The Role of Cyclic Adenosine Monophosphate (cAMP) in Modulating Glucocorticoid Receptor Signaling and Its Implications on Glucocorticoid-Related Collagen Loss
Abstract
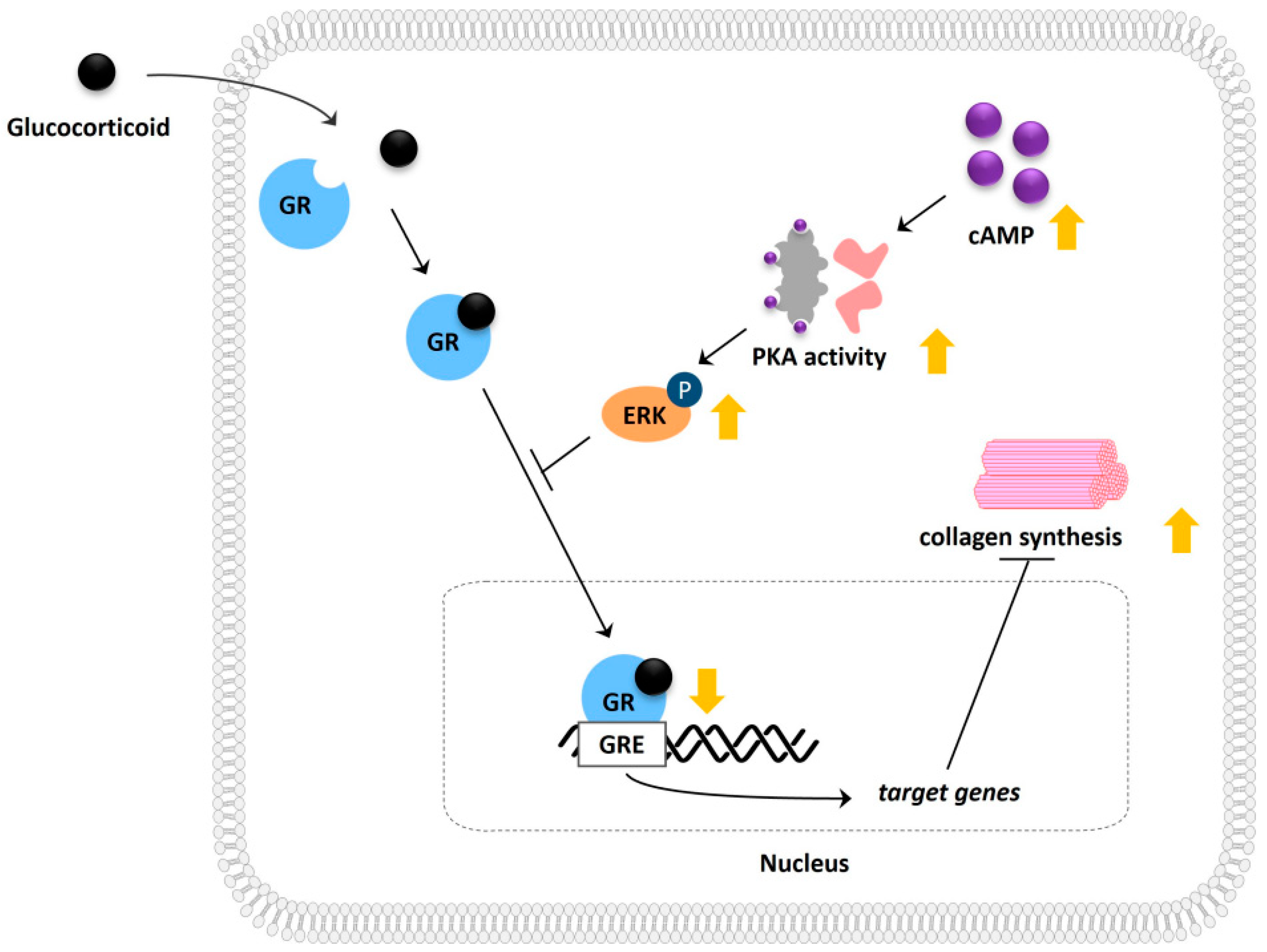
:1. Introduction
2. Results
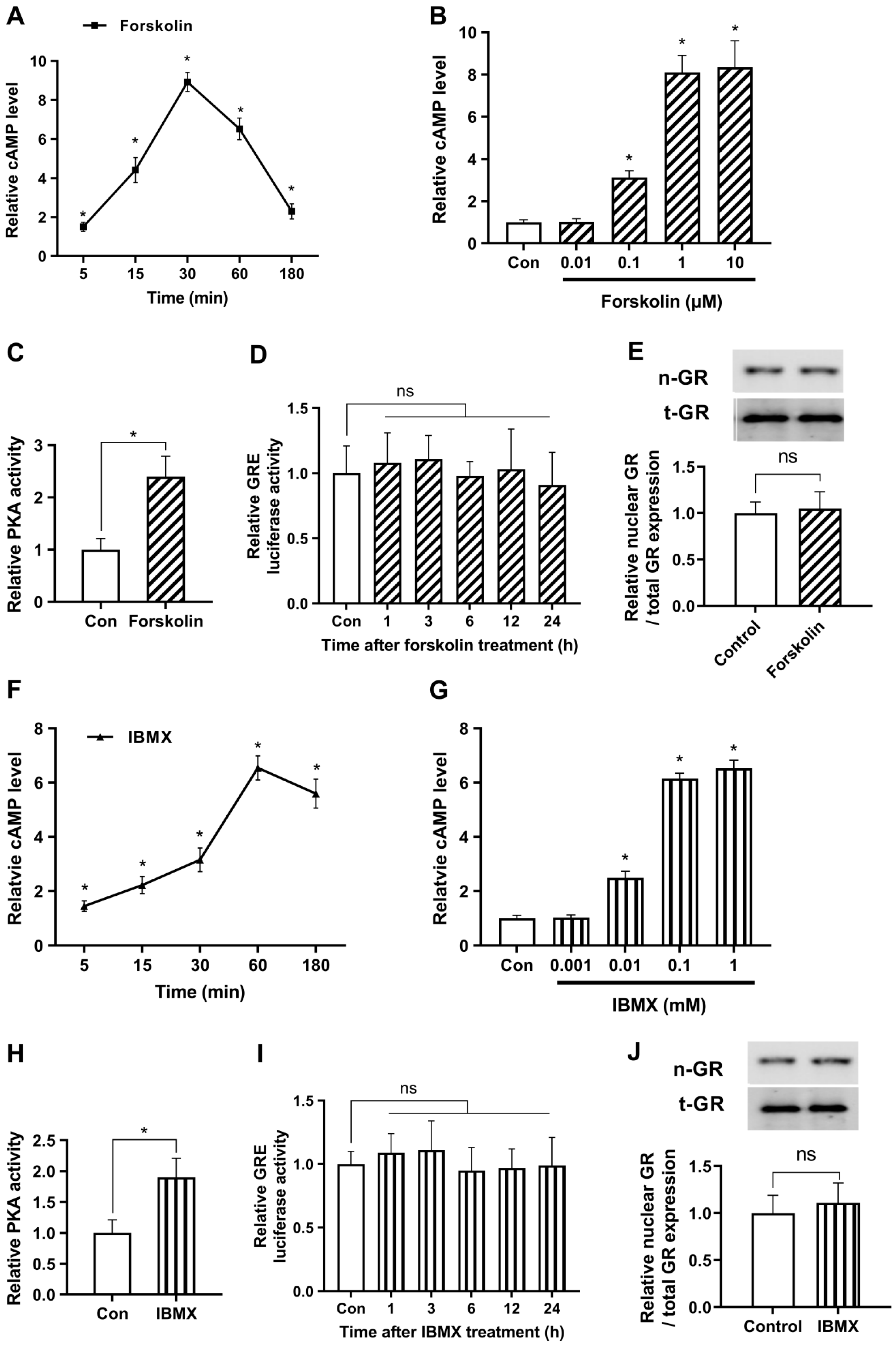
2.1. cAMP Does Not Affect Glucocorticoid Signaling under Non-Stressful Conditions
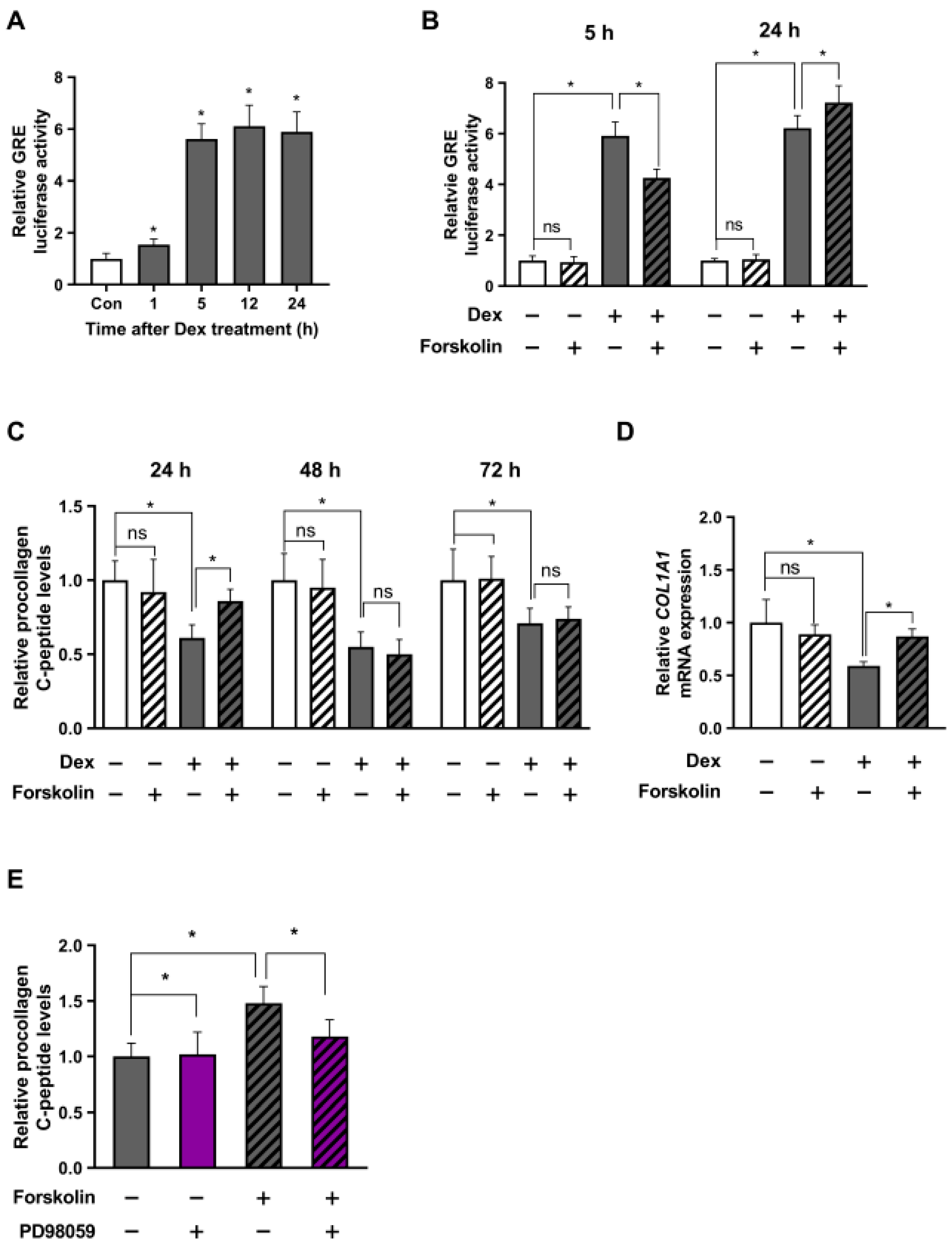
2.2. cAMP Alleviates Glucocorticoid Signaling under Stressful Conditions in a Short Period
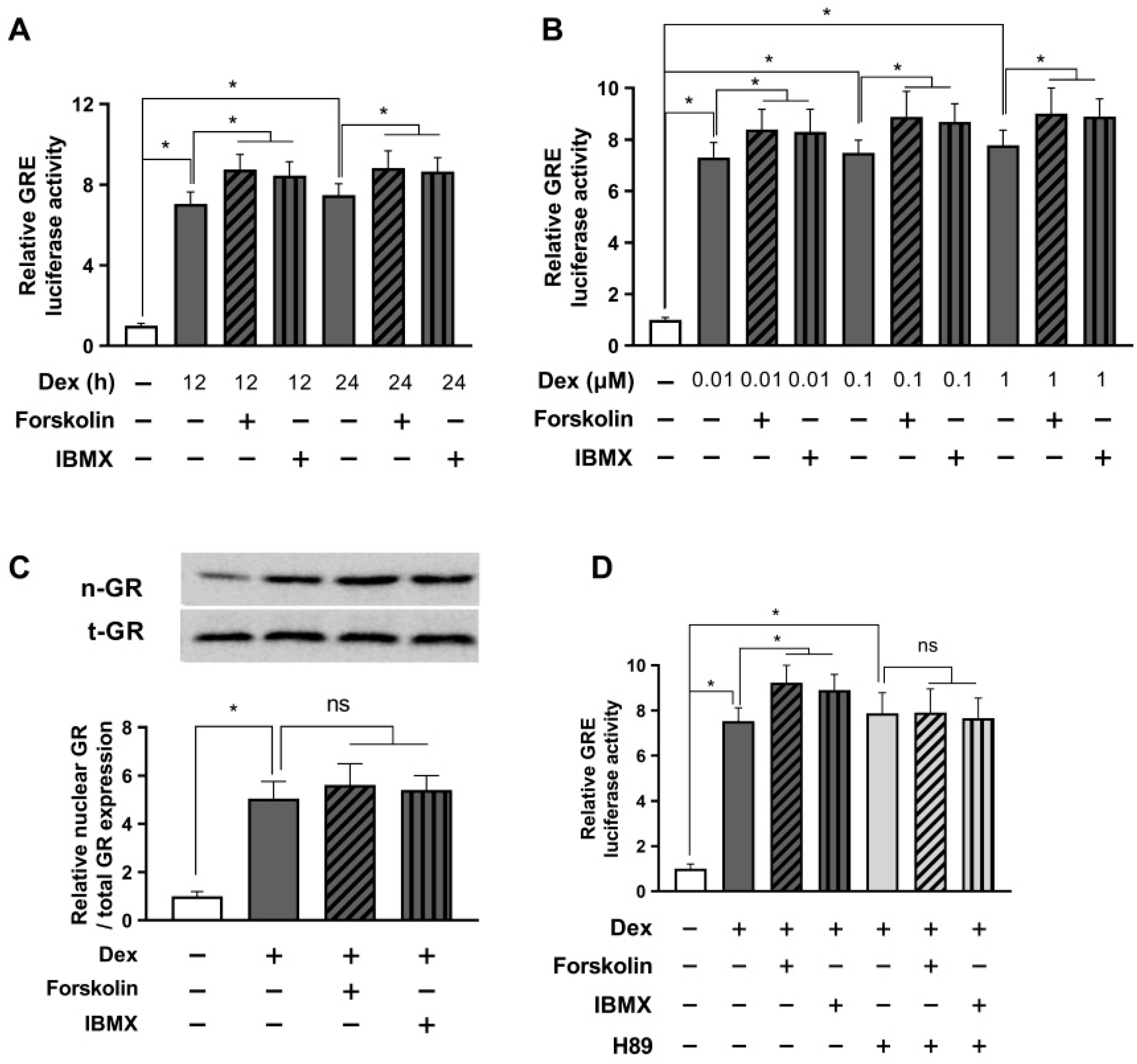
2.3. Prolonged Activation with cAMP Enhances Glucocorticoid Signaling
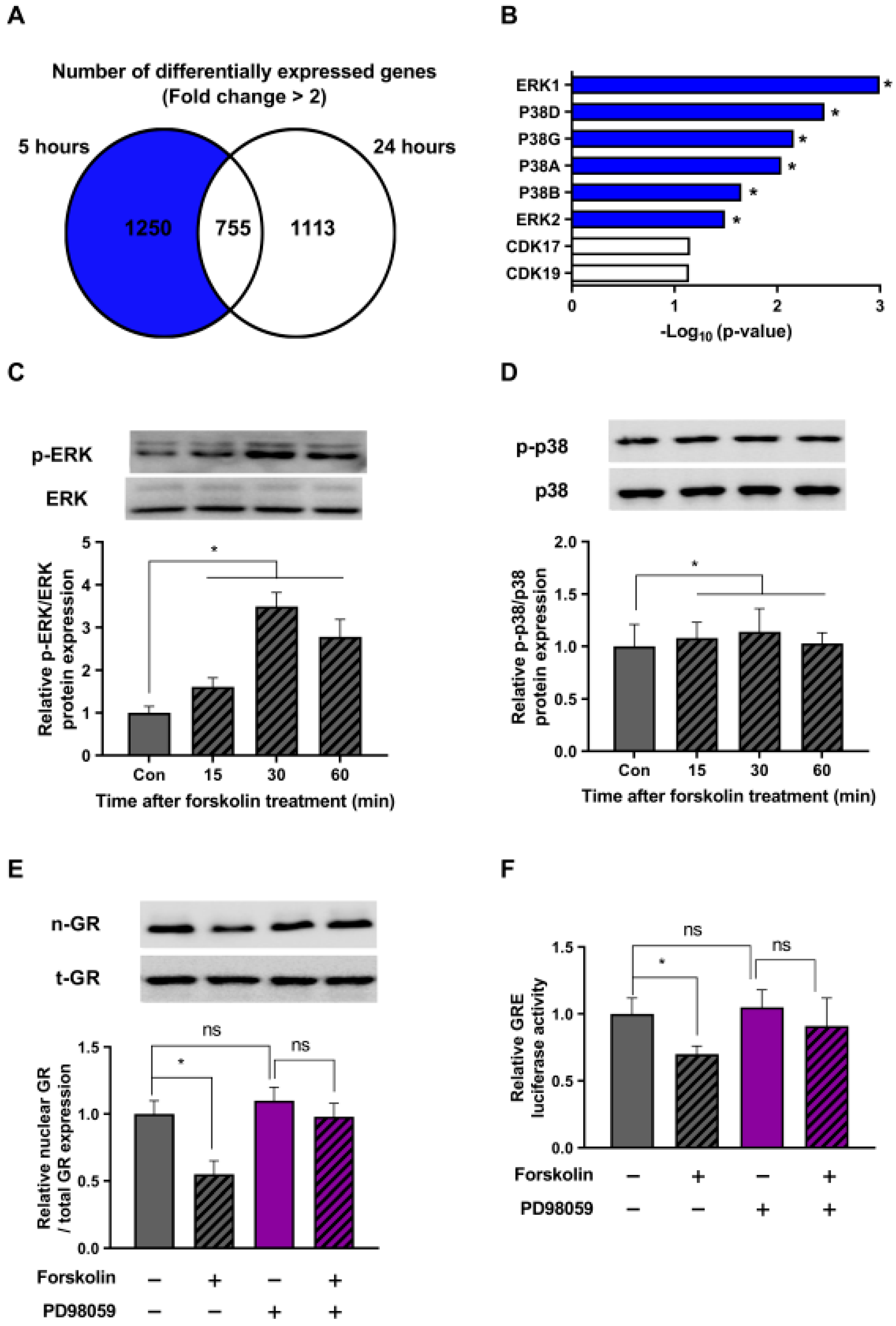
2.4. The ERK Pathway Is Involved in the Stress-Relieving Effects of cAMP
2.5. cAMP-Mediated Stress Reduction Improves Collagen Levels in Human Dermal Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Transient Transfection and Luciferase Assay
4.3. Protein Extraction and Western Blot Analysis
4.4. PKA Activity Measurements
4.5. Quantitative Assessment of Procollagen Secretion and cAMP
4.6. RNA Extraction and Real-Time PCR (RT-PCR)
4.7. RNA Sequencing and Bioinformatic Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010, 35, 109. [Google Scholar]
- Nicolaides, N.C.; Kyratzi, E.; Lamprokostopoulou, A.; Chrousos, G.P.; Charmandari, E. Stress, the stress system and the role of glucocorticoids. Neuroimmunomodulation 2014, 22, 6–19. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Tuckey, R.C.; Paus, R. Differential expression of HPA axis homolog in the skin. Mol. Cell. Endocrinol. 2007, 265, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Bucala, R. MIF rediscovered: Cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J. 1996, 10, 1607–1613. [Google Scholar] [CrossRef]
- Mayba, J.N.; Gooderham, M.J. Review of atopic dermatitis and topical therapies. J. Cutan. Med. Surg. 2017, 21, 227–236. [Google Scholar] [CrossRef]
- Newton, R. Molecular mechanisms of glucocorticoid action: What is important? Thorax 2000, 55, 603–613. [Google Scholar] [CrossRef] [Green Version]
