Metabolic Adaption of Flexor Carpi Radialis to Amplexus Behavior in Asiatic Toads (Bufo gargarizans)
Abstract
:1. Introduction
2. Results
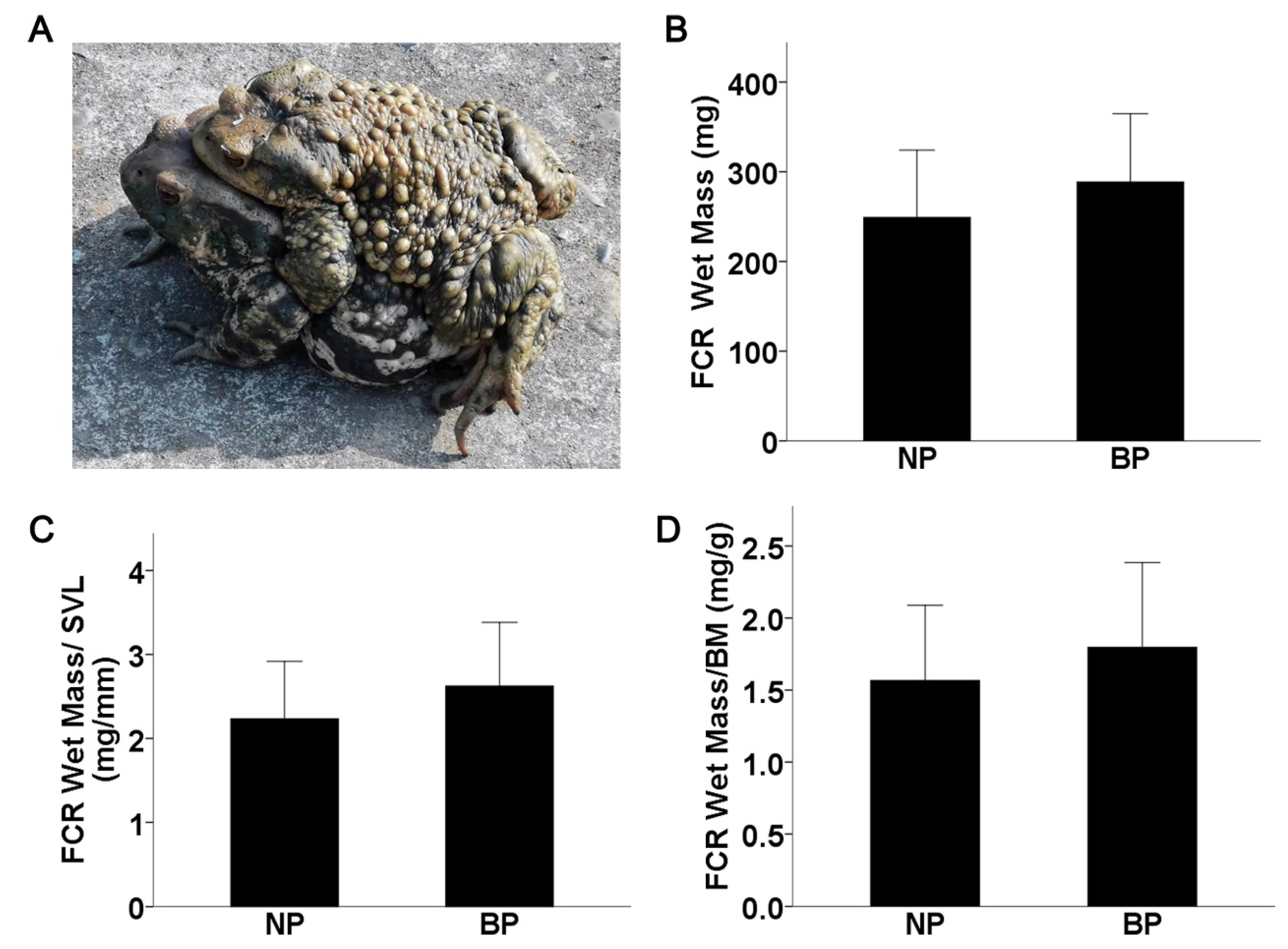
2.1. FCR Muscle Mass Is Not Altered in Amplectant Males
2.2. FCR Muscle of Amplectant Males in the Breeding Period Has a Distinct Metabolic Profile
2.3. Identification of Differential Metabolites in the Amplectant Males
2.4. KEGG Analysis of the Differential Metabolites
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Sample Preparation and Metabolite Extraction
4.3. GC-MS Analysis
4.4. GC-MS Qualitative and Quantitative Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crump, M.L. Anuran reproductive modes: Evolving perspectives. J. Herpetol. 2015, 49, 1–16. [Google Scholar] [CrossRef]
- Mi, Z.P. Sexual dimorphism in the forelimb muscles of the Asiatic toad Bufo gargarizans. Herpetol. J. 2012, 22, 219–224. [Google Scholar]
- Dole, J.W.; Durant, P. Movements and seasonal activity of Atelopus oxyrhynchus (Anura: Atelopodidae) in a Venezuelan cloud forest. Copeia 1974, 1974, 230–235. [Google Scholar] [CrossRef]
- Wells, K.D. The social behaviour of anuran amphibians. Anim. Behav. 1977, 25, 666–693. [Google Scholar] [CrossRef]
- Schwarzkopf, L. Measuring trade-offs: A review of studies of costs of reproduction in lizards. In Lizard Ecology; Princeton University Press: Princeton, NJ, USA, 1994; pp. 7–30. [Google Scholar]
- Clutton-Brock, T.H. The Evolution of Parental Care; Princeton University Press: Princeton, NJ, USA, 1991. [Google Scholar]
- Woolbright, L.L.; Stewart, M.M. Foraging success of the tropical frog, Eleutherodactylus coqui: The cost of calling. Copeia 1987, 1987, 69–75. [Google Scholar] [CrossRef]
- Given, M.F. Territoriality and aggressive interactions of male carpenter frogs, Rana virgatipes. Copeia 1988, 1988, 411–421. [Google Scholar] [CrossRef]
- Ryser, J. Weight loss, reproductive output, and the cost of reproduction in the common frog, Rana temporaria. Oecologia 1989, 78, 264–268. [Google Scholar] [CrossRef]
- McLister, J.D. The metabolic cost of amplexus in the grey tree frog (Hyla versicolor): Assessing the energetics of male mating success. Can. J. Zool. 2003, 81, 388–394. [Google Scholar] [CrossRef]
- Bowcock, H.; Brown, G.P.; Shine, R. Beastly bondage: The costs of amplexus in cane toads (Bufo marinus). Copeia 2009, 2009, 29–36. [Google Scholar] [CrossRef]
- Ryan, M.J. The Tungara Frog: A Study in Sexual Selection and Communication; University of Chicago Press: Chicago, IL, USA, 1985. [Google Scholar]
