Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes
Abstract
:1. Introduction
1.1. Pancreatic Cancer and Diabetes
1.2. Pancreatic Cancer-Related Diabetes (PCRD)
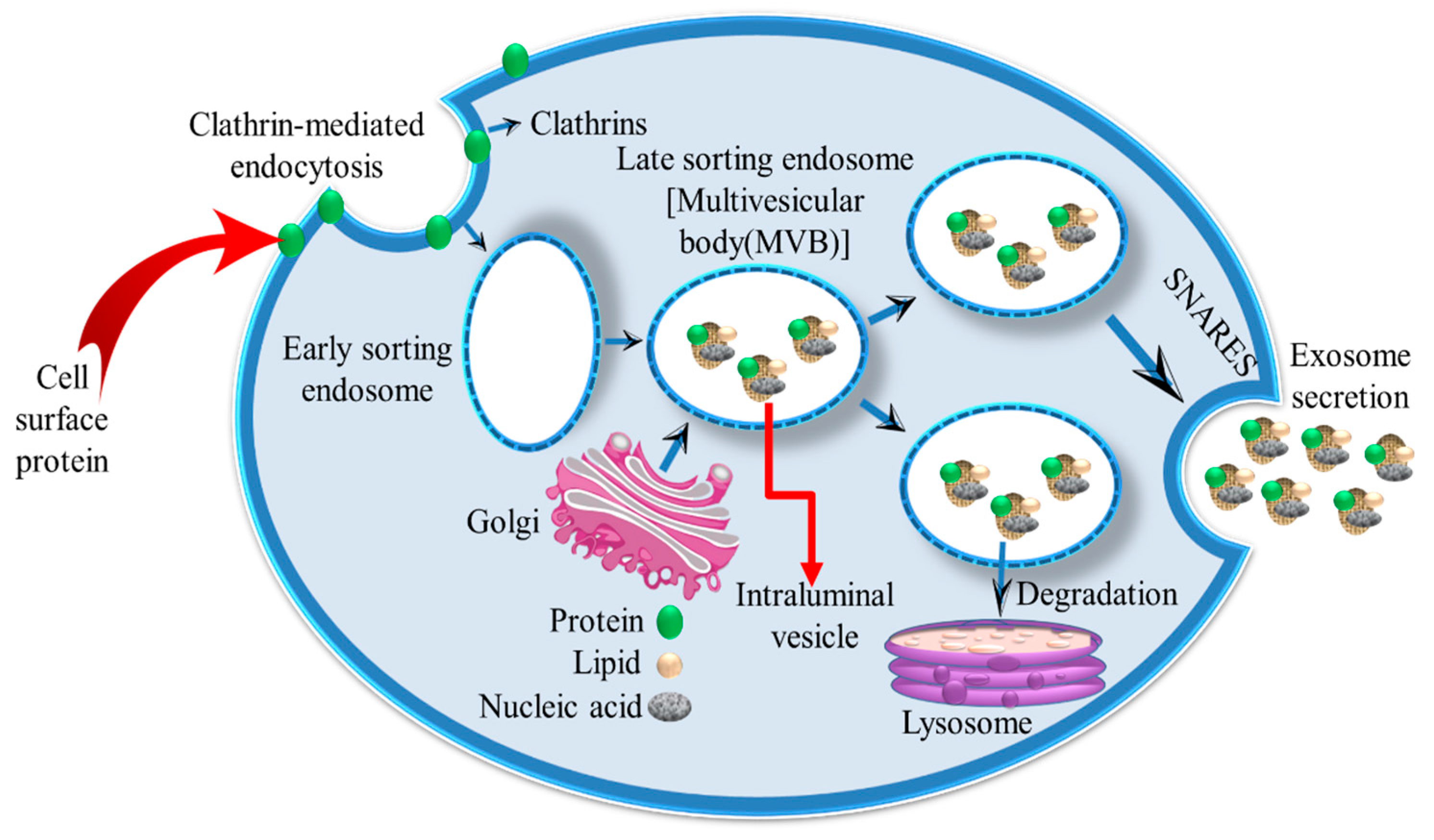
2. Exosomes
2.1. Exosome Formation and Characterization
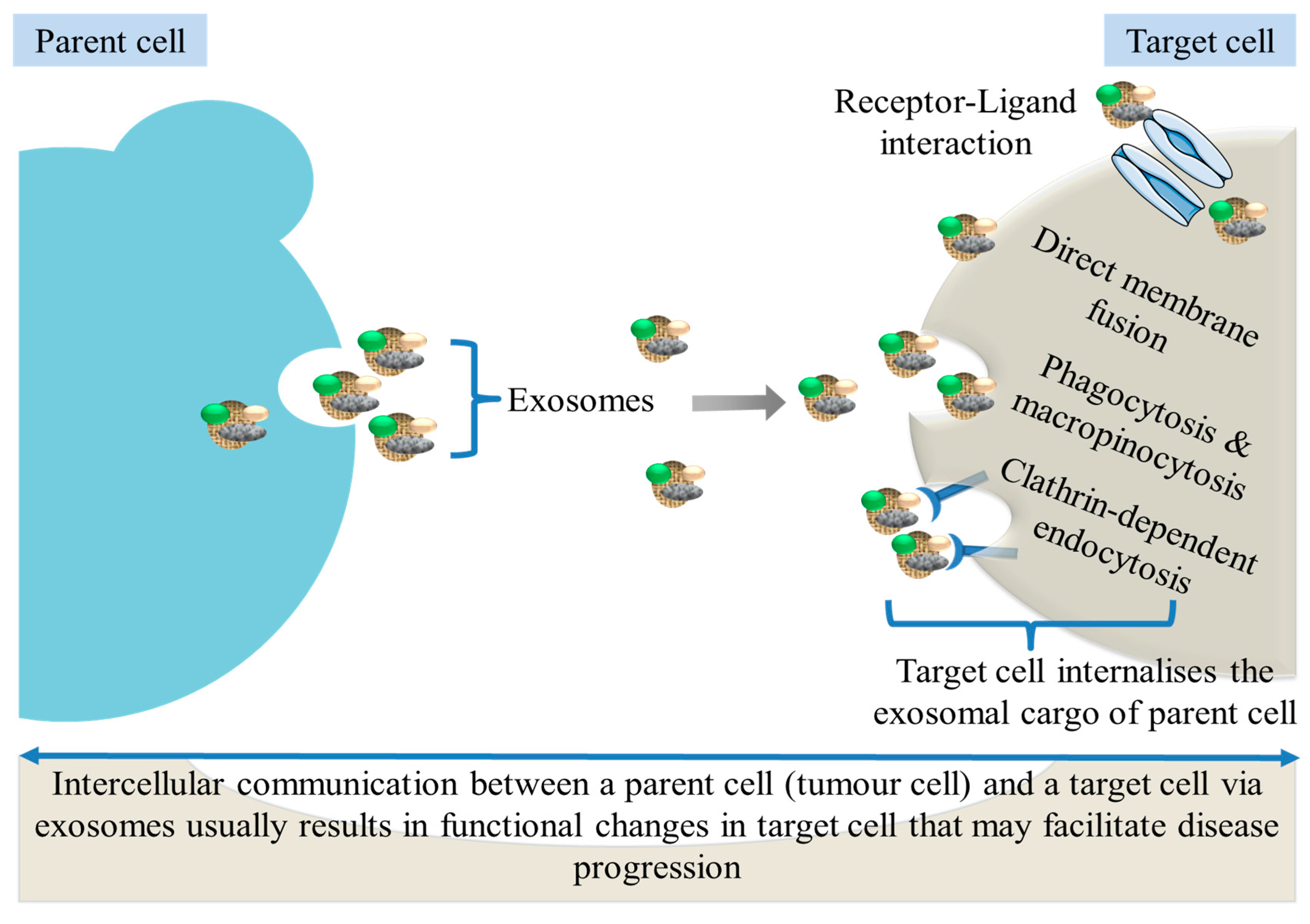
2.2. Uptake of Exosomes by Recipient Cells