- Timmermans, S.; Vandewalle, J.; Libert, C. Dimerization of the glucocorticoid receptor and its importance in (patho) physiology: A primer. Cells 2022, 11, 683. [Google Scholar] [CrossRef]
- Roumestan, C.; Gougat, C.; Jaffuel, D.; Mathieu, M. Glucocorticoids and their receptor: Mechanisms of action and clinical implications. La Rev. De Med. Interne 2004, 25, 636–647. [Google Scholar] [CrossRef]
- Le, Q.-V.; Wen, S.-Y.; Chen, C.-J.; Huang, C.-Y.; Kuo, W.-W. Reversion of glucocorticoid-induced senescence and collagen synthesis decrease by LY294002 is mediated through p38 in skin. Int. J. Biol. Sci. 2022, 18, 6102–6113. [Google Scholar] [CrossRef]
- Oikarinen, A.; Autio, P. New aspects of the mechanism of corticosteroid–induced dermal atrophy. Clin. Exp. Dermatol. 1991, 16, 416–419. [Google Scholar] [CrossRef]
- Kahan, V.; Andersen, M.; Tomimori, J.; Tufik, S. Stress, immunity and skin collagen integrity: Evidence from animal models and clinical conditions. Brain Behav. Immun. 2009, 23, 1089–1095. [Google Scholar] [CrossRef]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular organization of the cAMP signaling pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar] [CrossRef]
- Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- Raker, V.K.; Becker, C.; Steinbrink, K. The cAMP pathway as therapeutic target in autoimmune and inflammatory diseases. Front. Immunol. 2016, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.I.; Setaluri, V. Cyclic AMP (cAMP) signaling in melanocytes and melanoma. Arch. Biochem. Biophys. 2014, 563, 22–27. [Google Scholar] [CrossRef]
- Mika, D.; Leroy, J.; Vandecasteele, G.; Fischmeister, R. PDEs create local domains of cAMP signaling. J. Mol. Cell. Cardiol. 2012, 52, 323–329. [Google Scholar] [CrossRef]
- Soderling, S.H.; Beavo, J.A. Regulation of cAMP and cGMP signaling: New phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000, 12, 174–179. [Google Scholar] [CrossRef]
- Kovanich, D.; Low, T.Y.; Zaccolo, M. Using the Proteomics Toolbox to Resolve Topology and Dynamics of Compartmentalized cAMP Signaling. Int. J. Mol. Sci. 2023, 24, 4667. [Google Scholar] [CrossRef]
