Morphological Characterization of Human Lung Cancer Organoids Cultured in Type I Collagen Hydrogels: A Histological Approach
Abstract
1. Introduction
2. Results
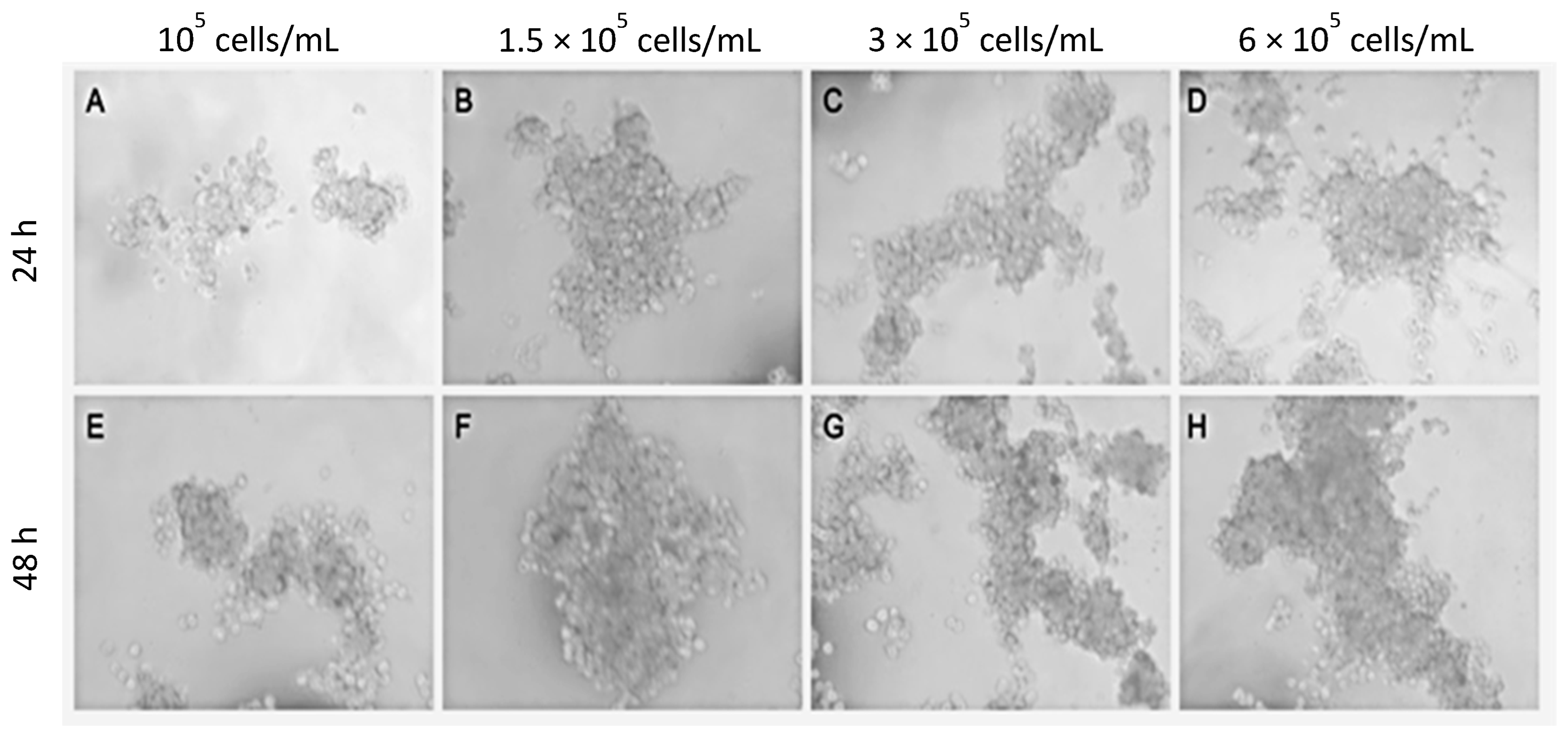
2.1. Optimization of Spheroid/Organoid Manufacture
2.2. Spheroid Morphology of A549 Cells
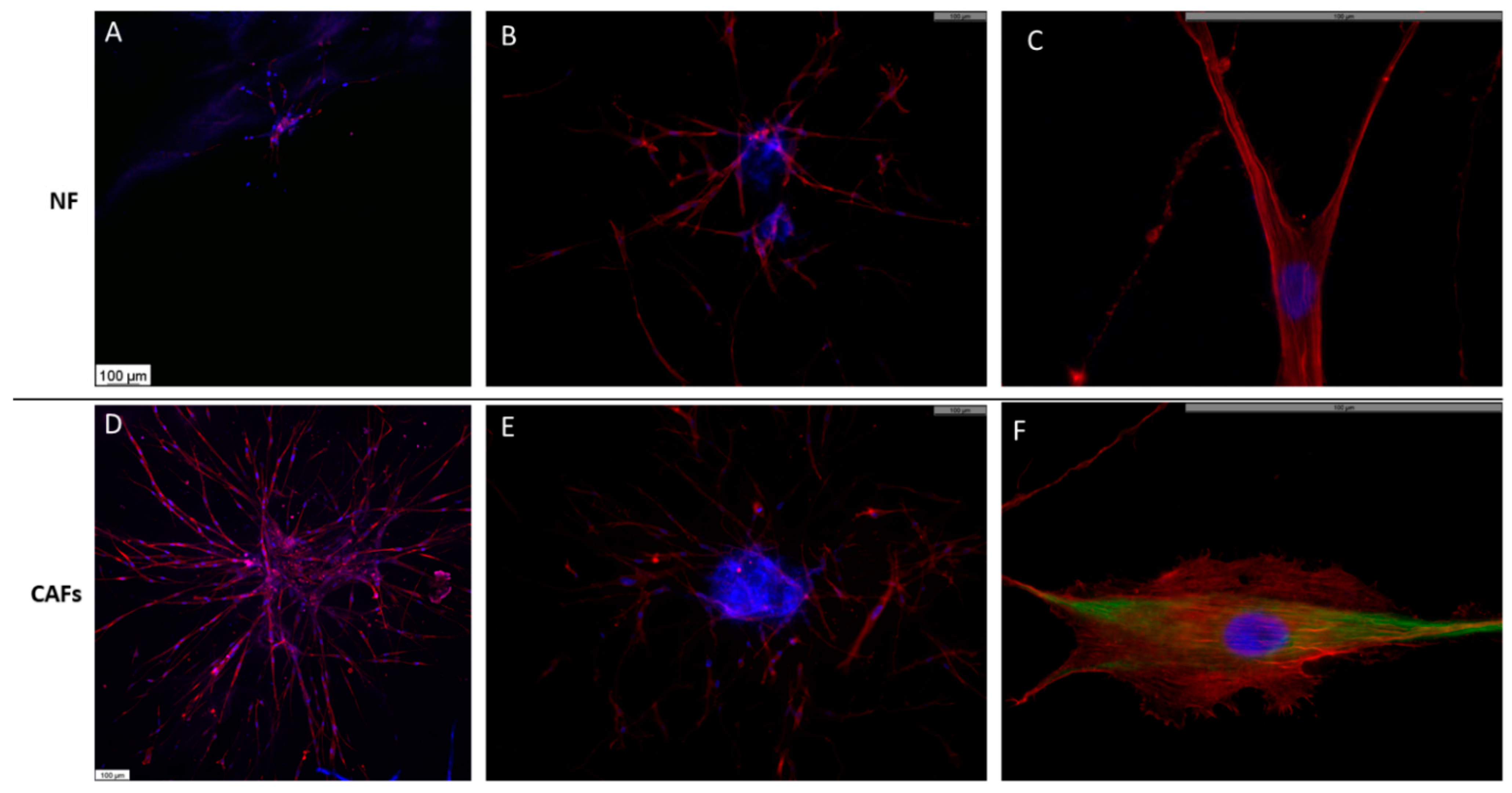
2.3. Morphology of NFs and CAFs Spheroids
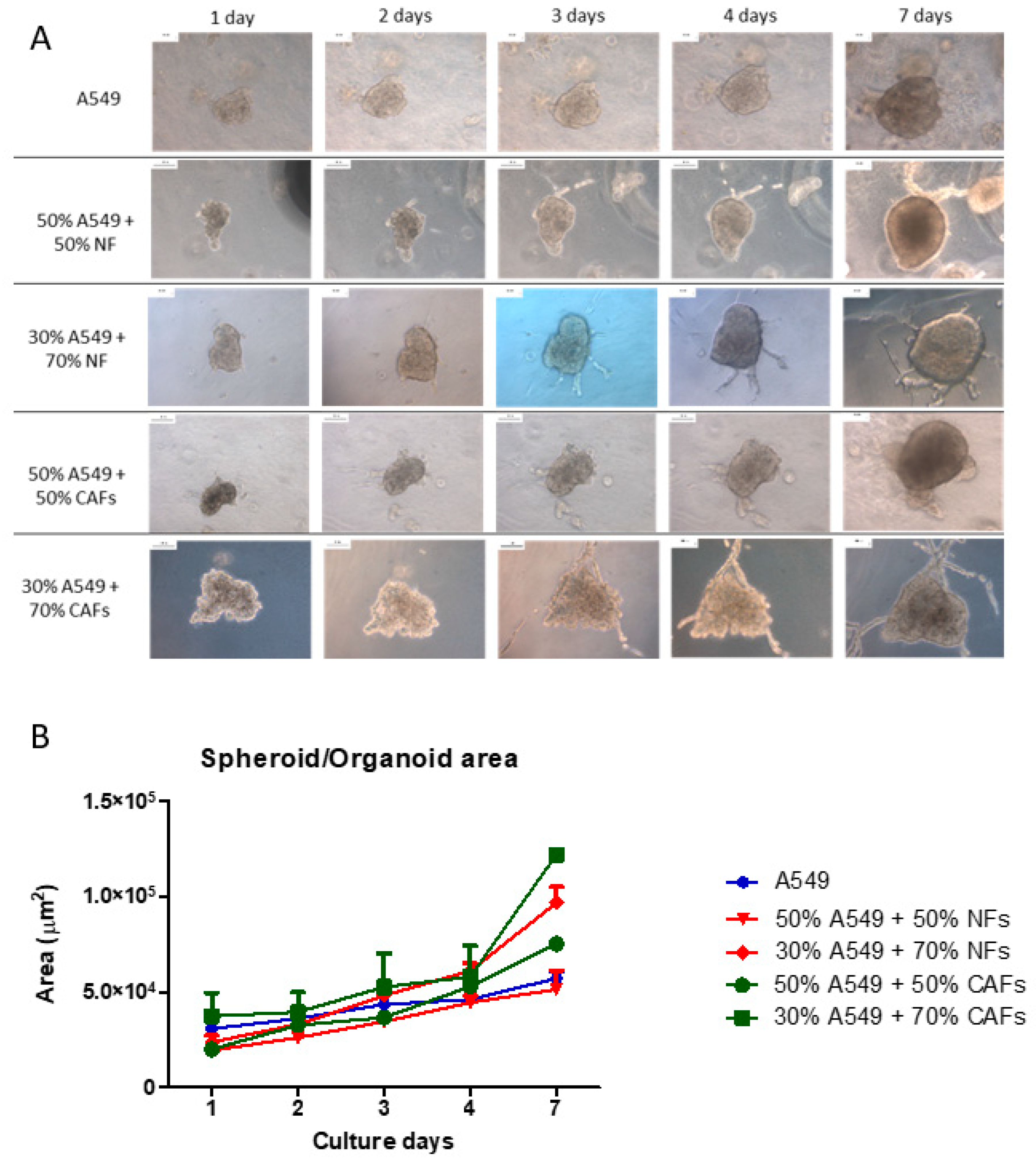
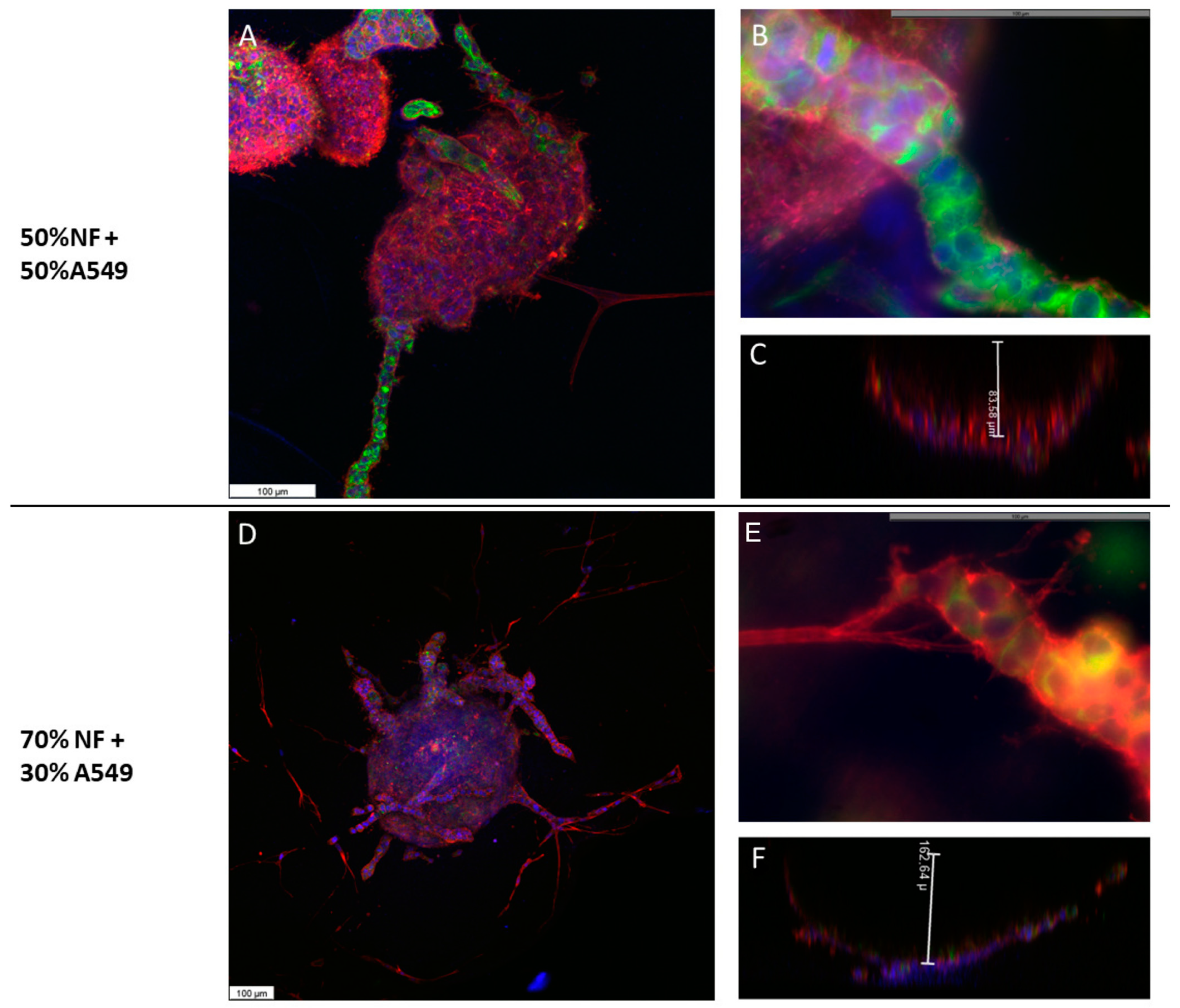
2.4. A549 + NFs Organoids Morphology
2.5. A549 + CAFs Organoids Morphology
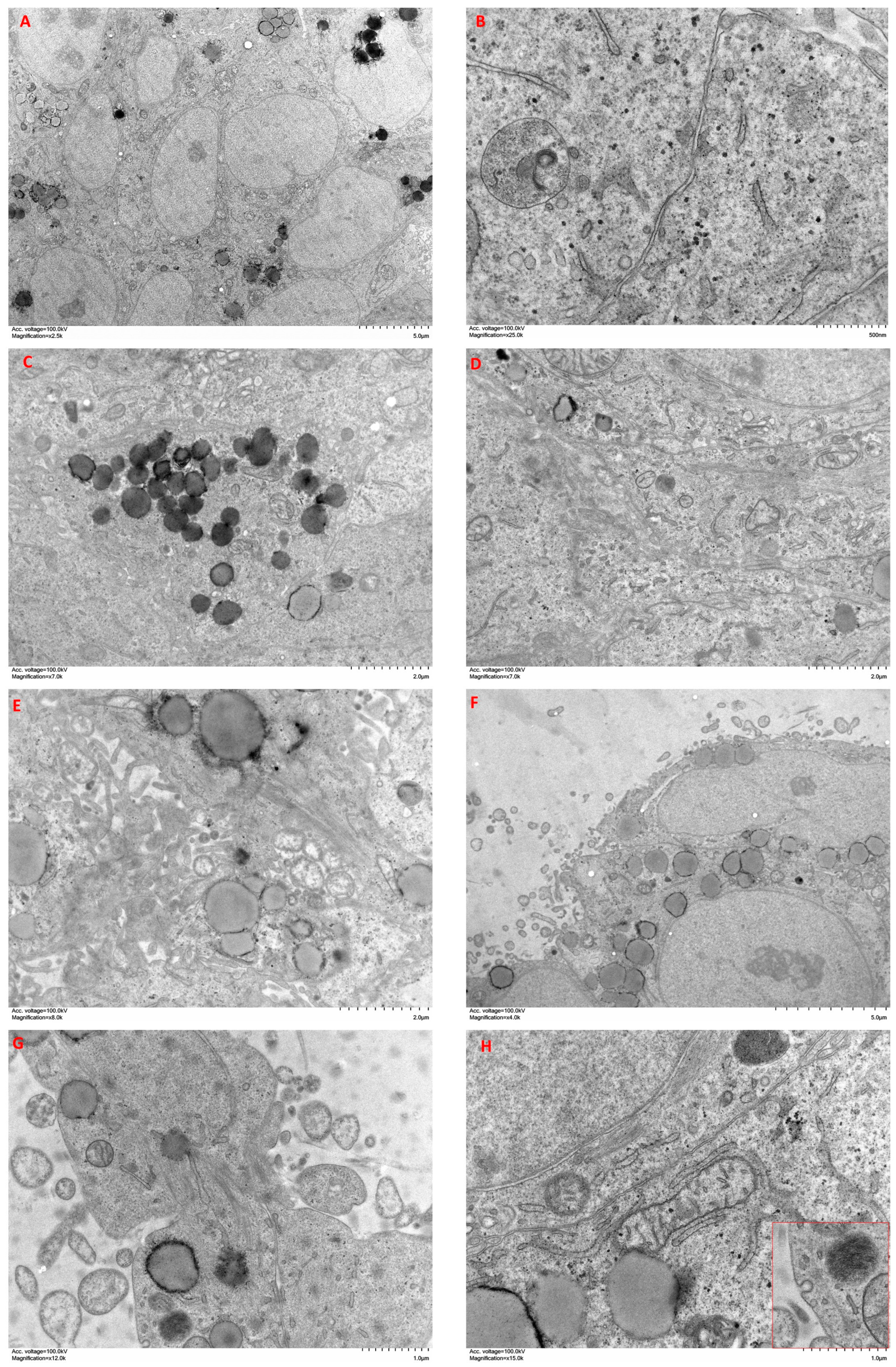
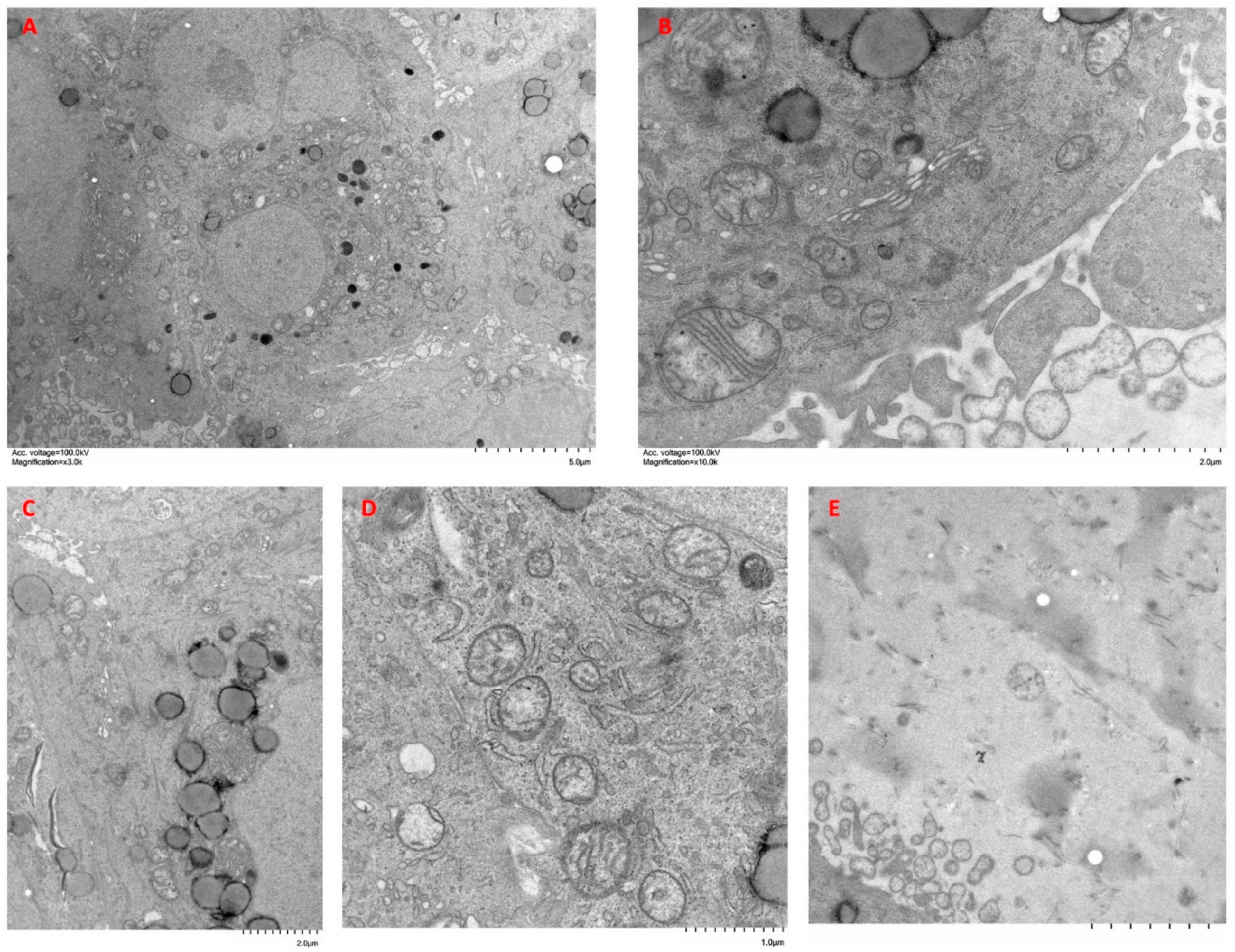
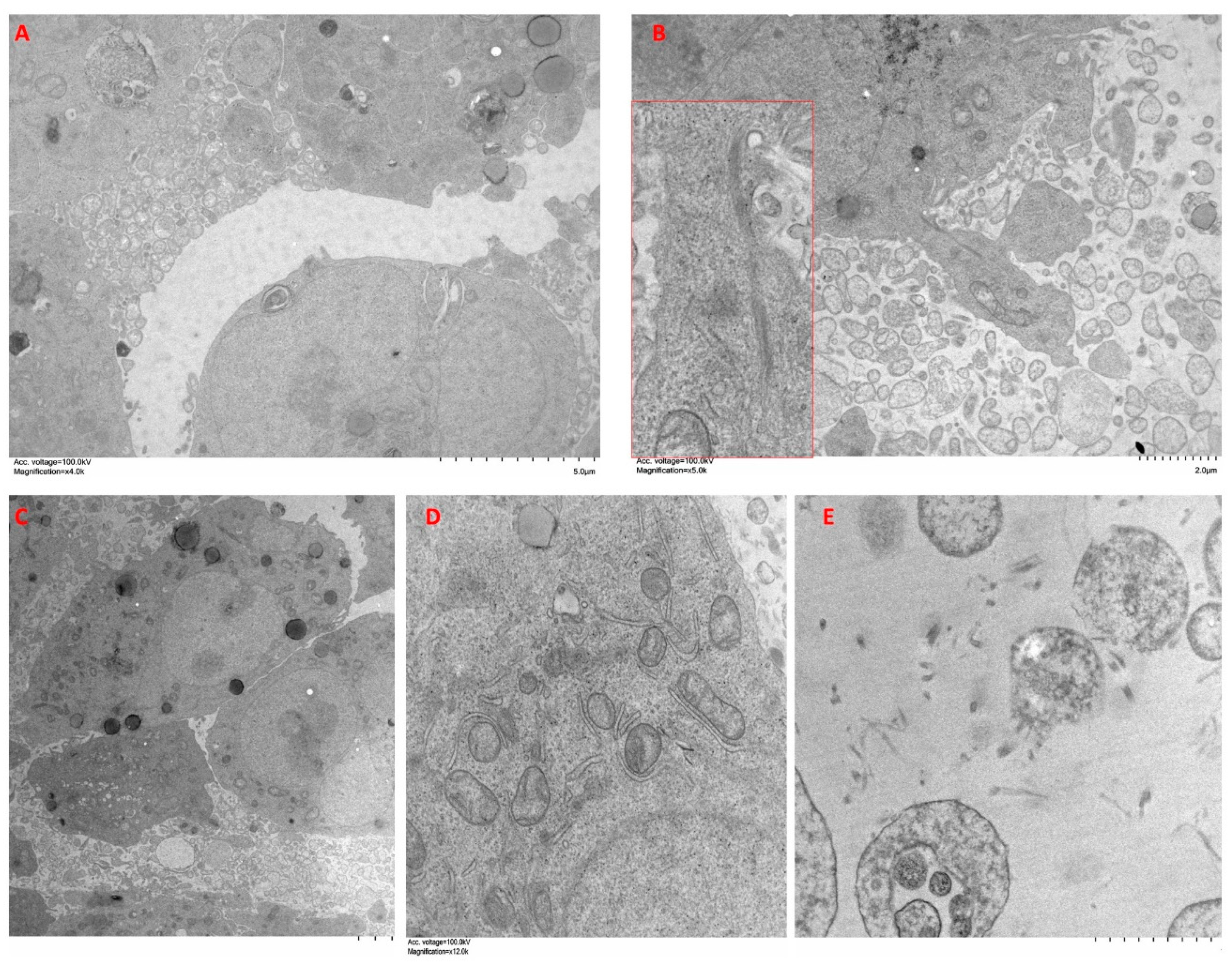
2.6. Ultrastructural Features of Cells Cultured in Spheroid/Organoids
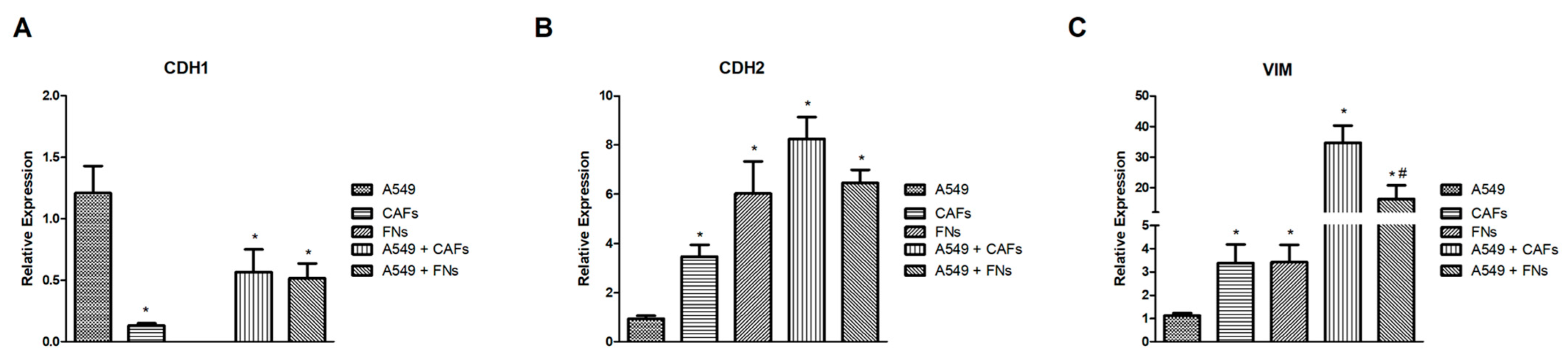
2.7. Analysis of the Relative Gene Expression of the Epithelial-Mesenchymal Transition (EMT)
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Cell Lines and Cell Isolation
4.3. Formation of Organoids/Spheroids
4.4. Organoid/Spheroid Culture in Type I Collagen Hydrogels
4.5. F-Actin Fluorescence Staining and Vimentin and Pankeratin Proteins Expression and Distribution in Organoids/Spheroids
4.6. Transmission Electron Microscopy (TEM)
