Zilberman, D.; Rulli, M.C. The global value of water in agriculture. Proc. Natl. Acad. Sci. USA 2020, 117, 21985–21993. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Cano-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W. Review: The World’s Water, 2000-2001: The Biennial Report on Freshwater Resources. Electron. Green J. 2002, 16, 2. [Google Scholar]
- Cui, Y.; Ouyang, S.; Zhao, Y.; Tie, L.; Shao, C.; Duan, H. Plant responses to high temperature and drought: A bibliometrics analysis. Front. Plant Sci. 2022, 13, 1052660. [Google Scholar] [CrossRef]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular Cloning and Functional Analysis of GmLACS2-3 Reveals Its Involvement in Cutin and Suberin Biosynthesis along with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Parrotta, L.; Aloisi, I.; Faleri, C.; Romi, M.; Del Duca, S.; Cai, G. Chronic heat stress affects the photosynthetic apparatus of Solanum lycopersicum L. cv Micro-Tom. Plant Physiol. Biochem. 2020, 154, 463–475. [Google Scholar] [CrossRef]
- Thompson, A.; Conley, M.; Herritt, M.; Thorp, K. Response of upland cotton (Gossypium hirsutum L.) leaf chlorophyll content to high heat and low-soil water in the Arizona low desert. Photosynthetica 2022, 60, 280–292. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, E. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Ogunkanmi, L.; MacCarthy, D.S.; Adiku, S.G.K. Impact of Extreme Temperature and Soil Water Stress on the Growth and Yield of Soybean (Glycine max (L.) Merrill). Agriculture 2021, 12, 43. [Google Scholar] [CrossRef]
- Hernandez-Santana, V.; Perez-Arcoiza, A.; Gomez-Jimenez, M.C.; Diaz-Espejo, A.; Gomez-Jimenez, M.C. Disentangling the link between leaf photosynthesis and turgor in fruit growth. Plant J. 2021, 107, 1788–1801. [Google Scholar] [CrossRef]
- Wan, W.; Liu, Z.; Li, J.; Xu, J.; Wu, H.; Xu, Z. Spatiotemporal patterns of maize drought stress and their effects on biomass in the Northeast and North China Plain from 2000 to 2019. Agr. Forest Meteorol. 2022, 315, 108821. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Gast, A.; Weber, C.; Daub, B.; Arneth, A. Water availability as dominant control of heat stress responses in two contrasting tree species. Tree Physiol. 2016, 36, 164–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bérard, A.; Ben Sassi, M.; Kaisermann, A.; Renault, P. Soil microbial community responses to heat wave components: Drought and high temperature. Clim. Res. 2015, 66, 243–264. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Wang, S.; Ren, F.; Wang, H. Climate warming extends growing season but not reproductive phase of terrestrial plants. Glob. Ecol. Biogeogr. 2021, 30, 950–960. [Google Scholar] [CrossRef]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Gatahi, D.M. Challenges and Opportunities in Tomato Production Chain and Sustainable Standards. Int. J. Hortic. Sci. Technol. 2020, 7, 235–262. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [Green Version]
- Erika, C.; Griebel, S.; Naumann, M.; Pawelzik, E. Biodiversity in Tomatoes: Is It Reflected in Nutrient Density and Nutritional Yields under Organic Outdoor Production? Front. Plant Sci. 2020, 11, 589692. [Google Scholar] [CrossRef]
- Bai, Y.; Lindhout, P. Domestication and Breeding of Tomatoes: What have We Gained and What Can We Gain in the Future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef]
- Chaudhary, J.; Khatri, P.; Singla, P.; Kumawat, S.; Kumari, A.R.V.; Vikram, A.; Jindal, S.K.; Kardile, H.; Kumar, R. Advances in Omics Approaches for Abiotic Stress Tolerance in Tomato. Biology 2019, 8, 90. [Google Scholar] [CrossRef] [Green Version]