3. Pancreatic Cancer-Derived Exosomes
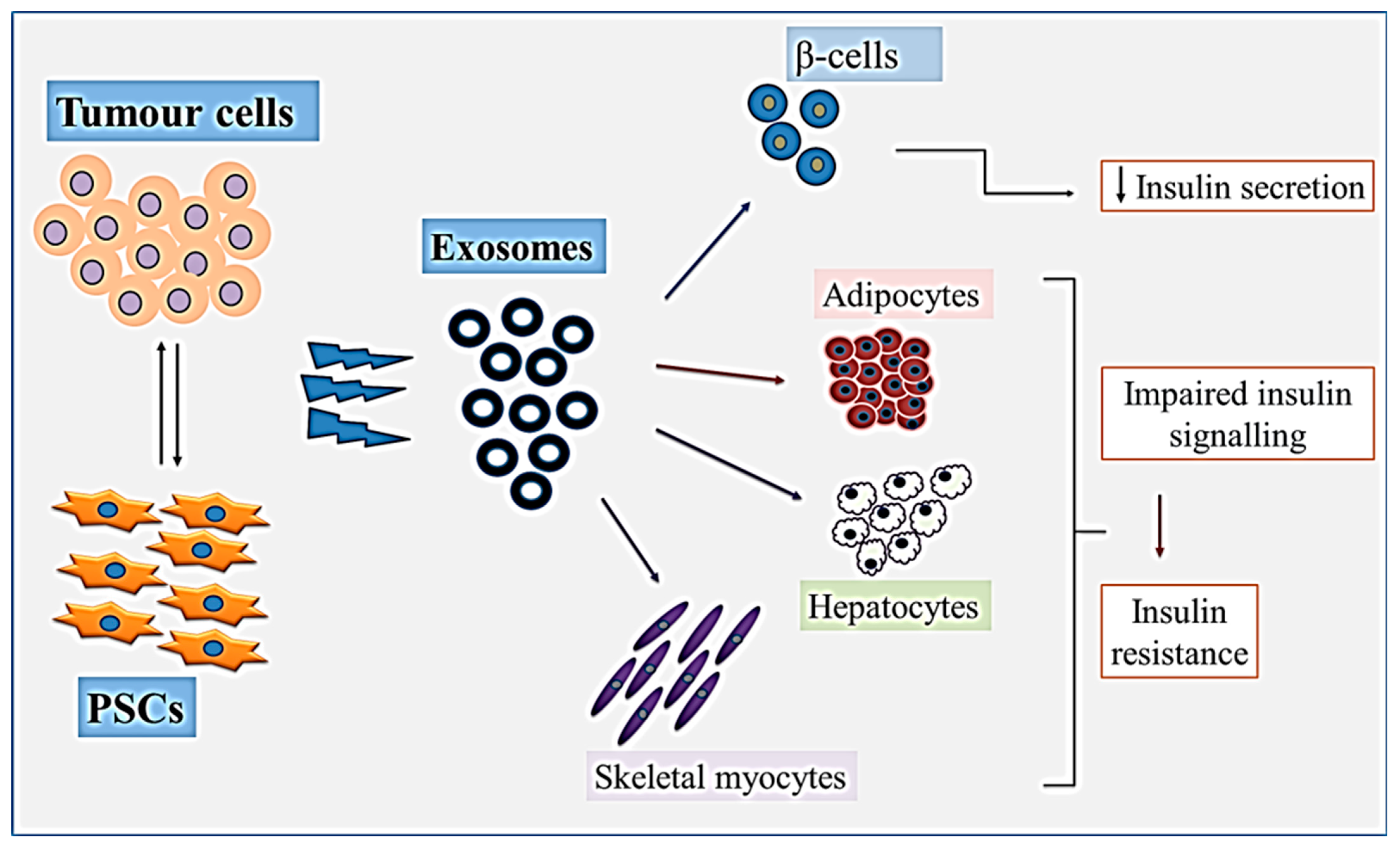
4. Role of Pancreatic Tumour-Derived Exosomes in PCRD
4.1. Exosomal Protein Cargo and PCRD
Adrenomedullin
- 1.