4.7. Determination of Relative Gene Expression Levels of CDH1, CDH2 and VIM
4.8. Morphometric Study
4.9. Data Presentation and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Cryer, A.M.; Thorley, A.J. Nanotechnology in the diagnosis and treatment of lung cancer. Pharmacol. Ther. 2019, 198, 189–205. [Google Scholar] [CrossRef]
- Miura, A.; Yamada, D.; Nakamura, M.; Tomida, S.; Shimizu, D.; Jiang, Y.; Takao, T.; Yamamoto, H.; Suzawa, K.; Shien, K.; et al. Oncogenic potential of human pluripotent stem cell-derived lung organoids with HER2 overexpression. Int. J. Cancer 2021, 149, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Gao, H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017, 126, 97–108. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wang, X.; Nguyen, H.T.; Zhuo, Y.; Cui, X.; Fewell, C.; Flemington, E.K.; Shan, B. Induction of long intergenic non-coding RNA HOTAIR in lung cancer cells by type I collagen. J. Hematol. Oncol. 2013, 6, 35. [Google Scholar] [CrossRef]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Fang, S.; Dai, Y.; Mei, Y.; Yang, M.; Hu, L.; Yang, H.; Guan, X.; Li, J. Clinical significance and biological role of cancer-derived Type I collagen in lung and esophageal cancers. Thorac. Cancer 2019, 10, 277–288. [Google Scholar] [CrossRef]