- Oren, S.; Ceylan, H.; Schnable, P.S.; Dong, L. High-Resolution Patterning and Transferring of Graphene-Based Nanomaterials onto Tape toward Roll-to-Roll Production of Tape-Based Wearable Sensors. Adv. Mater. Technol. 2017, 2, 1700223. [Google Scholar] [CrossRef]
- Janni, M.; Coppede, N.; Bettelli, M.; Briglia, N.; Petrozza, A.; Summerer, S.; Vurro, F.; Danzi, D.; Cellini, F.; Marmiroli, N.; et al. In Vivo Phenotyping for the Early Detection of Drought Stress in Tomato. Plant Phenomics 2019, 2019, 6168209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, G.; Liu, J.; Zhang, J.; Guo, J. Effects of Drought Stress on Photosynthetic and Physiological Parameters of Tomato. J. Am. Soc. Hortic. Sci. 2020, 145, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Ochogavía, J.M.; Gago, J.; Roldán, E.J.; Cifre, J.; Conesa, M. Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: Anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ. 2012, 36, 920–935. [Google Scholar] [CrossRef]
- Galmés, J.; Conesa, M.; Ochogavía, J.M.; Perdomo, J.A.; Francis, D.M.; Ribas-Carbó, M.; Savé, R.; Flexas, J.; Medrano, H.; Cifre, J. Physiological and morphological adaptations in relation to water use efficiency in Mediterranean accessions of Solanum lycopersicum. Plant Cell Environ. 2011, 34, 245–260. [Google Scholar] [CrossRef]
- Kulkarni, M.; Deshpande, U. Anatomical breeding for altered leaf parameters in tomato genotypes imparting drought resistance using Leaf Strength Index. Asian J. Plant Sci. 2006, 5, 414–420. [Google Scholar] [CrossRef] [Green Version]
- Consortium, T.T.G.S.; Aflitos, S.; Schijlen, E.; de Jong, H.; de Ridder, D.; Smit, S.; Finkers, R.; Wang, J.; Zhang, G.; Li, N.; et al. Exploring genetic variation in the tomato (Solanum section Lycopersicon) clade by whole-genome sequencing. Plant J. 2014, 80, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Meng, X.; Cai, J.; Li, G.; Dong, T.; Li, Z. Basic leucine zipper transcription factor SlbZIP1 mediates salt and drought stress tolerance in tomato. BMC Plant Biol. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Tao, X.; Tang, X.-M.; Xiao, L.; Sun, J.-L.; Yan, X.-F.; Li, D.; Deng, H.-Y.; Ma, X.-R. Comparative transcriptome analysis of tomato (Solanum lycopersicum) in response to exogenous abscisic acid. BMC Genom. 2013, 14, 841. [Google Scholar] [CrossRef] [Green Version]
- Eiovieno, P.; Punzo, P.; Eguida, G.; Emistretta, C.; Van Oosten, M.; Enurcato, R.; Bostan, H.; Ecolantuono, C.; Ecosta, A.; Ebagnaresi, P.; et al. Transcriptomic Changes Drive Physiological Responses to Progressive Drought Stress and Rehydration in Tomato. Front. Plant Sci. 2016, 7, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egea, I.; Albaladejo, I.; Meco, V.; Morales, B.; Sevilla, A.; Bolarin, M.C.; Flores, F.B. The drought-tolerant Solanum pennellii regulates leaf water loss and induces genes involved in amino acid and ethylene/jasmonate metabolism under dehydration. Sci. Rep. 2018, 8, 2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Jiang, N.; Zhou, X.X.; Hou, X.X.; Yang, G.L.; Meng, J.; Luan, Y.S. Tomato MYB49 enhances resistance to Phytophthora infestans and tolerance to water deficit and salt stress. Planta 2018, 248, 1487–1503. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, T.; Zhang, J.; Wang, Z.; Pei, T.; Yang, H.; Li, J.; Xu, X. Genome-Wide Analyses of the Genetic Screening of C2H2-Type Zinc Finger Transcription Factors and Abiotic and Biotic Stress Responses in Tomato (Solanum lycopersicum) Based on RNA-Seq Data. Front. Genet. 2020, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Foolad, M.R.; Panthee, D.R. Marker-Assisted Selection in Tomato Breeding. Crit. Rev. Plant Sci. 2012, 31, 93–123. [Google Scholar] [CrossRef]