- The PC-derived S-100A8 N-terminal peptide: a diabetogenic agent with reduced glucose consumption and lactate production by myoblasts in vitro [52]. This peptide also hindered the growth of myoblasts by inhibiting myotubular differentiation and increasing caspase-3 activation in cultured C2C12 myoblasts. S-100A8 is, therefore, suggested to be a cause of hyperglycemia because it impairs glucose catabolism in myoblasts and is speculated to be a promising biomarker for the diagnosis of PCRD. In addition, S-100A8 N-terminal alters β-cell insulin secretion by inhibiting glucose-stimulated insulin exocytosis (early response), which is characteristic of PCRD [53].
- 2.
- The insulin-like growth factor 1 (IGF-1) and Insulin-like growth factor-binding protein-2 (IGFBP-2) are biomarkers of pancreatic diseases, especially CP and PC. Since the expression of IGF-1 in CP and DM was elevated compared with that in PC and DM, IGF-1 may be an indicator that signals whether pancreatic diabetes is from CP or PC [54].
- 3.
- In the serum taken up to 4 years before a PC diagnosis, circulating Thrombospondin 1 (TSP-1) levels were found to be significantly reduced up to 24 months prior to diagnosis. Low serum TSP-1 levels in PC patients were associated with DM. Interestingly, TSP1 levels in PC patients with DM were lower compared to patients with T2DM alone. TSP-1 was also decreased in PC patients, compared to the healthy controls and patients with benign biliary obstruction at clinical diagnosis. Not only did circulating TSP-1 levels decrease up to 24 months before the diagnosis of PC, but a combination of TSP-1 and CA19-9 also produced a diagnostic capacity that significantly outperformed both markers alone. Decreased TSP-1 levels may be an indication of PCRD, and early PC detection strategies could include exploring the clinical relevance of TSP-1.
- 4.
- The vascular noninflammatory molecule 1 (Vanin 1/VNN1) is a pantetheinase that is anchored to the extracellular membrane of epithelial and myeloid cells. It belongs to an enzymatic pathway and causes oxidative stress, inflammation and cell migration and is suggested to be a biomarker for certain malignancies, including systemic lupus erythematosus and type 1 diabetic nephropathy [55,56]. It was overexpressed in PC and inhibited the growth of insulin-secreting cell lines (β-TC-6 and INS-1) when they were treated with conditioned media derived from PC cell lines. This loss of cell viability was even greater when the cells were exposed to conditioned media from PC cells transfected with a VNN1-overexpressing vector, indicating that the high expression of VNN1 causes injury to paraneoplastic insulin-secreting cells. The high expression of VNN1 also altered the expressions of oxidative stress-related factors, including the downregulation of the anti-oxidative stress/anticancer peroxisome-proliferator activated receptor γ (PPAR-γ), the upregulation of cysteamine which is a product of VNN1, the downregulation of the antioxidant glutathione (GSH) and upregulation of ROS. Hence, the overexpression of VNN1 in PC cells modulated the viability and function of β cells by promoting oxidative stress in the β cells’ microenvironment [57]. Additionally, VNN1, when used in combination with the matrix metalloproteinase 9 (MMP9), could be a more effective blood biomarker panel for the discrimination of PCRD from T2DM [58].