- Tsuji, H.; Lue, K.-Y. Temporal aspects of the amplexus and oviposition behavior of the fanged frog Rana kuhlii from Taiwan. Copeia 1998, 1998, 769–773. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Meth. 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Saoi, M.; Britz-McKibbin, P. New advances in tissue metabolomics: A review. Metabolites 2021, 11, 672. [Google Scholar] [CrossRef]
- Wishart, D.S.; Cheng, L.L.; Copié, V.; Edison, A.S.; Eghbalnia, H.R.; Hoch, J.C.; Gouveia, G.J.; Pathmasiri, W.; Powers, R.; Schock, T.B. NMR and metabolomics—A roadmap for the future. Metabolites 2022, 12, 678. [Google Scholar] [CrossRef]
- Li, P.; Tan, S.; Yao, Z.; Liu, G.; Fu, J.; Chen, J. Sex but not altitude, modulates phenotypic covariations between growth and physiological traits in adult Asiatic toads. Asian Herpetol. Res. 2022, 13, 34–42. [Google Scholar]
- Yu, T.; Lu, X. Sex recognition and mate choice lacking in male Asiatic toads (Bufo gargarizans). Ital. J. Zool. 2010, 77, 476–480. [Google Scholar] [CrossRef]
- Witte, K.; Chen, K.-C.; Wilczynski, W.; Ryan, M.J. Influence of amplexus on phonotaxis in the cricket frog Acris crepitans blanchardi. Copeia 2000, 2000, 257–261. [Google Scholar] [CrossRef]
- Kirby, A.C. Physiology of the sternoradialis muscle: Sexual dimorphism and role in amplexus in the Leopard frog (Rana pipiens). Comp. Biochem. Physiol. A 1983, 74, 705–709. [Google Scholar] [CrossRef]
- Moore, F.L.; Boyd, S.K.; Kelley, D.B. Historical perspective: Hormonal regulation of behaviors in amphibians. Horm. Behav. 2005, 48, 373–383. [Google Scholar] [CrossRef]
- Woodley, S.K. Hormones and reproductive behavior in amphibians. In Hormones and Reproduction of Vertebrates; Elsevier: Amsterdam, The Netherlands, 2011; pp. 143–169. [Google Scholar]
- Finkler, M.S.; Hayes, C.J.; Rifai, L. Sexual dimorphisms in metabolism, organ mass, and reproductive energetics in pre-breeding American toads (Anaxyrus americanus). Copeia 2014, 2014, 447–453. [Google Scholar] [CrossRef]
- Ophir, A.G.; Schrader, S.B.; Gillooly, J.F. Energetic cost of calling: General constraints and species-specific differences. J. Evol. Biol. 2010, 23, 1564–1569. [Google Scholar] [CrossRef]
- Bevier, C.R. Biochemical correlates of calling activity in neotropical frogs. Physiol. Zool. 1995, 68, 1118–1142. [Google Scholar] [CrossRef]
- Mercadal, I.T.; Ginzo, H.D. ‘Fasting male-feeding female’behaviour in Bombina orientalis during amplexus. Herpetol. Bull. 2006, 164, 23–25. [Google Scholar]