- Younis, A.; Ramzan, F.; Ramzan, Y.; Zulfiqar, F.; Ahsan, M.; Lim, K.B. Molecular Markers Improve Abiotic Stress Tolerance in Crops: A Review. Plants 2020, 9, 1374. [Google Scholar] [CrossRef]
- Sardaro, M.L.S.; Marmiroli, M.; Maestri, E.; Marmiroli, N. Genetic characterization of Italian tomato varieties and their traceability in tomato food products. Food Sci. Nutr. 2013, 1, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, V.; Mareri, L.; Faleri, C.; Nepi, M.; Romi, M.; Cai, G.; Cantini, C. Drought Stress Affects the Response of Italian Local Tomato (Solanum lycopersicum L.) Varieties in a Genotype-Dependent Manner. Plants 2019, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Naeem, M.; Muqarab, R.; Waseem, M. The Solanum melongena COP1 delays fruit ripening and influences ethylene signaling in tomato. J. Plant Physiol. 2019, 240, 152997. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Shahzad, K.; Saqib, S.; Shahzad, A.; Nasrullah; Younas, M.; Afridi, M.I. The Solanum melongena COP1LIKE manipulates fruit ripening and flowering time in tomato (Solanum lycopersicum). Plant Growth Regul. 2022, 96, 369–382. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, J.; Wang, Q.; Zhou, K.; Mao, K.; Ma, F. Overexpression of MdEPF2 improves water use efficiency and reduces oxidative stress in tomato. Environ. Exp. Bot. 2019, 162, 321–332. [Google Scholar] [CrossRef]
- Albacete, A.; Cantero-Navarro, E.; Großkinsky, D.K.; Arias, C.L.; Balibrea, M.E.; Bru, R.; Fragner, L.; Ghanem, M.E.; González, M.d.l.C.; Hernández, J.A.; et al. Ectopic overexpression of the cell wall invertase gene CIN1 leads to dehydration avoidance in tomato. J. Exp. Bot. 2015, 66, 863–878. [Google Scholar] [CrossRef]
- Wang, P.; Luo, Q.; Yang, W.; Ahammed, G.J.; Ding, S.; Chen, X.; Wang, J.; Xia, X.; Shi, K. A Novel Role of Pipecolic Acid Biosynthetic Pathway in Drought Tolerance through the Antioxidant System in Tomato. Antioxidants 2021, 10, 1923. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.B.; Iannacone, R.; Petrozza, A.; Mishra, A.; Armentano, N.; La Vecchia, G.; Trtílek, M.; Cellini, F.; Nedbal, L. Engineered drought tolerance in tomato plants is reflected in chlorophyll fluorescence emission. Plant Sci. 2012, 182, 79–86. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Li, S.; Zhang, L.; Qi, C.; Weeda, S.; Zhao, B.; Ren, S.; Guo, Y.-D. Plasma Membrane Intrinsic Proteins SlPIP2;1, SlPIP2;7 and SlPIP2;5 Conferring Enhanced Drought Stress Tolerance in Tomato. Sci. Rep. 2016, 6, 31814. [Google Scholar] [CrossRef] [Green Version]
- Goel, D.; Singh, A.K.; Yadav, V.; Babbar, S.B.; Bansal, K.C. Overexpression of osmotin gene confers tolerance to salt and drought stresses in transgenic tomato (Solanum lycopersicum L.). Protoplasma 2010, 245, 133–141. [Google Scholar] [CrossRef]
- Muñoz-Mayor, A.; Pineda, B.; Garcia-Abellán, J.O.; Antón, T.; Garcia-Sogo, B.; Sanchez-Bel, P.; Flores, F.B.; Atarés, A.; Angosto, T.; Pintor-Toro, J.A.; et al. Overexpression of dehydrin tas14 gene improves the osmotic stress imposed by drought and salinity in tomato. J. Plant Physiol. 2012, 169, 459–468. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Li, R.; Zhao, R.; Yang, M.; Sheng, J.; Shen, L. Reduced Drought Tolerance by CRISPR/Cas9-Mediated SlMAPK3 Mutagenesis in Tomato Plants. J. Agric. Food Chem. 2017, 65, 8674–8682. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhang, J.; Ma, Y.; Li, Y.; Zhang, F.; Zhang, Y.; Liang, Y. Overexpression of a Mitogen-Activated Protein Kinase SlMAPK3 Positively Regulates Tomato Tolerance to Cadmium and Drought Stress. Molecules 2019, 24, 556. [Google Scholar] [CrossRef] [Green Version]