4.2. Exosomal Lipid Cargo and PCRD
4.3. Exosomal RNA Cargo and PCRD
Exosomal miRNAs and PCRD
5. Tumour-Derived Exosomal Cargo and Pancreatic Cancer
6. Pancreatic Stellate Cell-Derived Exosomes and PCRD
7. Other Biomarkers for PCRD
- 1.
- Nitric oxide (NO), which is overexpressed in PCRD, has diabetogenic effects, and the absence of specific fragments of NO correlates with the development of DM [91]. Hence, PCRD might be a result of the diabetogenic effects of tumour products, possibly acting via NO.
- 2.
- In a study aimed at identifying the mediators of PCRD by comparing the gene expression between PCRD patients and PC patients without DM, low levels of Kinesin Family Member 22 (KIF22) and glycogen Phosphorylase L (PYGL) were found to be associated with good survival outcomes for PC patients with DM and may be prognostic biomarkers for PCRD. Additionally, bioinformatic predictions revealed that KIF22, PYGL, Ribosomal Protein S27a (RPS27A), and ubiquitin A-52 residue ribosomal protein fusion product 1 (UBA52) could be involved in the pathogenesis of PCRD [92].
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Hackeng, W.M.; Hruban, R.H.; Offerhaus, G.J.A.; Brosens, L.A.A. Surgical and molecular pathology of pancreatic neoplasms. Diagn. Pathol. 2016, 11, 47. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, J.; Xia, G.; Lei, S.; Huang, X.; Huang, X. Survival of pancreatic cancer patients is negatively correlated with age at diagnosis: A population-based retrospective study. Sci. Rep. 2020, 10, 7048. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Cancer Data in Australia [Internet]. Canberra: Australian Institute of Health and Welfare. 2022. Available online: https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia (accessed on 27 April 2023).
- Khadka, R.; Tian, W.J.; Hao, X.; Koirala, R. Risk factor, early diagnosis and overall survival on outcome of association between pancreatic cancer and diabetes mellitus: Changes and advances, a review. Int. J. Surg. 2018, 52, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Capasso, M.; Franceschi, M.; Rodriguez-Castro, K.I.; Crafa, P.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Leandro, G.; Meschi, T.; et al. Epidemiology and risk factors of pancreatic cancer. Acta Bio Med. Atenei Parm. 2018, 89, 141–146. [Google Scholar] [CrossRef]
- Lam, B.Q.; Shrivastava, S.K.; Shrivastava, A.; Shankar, S.; Srivastava, R.K. The Impact of obesity and diabetes mellitus on pancreatic cancer: Molecular mechanisms and clinical perspectives. J. Cell. Mol. Med. 2020, 24, 7706–7716. [Google Scholar] [CrossRef]
- Malka, D.; Hammel, P.; Maire, F.; Rufat, P.; Madeira, I.; Pessione, F.; Lévy, P.; Ruszniewski, P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut 2002, 51, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Pothuraju, R.; Rachagani, S.; Junker, W.M.; Chaudhary, S.; Saraswathi, V.; Kaur, S.; Batra, S.K. Pancreatic cancer associated with obesity and diabetes: An alternative approach for its targeting. J. Exp. Clin. Cancer Res. 2018, 37, 319. [Google Scholar] [CrossRef] [Green Version]
- Ewald, N. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J. Gastroenterol. 2013, 19, 7276. [Google Scholar] [CrossRef]