- Regnault, C.; Usal, M.; Veyrenc, S.; Couturier, K.; Batandier, C.; Bulteau, A.L.; Lejon, D.; Sapin, A.; Combourieu, B.; Chetiveaux, M.; et al. Unexpected metabolic disorders induced by endocrine disruptors in Xenopus tropicalis provide new lead for understanding amphibian decline. Proc. Natl. Acad. Sci. USA 2018, 115, E4416–E4425. [Google Scholar] [CrossRef] [Green Version]
- Usal, M.; Veyrenc, S.; Darracq-Ghitalla-Ciock, M.; Regnault, C.; Sroda, S.; Fini, J.B.; Canlet, C.; Tremblay-Franco, M.; Raveton, M.; Reynaud, S. Transgenerational metabolic disorders and reproduction defects induced by benzo[a]pyrene in Xenopus tropicalis. Environ. Pollut. 2021, 269, 116109. [Google Scholar] [CrossRef] [PubMed]
- Moreira, D.C.; Carvajalino-Fernandez, J.M.; Navas, C.A.; de Carvalho, J.E.; Hermes-Lima, M. Metabolic and redox biomarkers in skeletal muscle underlie physiological adaptations of two estivating anuran species in a South American semi-arid environment. Front Physiol 2021, 12, 769833. [Google Scholar] [CrossRef] [PubMed]
- Hervant, F.; Mathieu, J.; Durand, J. Behavioural, physiological and metabolic responses to long-term starvation and refeeding in a blind cave-dwelling (Proteus anguinus) and a surface-dwelling (Euproctus asper) salamander. J. Exp. Biol. 2001, 204, 269–281. [Google Scholar] [CrossRef]
- Karlsson, O.; Svanholm, S.; Eriksson, A.; Chidiac, J.; Eriksson, J.; Jerneren, F.; Berg, C. Pesticide-induced multigenerational effects on amphibian reproduction and metabolism. Sci. Total Environ. 2021, 775, 145771. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, X.; Zhang, H.; Xu, T.; Zhu, L.; Storey, K.B.; Chen, Q. Metabolic responses of plasma to extreme environments in overwintering Tibetan frogs Nanorana parkeri: A metabolome integrated analysis. Front Zool. 2021, 18, 41. [Google Scholar] [CrossRef]
- Shekhovtsov, S.V.; Bulakhova, N.A.; Tsentalovich, Y.P.; Zelentsova, E.A.; Meshcheryakova, E.N.; Poluboyarova, T.V.; Berman, D.I. Metabolomic analysis reveals that the moor frog Rana arvalis uses both glucose and glycerol as cryoprotectants. Animals 2022, 12, 1286. [Google Scholar] [CrossRef]
- Fitzpatrick, L.C. Life history patterns of storage and utilization of lipids for energy in amphibians. Am. Zool. 1976, 16, 725–732. [Google Scholar] [CrossRef] [Green Version]
- Brenes-Soto, A.; Dierenfeld, E.S.; Bosch, G.; Hendriks, W.H.; Janssens, G.P.J. Gaining insights in the nutritional metabolism of amphibians: Analyzing body nutrient profiles of the African clawed frog, Xenopus laevis. PeerJ 2019, 7, e7365. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef]