- Granat, L.M.; Kambhampati, O.; Klosek, S.; Niedzwecki, B.; Parsa, K.; Zhang, D. The promises and challenges of patient-derived tumor organoids in drug development and precision oncology. Anim. Model. Exp. Med. 2019, 2, 150–161. [Google Scholar] [CrossRef]
- Zhou, J.; Su, J.; Fu, X.; Zheng, L.; Yin, Z. Microfluidic device for primary tumor spheroid isolation. Exp. Hematol. Oncol. 2017, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Caponigro, G.; Sellers, W.R. Advances in the preclinical testing of cancer therapeutic hypotheses. Nat. Rev. Drug Discov. 2011, 10, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mun, H.; Sung, C.O.; Cho, E.J.; Jeon, H.J.; Chun, S.M.; Jung, D.J.; Shin, T.H.; Jeong, G.S.; Kim, D.K.; et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 2019, 10, 3991. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lyu, X.; Yi, M.; Zhao, W.; Song, Y.; Wu, K. Organoid technology and applications in cancer research. J. Hematol. Oncol. 2018, 11, 116. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Chen, H. Organoid models in lung regeneration and cancer. Cancer Lett. 2020, 475, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xin, W.; Yan, C.; Shi, Y.; Li, Y.; Hu, Y.; Ying, K. Organoids in lung cancer: A teenager with infinite growth potential. Lung Cancer 2022, 172, 100–107. [Google Scholar] [CrossRef]
- Velasco, V.; Shariati, S.A.; Esfandyarpour, R. Microtechnology-based methods for organoid models. Microsyst. Nanoeng. 2020, 6, 76. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.K.; Vu, K.P.; London, N.R., Jr. Organoid and Spheroid Tumor Models: Techniques and Applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Attieh, Y.; Clark, A.G.; Grass, C.; Richon, S.; Pocard, M.; Mariani, P.; Elkhatib, N.; Betz, T.; Gurchenkov, B.; Vignjevic, D.M. Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly. J. Cell Biol. 2017, 216, 3509–3520. [Google Scholar] [CrossRef]
- Altorki, N.K.; Markowitz, G.J.; Gao, D.; Port, J.L.; Saxena, A.; Stiles, B.; McGraw, T.; Mittal, V. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat. Rev. Cancer 2019, 19, 9–31. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, G.; Hu, P.C.; Wang, Y. Personalized medicine in non-small cell lung cancer: A review from a pharmacogenomics perspective. Acta