- Hong, K.P.; Spitzer, N.C.; Nicol, X. Improved molecular toolkit for cAMP studies in live cells. BMC Res. Notes 2011, 4, 241. [Google Scholar] [CrossRef]
- Averaimo, S.; Nicol, X. Intermingled cAMP, cGMP and calcium spatiotemporal dynamics in developing neuronal circuits. Front. Cell. Neurosci. 2014, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Roozendaal, B.; Quirarte, G.L.; McGaugh, J.L. Glucocorticoids interact with the basolateral amygdala β-adrenoceptor–cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002, 15, 553–560. [Google Scholar] [CrossRef]
- Koziol-White, C.; Johnstone, T.B.; Corpuz, M.L.; Cao, G.; Orfanos, S.; Parikh, V.; Deeney, B.; Tliba, O.; Ostrom, R.S.; Dainty, I. Budesonide enhances agonist-induced bronchodilation in human small airways by increasing cAMP production in airway smooth muscle. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L345–L355. [Google Scholar] [CrossRef]
- Thoresen, G.H.; Gjone, I.H.; Gladhaug, I.P.; Refsnes, M.; Østby, E.; Christoffersen, T. Studies of glucocorticoid enhancement of the capacity of hepatocytes to accumulate cyclic AMP. Pharmacol. Toxicol. 1989, 65, 175–180. [Google Scholar] [CrossRef]
- Levy, J.; Zhou, D.; Zippin, J. Cyclic adenosine monophosphate signaling in inflammatory skin disease. J. Clin. Exp. Derm. Res. 2016, 7, 2. [Google Scholar]
- Cheape, A.C.; Murrell, D.F. 2% Crisaborole topical ointment for the treatment of mild-to-moderate atopic dermatitis. Expert Rev. Clin. Immunol. 2017, 13, 415–423. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Hanifin, J.M.; Boguniewicz, M.; Wollenberg, A.; Bissonnette, R.; Purohit, V.; Kilty, I.; Tallman, A.M.; Zielinski, M.A. The role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp. Dermatol. 2019, 28, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Marquette, A.; André, J.; Bagot, M.; Bensussan, A.; Dumaz, N. ERK and PDE4 cooperate to induce RAF isoform switching in melanoma. Nat. Struct. Mol. Biol. 2011, 18, 584–591. [Google Scholar] [CrossRef]