- Thirumalaikumar, V.P.; Devkar, V.; Mehterov, N.; Ali, S.; Ozgur, R.; Turkan, I.; Mueller-Roeber, B.; Balazadeh, S. NAC transcription factor JUNGBRUNNEN1 enhances drought tolerance in tomato. Plant Biotechnol. J. 2018, 16, 354–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achard, P.; Gong, F.; Cheminant, S.; Alioua, M.; Hedden, P.; Genschik, P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 2008, 20, 2117–2129. [Google Scholar] [CrossRef] [Green Version]
- Achard, P.; Renou, J.P.; Berthome, R.; Harberd, N.P.; Genschik, P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr. Biol. 2008, 18, 656–660. [Google Scholar] [CrossRef] [Green Version]
- Faqir Napar, W.P.; Kaleri, A.R.; Ahmed, A.; Nabi, F.; Sajid, S.; Ćosić, T.; Yao, Y.; Liu, J.; Raspor, M.; Gao, Y. The anthocyanin-rich tomato genotype LA-1996 displays superior efficiency of mechanisms of tolerance to salinity and drought. J. Plant Physiol. 2022, 271, 153662. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Douthe, C.; El Aou-ouad, H.; Ribas-Carbó, M.; Galmés, J. Tomato landraces as a source to minimize yield losses and improve fruit quality under water deficit conditions. Agric. Water Manag. 2019, 223, 105722. [Google Scholar] [CrossRef]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of tomato landraces for drought tolerance based on growth and chlorophyll fluorescence analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Aloisi, I.; Del Duca, S.; Canelo, V.; Torrigiani, P.; Silva, H.; Biondi, S. Salares versus coastal ecotypes of quinoa: Salinity responses in Chilean landraces from contrasting habitats. Plant Physiol. Bioch. 2016, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.K.; Parveen, A.; Jamwal, G.; Basu, U.; Kumar, R.R.; Rai, P.K.; Sharma, J.P.; Alalawy, A.I.; Al-Duais, M.A.; Hossain, M.A.; et al. Leaf Proteome Response to Drought Stress and Antioxidant Potential in Tomato (Solanum lycopersicum L.). Atmosphere 2021, 12, 1021. [Google Scholar] [CrossRef]
- Kosová, K.; Vítámvás, P.; Urban, M.O.; Prášil, I.T.; Renaut, J. Plant Abiotic Stress Proteomics: The Major Factors Determining Alterations in Cellular Proteome. Front. Plant Sci. 2018, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Latef, A.A.H.A.; Rasool, S.; Akram, N.A.; Ashraf, M.; Gucel, S. Role of Proteomics in Crop Stress Tolerance. Front. Plant Sci. 2016, 7, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Palmer, M.; Zhou, J.; Bhatti, S.; Howe, K.J.; Fish, T.; Thannhauser, T.W. Differential Root Proteome Expression in Tomato Genotypes with Contrasting Drought Tolerance Exposed to Dehydration. J. Am. Soc. Hortic. Sci. 2013, 138, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.; Agrawal, V.; Gupta, S.C. Comparison of drought-induced polypeptides and ion leakage in three tomato cultivars. Biol. Plant. 2009, 53, 685–690. [Google Scholar] [CrossRef]
- Metwali, E.; Carle, R.; Schweiggert, R.; Almaghrabi, O.; Kaddasa, N. Genetic Variability Analysis for the Selection of Drought-Tolerant Tomato (Lycopersicon esculentum Mill.) Germplasm as Investigated by in Vitro Callus, Shoot, and Plantlet Cultures. J. Pure Appl. Microbiol. 2015, 9, 79–95. [Google Scholar]