Pharm. Sin. B 2018, 8, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Qin, T.; Qi, T. Precision Medicine in Lung Cancer Theranostics: Paving the Way from Traditional Technology to Advance Era. Cancer Control 2022, 29, 10732748221077351. [Google Scholar] [CrossRef]
- Politi, K.; Herbst, R.S. Lung cancer in the era of precision medicine. Clin. Cancer Res. 2015, 21, 2213–2220. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs. 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef]
- Zimmermann, M.; Box, C.; Eccles, S.A. Two-dimensional vs. three-dimensional in vitro tumor migration and invasion assays. Methods Mol. Biol. 2013, 986, 227–252. [Google Scholar] [CrossRef]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69–70, 29–41. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Xing, F.; Saidou, J.; Watabe, K. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. 2010, 15, 166–179. [Google Scholar] [CrossRef]
- Jia, C.; Wang, G.; Wang, T.; Fu, B.; Zhang, Y.; Huang, L.; Deng, Y.; Chen, G.; Wu, X.; Chen, J.; et al. Cancer-associated Fibroblasts induce epithelial-mesenchymal transition via the Transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in Hepatocellular Carcinoma. Int. J. Biol. Sci. 2020, 16, 2542–2558. [Google Scholar] [CrossRef]
- You, J.; Li, M.; Cao, L.M.; Gu, Q.H.; Deng, P.B.; Tan, Y.; Hu, C.P. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM 2019, 112, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Londoño-Berrio, M.; Castro, C.; Cañas, A.; Ortiz, I.; Osorio, M. Advances in Tumor Organoids for the Evaluation of Drugs: A Bibliographic Review. Pharmaceutics 2022, 14, 2709. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Billet, S.; Bhowmick, N.A. Understanding the role of stromal fibroblasts in cancer progression. Cell Adhes. Migr. 2012, 6, 231–235. [Google Scholar] [CrossRef]
- Louault, K.; Li, R.R.; DeClerck, Y.A. Cancer-Associated Fibroblasts: Understanding Their Heterogeneity. Cancers 2020, 12, 3108. [Google Scholar] [CrossRef] [PubMed]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín de Llano, J.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Tao, L.; Huang, G.; Song, H.; Chen, Y.; Chen, L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017, 14, 2611–2620. [Google Scholar] [CrossRef]
- Asif, P.J.; Longobardi, C.; Hahne, M.; Medema, J.P. The Role of Cancer-Associated Fibroblasts in Cancer Invasion and Metastasis. Cancers 2021, 13, 4720. [Google Scholar] [CrossRef]