- Lyons, J.; Bastian, B.C.; McCormick, F. MC1R and cAMP signaling inhibit cdc25B activity and delay cell cycle progression in melanoma cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13845–13850. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Murata, T.; Shimizu, K.; Morita, H.; Inui, M.; Tagawa, T. Phosphodiesterase 4 regulates the migration of B16-F10 melanoma cells. Exp. Ther. Med. 2012, 4, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Choi, D.; Park, S.; Park, T. Carvone decreases melanin content by inhibiting melanoma cell proliferation via the cyclic adenosine monophosphate (cAMP) pathway. Molecules 2020, 25, 5191. [Google Scholar] [CrossRef]
- Son, B.; Kang, W.; Park, S.; Choi, D.; Park, T. Dermal olfactory receptor OR51B5 is essential for survival and collagen synthesis in human dermal fibroblast (Hs68 cells). Int. J. Mol. Sci. 2021, 22, 9273. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Kang, W.; Park, S.; Son, B.; Park, T. β-ionone attenuates dexamethasone-induced suppression of collagen and hyaluronic acid synthesis in human dermal fibroblasts. Biomolecules 2021, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Choi, D.; Park, T. Decanal protects against UVB-induced photoaging in human dermal fibroblasts via the cAMP pathway. Nutrients 2020, 12, 1214. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.S.; Scott, J.D. A-kinase anchoring proteins: Protein kinase A and beyond. Curr. Opin. Cell Biol. 2000, 12, 217–221. [Google Scholar] [CrossRef]
- Søberg, K.; Skålhegg, B.S. The molecular basis for specificity at the level of the protein kinase a catalytic subunit. Front. Endocrinol. 2018, 9, 538. [Google Scholar]
- Turnham, R.E.; Scott, J.D. Protein kinase A catalytic subunit isoform PRKACA.; History, function and physiology. Gene 2016, 577, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Stork, P.J.; Schmitt, J.M. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002, 12, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Sevetson, B.R.; Kong, X.; Lawrence Jr, J.C. Increasing cAMP attenuates activation of mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 1993, 90, 10305–10309. [Google Scholar] [CrossRef] [Green Version]