- Illés, D.; Ivány, E.; Holzinger, G.; Kosár, K.; Adam, M.G.; Kamlage, B.; Zsóri, G.; Tajti, M.; Svébis, M.M.; Horváth, V.; et al. New Onset of DiabetEs in aSsociation with pancreatic ductal adenocarcinoma (NODES Trial): Protocol of a prospective, multicentre observational trial. BMJ Open 2020, 10, e037267. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes–Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.; Ede, J.; Collins, J.; Willens, D. Pancreatic Cancer Presenting as New-Onset Diabetes. Case Rep. Oncol. 2014, 7, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Mellenthin, C.; Balaban, V.D.; Dugic, A.; Cullati, S. Risk Factors for Pancreatic Cancer in Patients with New-Onset Diabetes: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4684. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, P.; Nguyen, T.L.; Prunier, C.; Razzaque, M.S.; Xu, K.; Atfi, A. Pancreatic cancer triggers diabetes through TGF-β–mediated selective depletion of islet β-cells. Life Sci. Alliance 2020, 3, e201900573. [Google Scholar] [CrossRef]
- Andersen, D.K.; Andren-Sandberg, Å.; Duell, E.J.; Goggins, M.; Korc, M.; Petersen, G.M.; Smith, J.P.; Whitcomb, D.C. Pancreatitis-Diabetes-Pancreatic Cancer: Summary of an NIDDK-NCI Workshop. Pancreas 2013, 42, 1227–1237. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, G.; Ramachandran, V.; Javeed, N.; Arumugam, T.; Dutta, S.; Klee, G.G.; Klee, E.W.; Smyrk, T.C.; Bamlet, W.; Han, J.J.; et al. Adrenomedullin is Up-regulated in Patients with Pancreatic Cancer and Causes Insulin Resistance in β Cells and Mice. Gastroenterology 2012, 143, 1510.e1–1517.e1. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, R.; Riveiro, M.E.; Berenguer-Daizé, C.; O’kane, A.; Gormley, J.; Touzelet, O.; Rezai, K.; Bekradda, M.; Ouafik, L. Targeting Adrenomedullin in Oncology: A Feasible Strategy with Potential as Much More Than an Alternative Anti-Angiogenic Therapy. Front. Oncol. 2021, 10, 589218. [Google Scholar] [CrossRef]
- Keleg, S.; Kayed, H.; Jiang, X.; Penzel, R.; Giese, T.; Büchler, M.W.; Friess, H.; Kleeff, J. Adrenomedullin is induced by hypoxia and enhances pancreatic cancer cell invasion. Int. J. Cancer 2007, 121, 21–32. [Google Scholar] [CrossRef]
- Permert, J.; Larsson, J.; Westermark, G.T.; Herrington, M.K.; Christmanson, L.; Pour, P.M.; Westermark, P.; Adrian, T.E. Islet Amyloid Polypeptide in Patients with Pancreatic Cancer and Diabetes. N. Engl. J. Med. 1994, 330, 313–318. [Google Scholar] [CrossRef]
- Hart, P.A.; Baichoo, E.; Bi, Y.; Hinton, A.; Kudva, Y.C.; Chari, S.T. Pancreatic polypeptide response to a mixed meal is blunted in pancreatic head cancer associated with diabetes mellitus. Pancreatology 2015, 15, 162–166. [Google Scholar] [CrossRef]
- Narasimhan, A.; Zhong, X.; Au, E.P.; Ceppa, E.P.; Nakeeb, A.; House, M.G.; Zyromski, N.J.; Schmidt, C.M.; Schloss, K.N.H.; Schloss, D.E.I.; et al. Profiling of Adipose and Skeletal Muscle in Human Pancreatic Cancer Cachexia Reveals Distinct Gene Profiles with Convergent Pathways. Cancers 2021, 13, 1975. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.C.; You, M.; Yu, S.-Y.; Luan, Y.; Eldani, M.; Caffrey, T.C.; Grandgenett, P.M.; O’connell, K.A.; Shukla, S.K.; Kattamuri, C.; et al. Visceral adipose tissue remodeling in pancreatic ductal adenocarcinoma cachexia: The role of activin A signaling. Sci. Rep. 2022, 12, 1659. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, O.-K. Potential Roles of Adipocyte Extracellular Vesicle–Derived miRNAs in Obesity-Mediated Insulin Resistance. Adv. Nutr. Int. Rev. J. 2021, 12, 566–574. [Google Scholar] [CrossRef]