- Qi, J.; Song, C.-P.; Wang, B.; Zhou, J.; Kangasjärvi, J.; Zhu, J.-K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative damage and antioxidant mechanism in tomatoes responding to drought and heat stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Noori, M.; Azar, A.M.; Saidi, M.; Panahandeh, J.; Haghi, D.Z. Evaluation of water deficiency impacts on antioxidant enzymes activity and lipid peroxidation in some tomato (Solanum lycopersicum L.) lines. Indian J. Agric. Res. 2018, 52, 228–235. [Google Scholar] [CrossRef]
- Çelik, Ö.; Ayan, A.; Atak, Ç. Enzymatic and non-enzymatic comparison of two different industrial tomato (Solanum lycopersicum) varieties against drought stress. Bot. Stud. 2017, 58, 32. [Google Scholar] [CrossRef] [Green Version]
- Landi, S.; De Lillo, A.; Nurcato, R.; Grillo, S.; Esposito, S. In-field study on traditional Italian tomato landraces: The constitutive activation of the ROS scavenging machinery reduces effects of drought stress. Plant Physiol. Bioch. 2017, 118, 150–160. [Google Scholar] [CrossRef]
- Rahman, S.M.L.; Mackay, W.A.; Nawata, E.; Sakuratani, T.; Uddin, A.S.M.M.; Quebedeaux, B. Superoxide Dismutase and Stress Tolerance of Four Tomato Cultivars. HortScience 2004, 39, 983–986. [Google Scholar] [CrossRef] [Green Version]
- Shamim, F.; Khan, K.; Athar, H.-u.-R.; Waheed, A. Protection of photosynthetic machinery by up-regulation of antioxidant enzymes in contrasting tomato genotypes under drought. Pak. J. Bot. 2015, 47, 1231–1239. [Google Scholar]
- Hajheidari, M.; Eivazi, A.; Buchanan, B.B.; Wong, J.H.; Majidi, I.; Salekdeh, G.H. Proteomics Uncovers a Role for Redox in Drought Tolerance in Wheat. J. Proteome Res. 2007, 6, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Perlikowski, D.; Kosmala, A.; Rapacz, M.; Kościelniak, J.; Pawłowicz, I.; Zwierzykowski, Z. Influence of short-term drought conditions and subsequent re-watering on the physiology and proteome of Lolium multiflorum/Festuca arundinacea introgression forms, with contrasting levels of tolerance to long-term drought. Plant Biol. 2014, 16, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Cresti, M.; Cai, G. Heat-shock protein 70 binds microtubules and interacts with kinesin in tobacco pollen tubes. Cytoskeleton 2013, 70, 522–537. [Google Scholar] [CrossRef]
- Conti, V.; Cantini, C.; Romi, M.; Cesare, M.M.; Parrotta, L.; Del Duca, S.; Cai, G. Distinct Tomato Cultivars Are Characterized by a Differential Pattern of Biochemical Responses to Drought Stress. Int. J. Mol. Sci. 2022, 23, 5412. [Google Scholar] [CrossRef]
- Parrotta, L.; Aloisi, I.; Suanno, C.; Faleri, C.; Kiełbowicz-Matuk, A.; Bini, L.; Cai, G.; Del Duca, S. A low molecular-weight cyclophilin localizes in different cell compartments of Pyrus communis pollen and is released in vitro under Ca2+ depletion. Plant Physiol. Bioch. 2019, 144, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Gläßer, C.; Haberer, G.; Finkemeier, I.; Pfannschmidt, T.; Kleine, T.; Leister, D.; Dietz, K.-J.; Häusler, R.E.; Grimm, B.; Mayer, K.F.X. Meta-Analysis of Retrograde Signaling in Arabidopsis thaliana Reveals a Core Module of Genes Embedded in Complex Cellular Signaling Networks. Mol. Plant 2014, 7, 1167–1190. [Google Scholar] [CrossRef] [Green Version]
- Dondini, L.; Bonazzi, S.; Del Duca, S.; Bregoli, A.M.; Serafini-Fracassini, D. Acclimation of chloroplast transglutaminase to high NaClconcentration in a polyamine-deficient variant strain of Dunaliella salina and in its wild type. J. Plant Physiol. 2001, 158, 185–197. [Google Scholar] [CrossRef]