- Thoresen, G.H.; Johasen, E.J.; Christoffersen, T. Effects of cAMP on ERK mitogen-activated protein kinase activity in hepatocytes do not parallel the bidirectional regulation of DNA synthesis. Cell Biol. Int. 1999, 23, 13–20. [Google Scholar] [CrossRef]
- d’Angelo, G.; Lee, H.; Weiner, R.I. cAMP-dependent protein kinase inhibits the mitogenic action of vascular endothelial growth factor and fibroblast growth factor in capillary endothelial cells by blocking Raf activation. J. Cell. Biochem. 1997, 67, 353–366. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Pelling, J.C.; Ramaswamy, N.T.; Eppler, J.W.; Wallace, D.P.; Nagao, S.; Rome, L.A.; Sullivan, L.P.; Grantham, J.J. cAMP stimulates the in vitro proliferation of renal cyst epithelial cells by activating the extracellular signal-regulated kinase pathway. Kidney Int. 2000, 57, 1460–1471. [Google Scholar] [CrossRef] [Green Version]
- Seger, R.; Hanoch, T.; Rosenberg, R.; Dantes, A.; Merz, W.E.; Strauss, J.F.; Amsterdam, A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2001, 276, 13957–13964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crépieux, P.; Marion, S.; Martinat, N.; Fafeur, V.; Vern, Y.L.; Kerboeuf, D.; Guillou, F.; Reiter, E. The ERK-dependent signalling is stage-specifically modulated by FSH, during primary Sertoli cell maturation. Oncogene 2001, 20, 4696–4709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; Mclauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lochner, A.; Moolman, J. The many faces of H89: A review. Cardiovasc. Drug Rev. 2006, 24, 261–274. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Son, B.; Park, S.; Park, T. Activation of OR10A3 by Suberic Acid Promotes Collagen Synthesis in UVB-Irradiated Dermal Fibroblasts via the cAMP-Akt Pathway. Cells 2022, 11, 3961. [Google Scholar] [CrossRef]
- Yano, N.; Suzuki, D.; Endoh, M.; Zhao, T.C.; Padbury, J.F.; Tseng, Y.-T. A novel phosphoinositide 3-kinase-dependent pathway for angiotensin II/AT-1 receptor-mediated induction of collagen synthesis in MES-13 mesangial cells. J. Biol. Chem. 2007, 282, 18819–18830. [Google Scholar] [CrossRef] [Green Version]