- Michal, G.; Schomburg, D. Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Das, I. Reproductive strategy and lipid mobilization in Rana hexadactyla at a seasonal locality. Ethol. Ecol. Evol. 1996, 8, 125–133. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Pérez-Felpete, N.; Fernández-Fernández, C.; Donapetry-García, C.; Pazos-García, C. Liver glucose metabolism in humans. Biosci. Rep. 2016, 36, e00416. [Google Scholar] [CrossRef] [Green Version]
- Zierler, K. Whole body glucose metabolism. Am. J. Physiol. 1999, 276, E409–E426. [Google Scholar] [CrossRef] [Green Version]
- Aas, V.; Rokling-Andersen, M.H.; Kase, E.T.; Thoresen, G.H.; Rustan, A.C. Eicosapentaenoic acid (20:5 n-3) increases fatty acid and glucose uptake in cultured human skeletal muscle cells. J. Lipid Res. 2006, 47, 366–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.-T.; Xu, J.; Wang, Y.-M.; Xue, C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, S. Structure and mechanism of ABC transporters. F1000prime Rep. 2015, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Negrutskii, B.S.; Deutscher, M.P. Channeling of aminoacyl-tRNA for protein synthesis in vivo. Proc. Natl. Acad. Sci. USA 1991, 88, 4991–4995. [Google Scholar] [CrossRef] [Green Version]
- Jobgen, W.; Meininger, C.J.; Jobgen, S.C.; Li, P.; Lee, M.J.; Smith, S.B.; Spencer, T.E.; Fried, S.K.; Wu, G. Dietary L-arginine supplementation reduces white fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J. Nutr. 2009, 139, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.W.; Escobar, J.; Nguyen, H.V.; Jobgen, S.C.; Jobgen, W.S.; Davis, T.A.; Wu, G. Oral N-carbamylglutamate supplementation increases protein synthesis in skeletal muscle of piglets. J. Nutr. 2007, 137, 315–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spry, C.; Kirk, K.; Saliba, K.J. Coenzyme A biosynthesis: An antimicrobial drug target. FEMS Microbiol. Rev. 2008, 32, 56–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, C.F.; Stonestrom, A.J.; Lorca, G.L.; Saier, M.H. Biochemical characterization of phosphoryl transfer involving HPr of the phosphoenolpyruvate-dependent phosphotransferase system in Treponema denticola, an organism that lacks PTS permeases. Biochemistry 2005, 44, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yao, K.; Yu, H.; Zhang, L.; Xu, Y.; Chen, L.; Sun, Z.; Zhu, Y.; Zhang, C.; Qian, Y.; et al. Metabolic remodelling during early mouse embryo development. Nat. Metab. 2021, 3, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Xing, T.; Li, C.; Xu, X.; Zhou, G. The effect of breed and age on the growth performance, carcass traits and metabolic profile in breast muscle of Chinese indigenous chickens. Foods 2022, 11, 483. [Google Scholar] [CrossRef]