- Danai, L.V.; Babic, A.; Rosenthal, M.H.; Dennstedt, E.A.; Muir, A.; Lien, E.C.; Mayers, J.R.; Tai, K.; Lau, A.N.; Jones-Sali, P.; et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 2018, 558, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.F. How pancreatic adenocarcinoma might cause diabetes? The role of TGF-β. Int. J. Clin. Gastroenterol. Hepatol. 2021, 3, 5–10. [Google Scholar]
- Motohashi, N.; Alexander, M.S.; Shimizu-Motohashi, Y.; Myers, J.A.; Kawahara, G.; Kunkel, L.M. Regulation of IRS1/Akt insulin signaling by microRNA-128a during myogenesis. J. Cell Sci. 2013, 126, 2678–2691. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Li, Y.; Yang, X.; He, X.; Zhang, H.; Zhang, L.; He, J. The Feedback Regulation of PI3K-miR-19a, and MAPK-miR-23b/27b in Endothelial Cells under Shear Stress. Molecules 2012, 18, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Sahoo, J.; Kamalanathan, S.; Naik, D.; Mohan, P.; Kalayarasan, R. Diabetes and pancreatic cancer: Exploring the two-way traffic. World J. Gastroenterol. 2021, 27, 4939–4962. [Google Scholar] [CrossRef]
- De Souza, A.; Irfan, K.; Masud, F.; Saif, M.W. Diabetes Type 2 and Pancreatic Cancer: A History Unfolding. JOP J. Pancreas 2016, 17, 144. [Google Scholar]
- Albury-Warren, T. Diabetes Phenotypes in Transgenic Pancreatic Cancer Mouse Models. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2015. [Google Scholar]
- Gupta, S.; Vittinghoff, E.; Bertenthal, D.; Corley, D.; Shen, H.; Walter, L.C.; McQuaid, K. New-Onset Diabetes and Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2006, 4, 1366–1372. [Google Scholar] [CrossRef]
- Gallo, M.; Adinolfi, V.; Morviducci, L.; Acquati, S.; Tuveri, E.; Ferrari, P.; Zatelli, M.; Faggiano, A.; Argentiero, A.; Natalicchio, A.; et al. Early prediction of pancreatic cancer from new-onset diabetes: An Associazione Italiana Oncologia Medica (AIOM)/Associazione Medici Diabetologi (AMD)/Società Italiana Endocrinologia (SIE)/Società Italiana Farmacologia (SIF) multidisciplinary consensus position paper. ESMO Open 2021, 6, 100155. [Google Scholar] [CrossRef]
- Lazo, S.; Hooten, N.N.; Green, J.; Eitan, E.; Mode, N.A.; Liu, Q.; Zonderman, A.B.; Ezike, N.; Mattson, M.P.; Ghosh, P.; et al. Mitochondrial DNA in extracellular vesicles declines with age. Aging Cell 2020, 20, e13283. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [Green Version]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.F.; Noren Hooten, N.; Freeman, D.W.; Mode, N.A.; Zonderman, A.B.; Evans, M.K. Extracellular vesicles in diabetes mellitus induce alterations in endothelial cell morphology and migration. J. Transl. Med. 2020, 18, 230. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zheng, L.; Zou, X.; Wang, J.; Zhong, J.; Zhong, T. Extracellular vesicles in type 2 diabetes mellitus: Key roles in pathogenesis, complications, and therapy. J. Extracell. Vesicles 2019, 8, 1625677. [Google Scholar] [CrossRef] [Green Version]
- Moeng, S.; Son, S.W.; Lee, J.S.; Lee, H.Y.; Kim, T.H.; Choi, S.Y.; Kuh, H.J.; Park, J.K. Extracellular Vesicles (EVs) and Pancreatic Cancer: From the Role of EVs to the Interference with EV-Mediated Reciprocal Communication. Biomedicines 2020, 8, 267. [Google Scholar] [CrossRef]