- Weng, L.; Wang, W.; Su, X.; Huang, Y.; Su, L.; Liu, M.; Sun, Y.; Yang, B.; Zhou, H. The effect of cAMP-PKA activation on TGF-β1-induced profibrotic signaling. Cell. Physiol. Biochem. 2015, 36, 1911–1927. [Google Scholar] [CrossRef]
- Tao, Y.; Pan, M.; Liu, S.; Fang, F.; Lu, R.; Lu, C.; Zheng, M.; An, J.; Xu, H.; Zhao, F. cAMP level modulates scleral collagen remodeling, a critical step in the development of myopia. PLoS ONE 2013, 8, e71441. [Google Scholar] [CrossRef] [Green Version]
- Swaney, J.S.; Roth, D.M.; Olson, E.R.; Naugle, J.E.; Meszaros, J.G.; Insel, P.A. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc. Natl. Acad. Sci. USA 2005, 102, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Wettlaufer, S.H.; Hogaboam, C.; Aronoff, D.M.; Peters-Golden, M. Prostaglandin E2 inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 292, L405–L413. [Google Scholar] [CrossRef] [Green Version]
- Perez-Aso, M.; Fernandez, P.; Mediero, A.; Chan, E.S.; Cronstein, B.N. Adenosine 2A receptor promotes collagen production by human fibroblasts via pathways involving cyclic AMP and AKT but independent of Smad2/3. FASEB J. 2014, 28, 802. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Eksterowicz, J.; Zhou, H.; Rew, Y.; Zhu, L.; Yan, X.; Medina, J.C.; Huang, T.; Chen, X.; Sutimantanapi, D. Discovery of a Potent Steroidal Glucocorticoid Receptor Antagonist with Enhanced Selectivity against the Progesterone and Androgen Receptors (OP-3633). J. Med. Chem. 2019, 62, 6751–6764. [Google Scholar] [CrossRef]
- Nie, R.; Lu, J.; Xu, R.; Yang, J.; Shen, X.; Ouyang, X.; Zhu, D.; Huang, Y.; Zhao, T.; Zhao, X. Ipriflavone as a non-steroidal glucocorticoid receptor antagonist ameliorates diabetic cognitive impairment in mice. Aging Cell 2022, 21, e13572. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M.; Maneechotesuwan, K.; Usmani, O. Molecular interactions between glucocorticoids and long-acting β2-agonists. J. Allergy Clin. Immunol. 