- Diamantidou, D.; Deda, O.; Zervos, I.; Taitzoglou, I.; Gika, H.; Theodoridis, G.; Michopoulos, F. Hepatic metabolic profiling of lifelong exercise training rats. J. Proteome Res. 2022, 21, 2075–2084. [Google Scholar] [CrossRef]
- Liao, W.B.; Liao, Y.M.; Xiao, W.M.; Chen, W.; Mi, Z.P.; Li, C. Sexual dimorphism in hind limb muscle mass of the Andrew’s Toad (Bufo andrewsi) in relation to sexual selection. North-West. J. Zool. 2012, 8, 252–256. [Google Scholar]



| Class | Metabolites | VIP | p-Value | log2(FC) | FC | Type |
|---|---|---|---|---|---|---|
| Amino acids, peptides, and analogues | L-aspartic Acid | 2.99 | 4.30 × 10−6 | 3.08 | 8.43 | Up |
| Amino acids, peptides, and analogues | 3-methylhistidine | 2.51 | 3.70 × 10−5 | 2.28 | 4.86 | Up |
| Amino acids, peptides, and analogues | Dl-pyroglutamic acid | 2.91 | 0.00056 | 2.25 | 4.77 | Up |
| Amino acids, peptides, and analogues | L-methionine | 2.40 | 6.80 × 10−7 | 2.14 | 4.40 | Up |
| Amino acids, peptides, and analogues | 1-methylhistidine | 3.11 | 1.80 × 10−5 | 2.10 | 4.30 | Up |
| Amino acids, peptides, and analogues | 2,3-diaminopropionic acid | 1.75 | 0.0066 | 2.08 | 4.23 | Up |
| Amino acids, peptides, and analogues | 4-hydroxyproline | 1.97 | 2.10 × 10−6 | 1.57 | 2.97 | Up |
| Amino acids, peptides, and analogues | (2r,3r)-2-amino-3-hydroxybutanedioic acid | 1.06 | 0.01218 | 0.91 | 1.88 | Up |
| Amino acids, peptides, and analogues | beta-alanine | 1.30 | 0.01267 | 0.90 | 1.87 | Up |
| Amino acids, peptides, and analogues | ornithine | 1.22 | 0.00903 | 0.86 | 1.81 | Up |
| Amino acids, peptides, and analogues | Dl-homocystine | 1.20 | 0.00319 | 0.80 | 1.74 | Up |
| Amino acids, peptides, and analogues | L-cysteine | 1.11 | 0.00576 | 0.68 | 1.60 | Up |
| Amino acids, peptides, and analogues | L-tyrosine | 1.11 | 0.00045 | 0.60 | 1.52 | Up |
| Amino acids, peptides, and analogues | pyroglutamic acid | 1.11 | 0.0034 | −0.57 | 0.67 | Down |
| Amino acids, peptides, and analogues | L-proline | 1.09 | 0.0479 | −0.90 | 0.53 | Down |
| Amino acids, peptides, and analogues | pipecolic acid | 1.90 | 0.00055 | −1.58 | 0.33 | Down |
| Amino acids, peptides, and analogues | glutamine | 2.06 | 0.00341 | −2.85 | 0.14 | Down |
| Amino acids, peptides, and analogues | L-glutamine | 3.22 | 0.00227 | −4.95 | 0.03 | Down |
| Benzenoids | oxymetazoline | 1.58 | 0.03875 | 1.48 | 2.80 | Up |
| Benzenoids | bisphenol a | 1.39 | 0.03241 | 0.96 | 1.94 | Up |