- Tamburino, R.; Vitale, M.; Ruggiero, A.; Sassi, M.; Sannino, L.; Arena, S.; Costa, A.; Batelli, G.; Zambrano, N.; Scaloni, A.; et al. Chloroplast proteome response to drought stress and recovery in tomato (Solanum lycopersicum L.). BMC Plant Bio. 2017, 17, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parry, M.A.J.; Andralojc, P.J.; Khan, S.; Lea, P.J.; Keys, A.J. Rubisco Activity: Effects of Drought Stress. Ann. Bot. 2002, 89, 833–839. [Google Scholar] [CrossRef] [Green Version]
- Feller, U.; Anders, I.; Demirevska, K. Degradation of rubisco and other chloroplast proteins under abiotic stress. Gen. Appl. Plant Physiol. 2008, 34, 5–18. [Google Scholar]
- Demirevska, K.; Zasheva, D.; Dimitrov, R.; Simova-Stoilova, L.; Stamenova, M.; Feller, U. Drought stress effects on Rubisco in wheat: Changes in the Rubisco large subunit. Acta Physiol. Plant. 2009, 31, 1129–1138. [Google Scholar] [CrossRef]
- Hasanagić, D.; Koleška, I.; Kojić, D.; Vlaisavljević, S.; Janjić, N.; Kukavica, B. Long term drought effects on tomato leaves: Anatomical, gas exchange and antioxidant modifications. Acta Physiol. Plant. 2020, 42, 121. [Google Scholar] [CrossRef]
- Piccini, C.; Cai, G.; Dias, M.C.; Araújo, M.; Parri, S.; Romi, M.; Faleri, C.; Cantini, C. Olive Varieties under UV-B Stress Show Distinct Responses in Terms of Antioxidant Machinery and Isoform/Activity of RubisCO. Int. J. Mol. Sci. 2021, 22, 11214. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Jin, Y.; Yang, Y.-J.; Li, G.-J.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Beena, R.; Laksmi, G.; Soni, K.B.; Alex, S.; Viji, M.M. Changes in sucrose metabolic enzymes to water stress in contrasting rice genotypes. Plant Stress 2022, 5, 100088. [Google Scholar] [CrossRef]
- Somanaboina, A.; Narasu, L.; Varshney, R.; Kishor, P. Development of transgenic tomato (Solanum lycoperscicum L.) by heterologous expression of osmotin-like protein (OLP) and chitinase (Chi11) genes for salt and drought stress tolerance. In Proceedings of the InterDrought-V, Hyderabad, India, 21–25 February 2017. [Google Scholar]
- Giorio, P.; Guida, G.; Mistretta, C.; Sellami, M.H.; Oliva, M.; Punzo, P.; Iovieno, P.; Arena, C.; De Maio, A.; Grillo, S.; et al. Physiological, biochemical and molecular responses to water stress and rehydration in Mediterranean adapted tomato landraces. Plant Biol. 2018, 20, 995–1004. [Google Scholar] [CrossRef]
- Yu, L.-X.; Djebrouni, M.; Chamberland, H.; Lafontaine, J.G.; Tabaeizadeh, Z. Chitinase: Differential induction of gene expression and enzyme activity by drought stress in the wild (Lycopersicon chilense Dun.) and cultivated (L. esculentum Mill.) tomatoes. J. Plant Physiol. 1998, 153, 745–753. [Google Scholar] [CrossRef]
- Leoni, C.; Volpicella, M.; Dileo, M.C.G.; Gattulli, B.A.R.; Ceci, L.R. Chitinases as Food Allergens. Molecules 2019, 24, 2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Włodarczyk, K.; Smolińska, B.; Majak, I. Tomato Allergy: The Characterization of the Selected Allergens and Antioxidants of Tomato (Solanum lycopersicum)—A Review. Antioxidants 2022, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Devadasu, E.; Saini, D.; Dhokne, K.; Marriboina, S.; Raghavendra, A.S.; Subramanyam, R. Reversible changes in structure and function of photosynthetic apparatus of pea (Pisum sativum) leaves under drought stress. Plant J. 2023, 113, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Liu, H.; Chen, Y.-E.; Yin, Y.; Zhang, Z.-W.; Song, J.; Chang, L.-J.; Zhang, F.-L.; Wang, D.; Dai, X.-H.; et al. Chloroplastic photoprotective strategies differ between bundle sheath and mesophyll cells in maize (Zea mays L.) Under drought. Front. Plant Sci. 2022, 13, 885781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Lei, H.; Xu, C.; Chen, G.X. Long-term drought resistance in rice (Oryza sativa L.) during leaf senescence: A photosynthetic view. Plant Growth Regul. 2019, 88, 253–266. [Google Scholar] [CrossRef]