2002, 110, S261–S268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galliher-Beckley, A.J.; Cidlowski, J.A. Emerging roles of glucocorticoid receptor phosphorylation in modulating glucocorticoid hormone action in health and disease. IUBMB Life 2009, 61, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, L.M.; Jiménez-Panizo, A.; Alegre-Martí, A.; Estébanez-Perpiñá, E.; Caelles, C.; Pérez, P. Glucocorticoid resistance: Interference between the glucocorticoid receptor and the MAPK signalling pathways. Int. J. Mol. Sci. 2021, 22, 10049. [Google Scholar] [CrossRef] [PubMed]
- Necela, B.M.; Cidlowski, J.A. Mechanisms of glucocorticoid receptor action in noninflammatory and inflammatory cells. Proc. Am. Thorac. Soc. 2004, 1, 239–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, K.R. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 2017, 18, 159–174. [Google Scholar] [CrossRef]
- Præstholm, S.M.; Correia, C.M.; Grøntved, L. Multifaceted control of GR signaling and its impact on hepatic transcriptional networks and metabolism. Front. Endocrinol. 2020, 11, 572981. [Google Scholar] [CrossRef]
- Lim, B.; Samper, N.; Lu, H.; Rushlow, C.; Jiménez, G.; Shvartsman, S.Y. Kinetics of gene derepression by ERK signaling. Proc. Natl. Acad. Sci. USA 2013, 110, 10330–10335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyssonnaux, C.; Eychène, A. The Raf/MEK/ERK pathway: New concepts of activation. Biol. Cell 2001, 93, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Rogatsky, I.; Logan, S.K.; Garabedian, M.J. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 2050–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.J.; Sanjanwala, A.R.; Morin, E.E.; Rowland-Fisher, E.; Anderson, K.; Schwendeman, A.; Rainey, W.E. Synthetic high-density lipoprotein (sHDL) inhibits steroid production in HAC15 adrenal cells. Endocrinology 2016, 157, 3122–3129. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Edwards, M.A.; Ahlem, C.; Kennedy, M.; Cohen, A.; Gomez-Sanchez, C.E.; Rainey, W.E. The effects of adrenocorticotrophic hormone on steroid metabolomic profiles in human adrenal cells. J. Endocrinol. 2011, 209, 327–335. [Google Scholar] [CrossRef] [Green Version]