| Benzenoids | 3-hydroxymandelic acid | 1.38 | 0.00989 | −1.29 | 0.41 | Down |
| Benzenoids | oxycodone | 1.79 | 0.02694 | −2.83 | 0.14 | Down |
| Carbohydrates and carbohydrate conjugates | fructose 1-phosphate | 2.10 | 0.04177 | 4.47 | 22.10 | Up |
| Carbohydrates and carbohydrate conjugates | sorbitol | 3.20 | 0.0008 | 3.60 | 12.15 | Up |
| Carbohydrates and carbohydrate conjugates | D-(−)-ribofuranose | 2.91 | 2.50 × 10−5 | 2.75 | 6.75 | Up |
| Carbohydrates and carbohydrate conjugates | gluconic acid | 2.12 | 0.00145 | 2.63 | 6.17 | Up |
| Carbohydrates and carbohydrate conjugates | mannitol 1-phosphate | 2.14 | 8.40 × 10−5 | 1.73 | 3.32 | Up |
| Carbohydrates and carbohydrate conjugates | D-ribofuranose | 1.82 | 0.00144 | 1.42 | 2.68 | Up |
| Carbohydrates and carbohydrate conjugates | cellobiose | 1.62 | 1.70 × 10−5 | 1.03 | 2.04 | Up |
| Carbohydrates and carbohydrate conjugates | L-fucose | 1.43 | 0.00019 | 0.92 | 1.90 | Up |
| Carbohydrates and carbohydrate conjugates | maltotriose | 1.21 | 0.01209 | 0.86 | 1.82 | Up |
| Carbohydrates and carbohydrate conjugates | D-mannose 6-phosphate | 1.19 | 0.04133 | 0.73 | 1.66 | Up |
| Carbohydrates and carbohydrate conjugates | D-(+)-maltose | 1.18 | 0.00139 | 0.66 | 1.58 | Up |
| Carbohydrates and carbohydrate conjugates | 6-phosphogluconic acid | 1.12 | 0.03054 | −1.51 | 0.35 | Down |
| Lipids and lipid-like molecules | 4-hydroxybutyric acid | 1.54 | 0.00218 | 1.06 | 2.09 | Up |
| Lipids and lipid-like molecules | 2-ethylhexanoic acid | 1.56 | 9.60 × 10−6 | 1.04 | 2.06 | Up |
| Lipids and lipid-like molecules | stearyl alcohol | 1.61 | 0.00065 | 1.01 | 2.01 | Up |
| Lipids and lipid-like molecules | docosahexaenoic acid | 1.56 | 6.30 × 10−5 | 0.96 | 1.94 | Up |
| Lipids and lipid-like molecules | eicosapentaenoic acid | 1.35 | 0.01958 | 0.90 | 1.86 | Up |
| Lipids and lipid-like molecules | lactitol | 1.40 | 0.00089 | 0.88 | 1.85 | Up |
| Lipids and lipid-like molecules | beta-glycerophosphoric acid | 1.15 | 0.00056 | 0.58 | 1.50 | Up |
| Lipids and lipid-like molecules | sitosterol | 1.29 | 0.0069 | −0.83 | 0.56 | Down |
| Organic acids and derivatives | ethylphosphonic acid | 2.30 | 0.00077 | 2.07 | 4.18 | Up |
| Organic acids and derivatives | o-phosphoethanolamine | 1.18 | 0.00077 | 0.64 | 1.56 | Up |
| Organic acids and derivatives | fumaric acid | 1.15 | 0.00193 | 0.57 | 1.49 | Up |
| Organic acids and derivatives | oxalic acid | 1.04 | 8.80 × 10−5 | 0.43 | 1.35 | Up |
| Organic acids and derivatives | 2-hydroxyglutaric acid | 1.81 | 0.00024 | −1.50 | 0.35 | Down |