- Sapeta, H.; Yokono, M.; Takabayashi, A.; Ueno, Y.; Cordeiro, A.M.; Hara, T.; Tanaka, A.; Akimoto, S.; Oliveira, M.M.; Tanaka, R. Reversible down-regulation of photosystems I and II leads to fast photosynthesis recovery after long-term drought in Jatropha curcas. J. Exp. Bot. 2023, 74, 336–351. [Google Scholar] [CrossRef]
- Stefanov, M.; Rashkov, G.; Borisova, P.; Apostolova, E. Sensitivity of the Photosynthetic Apparatus in Maize and Sorghum under Different Drought Levels. Plants 2023, 12, 1863. [Google Scholar] [CrossRef] [PubMed]
- Borba, M.E.A.; Maciel, G.M.; Fraga Júnior, E.F.; Machado Júnior, C.S.; Marquez, G.R.; Silva, I.G.; Almeida, R.S. Gas exchanges and water use efficiency in the selection of tomato genotypes tolerant to water stress. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Bsoul, E.Y.; Al-Afaeshat, A.F.; Qaryouti, M. Growth, water relation and physiological responses of drought stressed Irhaba tomato landrace as compared with Amani and GS-12 cultivars. J. Food Agric. Environ. 2016, 14, 78–84. [Google Scholar]
- Wahb-Allah, M.A.; Alsadon, A.A.; Ibrahim, A.A. Drought tolerance of several tomato genotypes under greenhouse conditions. World Appl. Sci. J. 2011, 15, 933–940. [Google Scholar]
- Aghaie, P.; Hosseini Tafreshi, S.A.; Ebrahimi, M.A.; Haerinasab, M. Tolerance evaluation and clustering of fourteen tomato cultivars grown under mild and severe drought conditions. Sci. Hortic. 2018, 232, 1–12. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Parri, S.; Aloisi, I.; Marino, G.; Cai, G.; Cantini, C. Morpho-Physiological Classification of Italian Tomato Cultivars (Solanum lycopersicum L.) According to Drought Tolerance during Vegetative and Reproductive Growth. Plants 2021, 10, 1826. [Google Scholar] [CrossRef]
- Sivakumar, R.; Durga Devi, D.; Chandrasekar, C.N.; Santhi, R.; Vijayakumar, R.M. Impact of drought on gas exchange and physiological parameters and yield in contrasting genotypes of tomato (Solanum lycopersicum). Indian J. Plant Physiol. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- Moles, T.M.; Mariotti, L.; De Pedro, L.F.; Guglielminetti, L.; Picciarelli, P.; Scartazza, A. Drought induced changes of leaf-to-root relationships in two tomato genotypes. Plant Physiol. Bioch. 2018, 128, 24–31. [Google Scholar] [CrossRef]
- Rigano, M.M.; Arena, C.; Di Matteo, A.; Sellitto, S.; Frusciante, L.; Barone, A. Eco-physiological response to water stress of drought-tolerant and drought-sensitive tomato genotypes. Plant Biosystems 2016, 150, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.D.M.; Ríos, J.J.; Blasco, B.; Rosales, M.Á.; Melgarejo, R.; Romero, L.; Ruiz, J.M. Ammonia production and assimilation: Its importance as a tolerance mechanism during moderate water deficit in tomato plants. J. Plant Physiol. 2011, 168, 816–823. [Google Scholar] [CrossRef]
- Amuji, C.F.; Beaumont, L.J.; Atwell, B.J. The effect of co-occurring heat and water stress on reproductive traits and yield of tomato (Solanum lycopersicum). Hortic. J. 2020, 89, 530–536. [Google Scholar] [CrossRef]
- Nankishore, A.; Farrell, A.D. The response of contrasting tomato genotypes to combined heat and drought stress. J. Plant Physiol. 2016, 202, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kautz, B.; Noga, G.; Hunsche, M. Controlled long-term water deficiency and its impact on the fluorescence emission of tomato leaves during stress and re-watering. Eur. J. Hortic. Sci. 2014, 79, 60–69. [Google Scholar]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Fullana-Pericàs, M.; Conesa, M.À.; Ribas-Carbó, M.; Galmés, J. The use of a tomato landrace as rootstock improves the response of commercial tomato under water deficit conditions. Agronomy 2020, 10, 748. [Google Scholar] [CrossRef]