- van den Akker, E.L.; Koper, J.W.; van Rossum, E.F.; Dekker, M.J.; Russcher, H.; de Jong, F.H.; Uitterlinden, A.G.; Hofman, A.; Pols, H.A.; Witteman, J.C. Glucocorticoid receptor gene and risk of cardiovascular disease. Arch. Intern. Med. 2008, 168, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Rosmond, R. The glucocorticoid receptor gene and its association to metabolic syndrome. Obes. Res. 2002, 10, 1078–1086. [Google Scholar] [CrossRef]
- Morale, C.; Brouwer, J.; Testa, N.; Tirolo, C.; Barden, N.; Dijkstra, C.; Amor, S.; Marchetti, B. Stress, glucocorticoids and the susceptibility to develop autoimmune disorders of the central nervous system. Neurol. Sci. 2001, 22, 159–162. [Google Scholar] [CrossRef]
- DeRijk, R.H.; van Leeuwen, N.; Klok, M.D.; Zitman, F.G. Corticosteroid receptor-gene variants: Modulators of the stress-response and implications for mental health. Eur. J. Pharmacol. 2008, 585, 492–501. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.; Choi, D.; Roh, J.; Jung, Y.; Ha, Y.; Yang, S.; Park, T. The Role of Cyclic Adenosine Monophosphate (cAMP) in Modulating Glucocorticoid Receptor Signaling and Its Implications on Glucocorticoid-Related Collagen Loss. Int. J. Mol. Sci. 2023, 24, 10180. https://doi.org/10.3390/ijms241210180
Kang W, Choi D, Roh J, Jung Y, Ha Y, Yang S, Park T. The Role of Cyclic Adenosine Monophosphate (cAMP) in Modulating Glucocorticoid Receptor Signaling and Its Implications on Glucocorticoid-Related Collagen Loss. International Journal of Molecular Sciences. 2023; 24(12):10180. https://doi.org/10.3390/ijms241210180
Chicago/Turabian StyleKang, Wesuk, Dabin Choi, Jiyun Roh, Yearim Jung, Yoojeong Ha, Suhjin Yang, and Taesun Park. 2023. "The Role of Cyclic Adenosine Monophosphate (cAMP) in Modulating Glucocorticoid Receptor Signaling and Its Implications on Glucocorticoid-Related Collagen Loss" International Journal of Molecular Sciences 24, no. 12: 10180. https://doi.org/10.3390/ijms241210180
APA StyleKang, W., Choi, D., Roh, J., Jung, Y., Ha, Y., Yang, S., & Park, T. (2023). The Role of Cyclic Adenosine Monophosphate (cAMP) in Modulating Glucocorticoid Receptor Signaling and Its Implications on Glucocorticoid-Related Collagen Loss. International Journal of Molecular Sciences, 24(12), 10180. https://doi.org/10.3390/ijms241210180