| Organic acids and derivatives | taurine | 1.10 | 0.02924 | −1.95 | 0.26 | Down |
| Organic nitrogen compounds | ethanolamine | 1.72 | 9.40 × 10−5 | 1.17 | 2.26 | Up |
| Organic nitrogen compounds | ritalinic acid | 1.28 | 0.01292 | 0.75 | 1.68 | Up |
| Organic nitrogen compounds | 1,2-ethanediamine | 1.39 | 0.00014 | −0.80 | 0.58 | Down |
| Organic nitrogen compounds | putrescine | 1.41 | 0.01117 | −1.10 | 0.47 | Down |
| Organic oxygen compounds | myo-inositol | 1.66 | 0.00023 | 1.21 | 2.31 | Up |
| Organic oxygen compounds | pantothenic acid | 1.74 | 0.00819 | 1.15 | 2.21 | Up |
| Organic oxygen compounds | shikimic acid | 1.31 | 0.01608 | 0.83 | 1.78 | Up |
| Organoheterocyclic compounds | cyanuric acid | 1.32 | 1.60 × 10−5 | 0.74 | 1.67 | Up |
| Organoheterocyclic compounds | 1h-1,2,4-triazol-5-amine | 1.14 | 0.00714 | 0.58 | 1.50 | Up |
| Organoheterocyclic compounds | cytosine | 1.10 | 0.00108 | 0.52 | 1.44 | Up |
| Organoheterocyclic compounds | hypoxanthine | 1.02 | 0.00018 | 0.47 | 1.39 | Up |
| Others | cis-piceid | 1.86 | 0.01863 | 1.93 | 3.80 | Up |
| Others | conduritol-beta-epoxide | 1.76 | 0.00551 | 1.62 | 3.06 | Up |
| Others | 1-propionylproline | 1.47 | 0.00031 | 0.92 | 1.89 | Up |
| Others | (2-hydroxy-3-phosphonooxypropyl) dihydrogen phosphate | 1.31 | 0.0013 | 0.81 | 1.75 | Up |
| Others | 4-(2-hydroxypropyl) morpholine | 1.19 | 0.00019 | 0.69 | 1.61 | Up |
| Others | 5′-methylthioadenosine | 1.70 | 0.042 | −2.04 | 0.24 | Down |
| Others | (2s)-2-(cyanoamino) propanoic acid | 1.20 | 0.00836 | −0.93 | 0.53 | Down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Ma, H.; Yang, Y.; Mi, Z. Metabolic Adaption of Flexor Carpi Radialis to Amplexus Behavior in Asiatic Toads (Bufo gargarizans). Int. J. Mol. Sci. 2023, 24, 10174. https://doi.org/10.3390/ijms241210174
Yan C, Ma H, Yang Y, Mi Z. Metabolic Adaption of Flexor Carpi Radialis to Amplexus Behavior in Asiatic Toads (Bufo gargarizans). International Journal of Molecular Sciences. 2023; 24(12):10174. https://doi.org/10.3390/ijms241210174
Chicago/Turabian StyleYan, Chengzhi, Hui Ma, Yuejun Yang, and Zhiping Mi. 2023. "Metabolic Adaption of Flexor Carpi Radialis to Amplexus Behavior in Asiatic Toads (Bufo gargarizans)" International Journal of Molecular Sciences 24, no. 12: 10174. https://doi.org/10.3390/ijms241210174
APA StyleYan, C., Ma, H., Yang, Y., & Mi, Z. (2023). Metabolic Adaption of Flexor Carpi Radialis to Amplexus Behavior in Asiatic Toads (Bufo gargarizans). International Journal of Molecular Sciences, 24(12), 10174. https://doi.org/10.3390/ijms241210174