- Erdogan, B.; Webb, D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans. 2017, 45, 229–236. [Google Scholar] [CrossRef]
- Milián, L.; Monleón-Guinot, I.; Sancho-Tello, M.; Galbis, J.M.; Cremades, A.; Almenar-Ordaz, M.; Peñaroja-Martinez, J.; Farras, R.; Martín de Llano, J.J.; Carda, C.; et al. In Vitro Effect of Δ9-Tetrahydrocannabinol and Cannabidiol on Cancer-Associated Fibroblasts Isolated from Lung Cancer. Int. J. Mol. Sci. 2022, 23, 6766. [Google Scholar] [CrossRef]
- Oliver-Ferrándiz, M.; Milián, L.; Sancho-Tello, M.; Martín de Llano, J.J.; Gisbert Roca, F.; Martínez-Ramos, C.; Carda, C.; Mata, M. Alginate-Agarose Hydrogels Improve the In Vitro Differentiation of Human Dental Pulp Stem Cells in Chondrocytes. A Histological Study. Biomedicines 2021, 9, 834. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monleón-Guinot, I.; Milian, L.; Martínez-Vallejo, P.; Sancho-Tello, M.; Llop-Miguel, M.; Galbis, J.M.; Cremades, A.; Carda, C.; Mata, M. Morphological Characterization of Human Lung Cancer Organoids Cultured in Type I Collagen Hydrogels: A Histological Approach. Int. J. Mol. Sci. 2023, 24, 10131. https://doi.org/10.3390/ijms241210131