- Badawy, M.A.; Abdel-Wahab, A.; Sayed, E.G. Increasing tomato (Solanum Lycopersicum, L.) tolerance of water stress conditions by using some agricultural practices. Plant Arch. 2020, 20, 2655–2676. [Google Scholar]
- Pazzagli, P.T.; Weiner, J.; Liu, F. Effects of CO2 elevation and irrigation regimes on leaf gas exchange, plant water relations, and water use efficiency of two tomato cultivars. Agric. Water Manag. 2016, 169, 26–33. [Google Scholar] [CrossRef]
- Guida, G.; Sellami, M.H.; Mistretta, C.; Oliva, M.; Buonomo, R.; De Mascellis, R.; Patanè, C.; Rouphael, Y.; Albrizio, R.; Giorio, P. Agronomical, physiological and fruit quality responses of two Italian long-storage tomato landraces under rain-fed and full irrigation conditions. Agric. Water Manag. 2017, 180, 126–135. [Google Scholar] [CrossRef]
- Conti, V.; Romi, M.; Guarnieri, M.; Cantini, C.; Cai, G. Italian Tomato Cultivars under Drought Stress Show Different Content of Bioactives in Pulp and Peel of Fruits. Foods 2022, 11, 270. [Google Scholar] [CrossRef]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamasi, G.; Pardini, A.; Bonechi, C.; Donati, A.; Pessina, F.; Marcolongo, P.; Gamberucci, A.; Leone, G.; Consumi, M.; Magnani, A.; et al. Characterization of nutraceutical components in tomato pulp, skin and locular gel. Eur. Food Res. Technol. 2019, 245, 907–918. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dere, S.; Kusvuran, S.; Dasgan, H.Y. Does drought increase the antioxidant nutrient capacity of tomatoes? Int. J. Food Sci. Technol. 2022, 57, 6633–6645. [Google Scholar] [CrossRef]
- Bogale, A.; Nagle, M.; Latif, S.; Aguila, M.; Müller, J. Regulated deficit irrigation and partial root-zone drying irrigation impact bioactive compounds and antioxidant activity in two select tomato cultivars. Sci. Hortic. 2016, 213, 115–124. [Google Scholar] [CrossRef]
- Patanè, C.; Siah, S.; Pellegrino, A.; Cosentino, S.L.; Siracusa, L. Fruit yield, polyphenols, and carotenoids in long shelf-life tomatoes in response to drought stress and rewatering. Agronomy 2021, 11, 1943. [Google Scholar] [CrossRef]
- Dariva, F.D.; Pessoa, H.P.; Copati, M.G.F.; de Almeida, G.Q.; de Castro Filho, M.N.; Picoli, E.A.D.T.; da Cunha, F.F.; Nick, C. Yield and fruit quality attributes of selected tomato introgression lines subjected to long-term deficit irrigation. Sci. Hortic. 2021, 289, 110426. [Google Scholar] [CrossRef]
- Sivakumar, R.; Srividhya, S. Impact of drought on flowering, yield and quality parameters in diverse genotypes of tomato (Solanum lycopersicum L.). Adv. Hortic. Sci. 2016, 30, 3–11. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conti, V.; Parrotta, L.; Romi, M.; Del Duca, S.; Cai, G. Tomato Biodiversity and Drought Tolerance: A Multilevel Review. Int. J. Mol. Sci. 2023, 24, 10044. https://doi.org/10.3390/ijms241210044
Conti V, Parrotta L, Romi M, Del Duca S, Cai G. Tomato Biodiversity and Drought Tolerance: A Multilevel Review. International Journal of Molecular Sciences. 2023; 24(12):10044. https://doi.org/10.3390/ijms241210044
Chicago/Turabian StyleConti, Veronica, Luigi Parrotta, Marco Romi, Stefano Del Duca, and Giampiero Cai. 2023. "Tomato Biodiversity and Drought Tolerance: A Multilevel Review" International Journal of Molecular Sciences 24, no. 12: 10044. https://doi.org/10.3390/ijms241210044