Monleón-Guinot I, Milian L, Martínez-Vallejo P, Sancho-Tello M, Llop-Miguel M, Galbis JM, Cremades A, Carda C, Mata M. Morphological Characterization of Human Lung Cancer Organoids Cultured in Type I Collagen Hydrogels: A Histological Approach. International Journal of Molecular Sciences. 2023; 24(12):10131. https://doi.org/10.3390/ijms241210131
Chicago/Turabian StyleMonleón-Guinot, Irene, Lara Milian, Patricia Martínez-Vallejo, María Sancho-Tello, Mauro Llop-Miguel, José Marcelo Galbis, Antonio Cremades, Carmen Carda, and Manuel Mata. 2023. "Morphological Characterization of Human Lung Cancer Organoids Cultured in Type I Collagen Hydrogels: A Histological Approach" International Journal of Molecular Sciences 24, no. 12: 10131. https://doi.org/10.3390/ijms241210131
APA StyleMonleón-Guinot, I., Milian, L., Martínez-Vallejo, P., Sancho-Tello, M., Llop-Miguel, M., Galbis, J. M., Cremades, A., Carda, C., & Mata, M. (2023). Morphological Characterization of Human Lung Cancer Organoids Cultured in Type I Collagen Hydrogels: A Histological Approach. International Journal of Molecular Sciences, 24(12), 10131. https://doi.org/10.3390/ijms241210131