- Javeed, N.; Sagar, G.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Bhattacharya, S.; Truty, M.; Petersen, G.M.; Kaufman, R.J.; Chari, S.T.; et al. Pancreatic Cancer–Derived Exosomes Cause Paraneoplastic β-cell Dysfunction. Clin. Cancer Res. 2015, 21, 1722–1733. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.; Yao, W.; Dai, X.; Zhang, A.; Hou, L.; Wang, L.; Wang, Y.; Huang, X.; Meng, X.; Li, L. Pancreatic cancer-derived exosomal microRNA-19a induces β-cell dysfunction by targeting ADCY1 and EPAC2. Int. J. Biol. Sci. 2021, 17, 3622–3633. [Google Scholar] [CrossRef] [PubMed]
- Halvaei, S.; Daryani, S.; Eslami-S, Z.; Samadi, T.; Jafarbeik-Iravani, N.; Bakhshayesh, T.O.; Majidzadeh-A, K.; Esmaeili, R. Exosomes in Cancer Liquid Biopsy: A Focus on Breast Cancer. Mol. Ther. Nucleic Acids 2017, 10, 131–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verel-Yilmaz, Y.; Fernández, J.P.; Schäfer, A.; Nevermann, S.; Cook, L.; Gercke, N.; Helmprobst, F.; Jaworek, C.; von Strandmann, E.P.; Pagenstecher, A.; et al. Extracellular Vesicle-Based Detection of Pancreatic Cancer. Front. Cell Dev. Biol. 2021, 9, 697939. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Pauklin, S. Extracellular vesicles in pancreatic cancer progression and therapies. Cell Death Dis. 2021, 12, 973. [Google Scholar] [CrossRef]
- Channon, L.M.; Tyma, V.M.; Xu, Z.; Greening, D.W.; Wilson, J.S.; Perera, C.J.; Apte, M.V. Small extracellular vesicles (exosomes) and their cargo in pancreatic cancer: Key roles in the hallmarks of cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2022, 1877, 188728. [Google Scholar] [CrossRef] [PubMed]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.R.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef]
- Cueille, C.; Birot, O.; Bigard, X.; Hagner, S.; Garel, J.-M. Post-transcriptional regulation of CRLR expression during hypoxia. Biochem. Biophys. Res. Commun. 2004, 326, 23–29. [Google Scholar] [CrossRef]
- Sagar, G.; Sah, R.P.; Javeed, N.; Dutta, S.K.; Smyrk, T.C.; Lau, J.S.; Giorgadze, N.; Tchkonia, T.; Kirkland, J.L.; Chari, S.T.; et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 2015, 65, 1165–1174. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.; Greco, E.; Fogar, P.; Pucci, P.; Flagiello, A.; Baldo, G.; Giunco, S.; Valerio, A.; Navaglia, F.; Zambon, C.-F.; et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: A diabetes cause? Clin. Chim. Acta 2006, 372, 120–128. [Google Scholar] [CrossRef]
- Padoan, A.; Greco, E.; Basso, D.; Fogar, P.; Valerio, A.N.N.A.; Fadi, E.; Schiavon, S.; Fasolo, M.; Vigolo, S.; DE CARLO, E.; et al. The pancreatic cancer derived N-Terminal peptide of S100A8 inhibits insulin exocytosis and stimulates cancer cell growth. Biochim. Clin. 2007, 31, 432. [Google Scholar]
- Włodarczyk, B.; Borkowska, A.; Włodarczyk, P.; Małecka-Panas, E.; Gąsiorowska, A. Insulin-like growth factor 1 and insulin-like growth factor binding protein 2 serum levels as potential biomarkers in differential diagnosis between chronic pancreatitis and pancreatic adenocarcinoma in reference to pancreatic diabetes. Gastroenterol. Rev. 2021, 16, 36–42. [Google Scholar] [CrossRef]
- Sánchez-Muñoz, F.; Amezcua-Guerra, L.; Macías-Palacios, M.; Márquez-Velasco, R.; Bojalil, R. Vanin-1 as a potential novel biomarker for active nephritis in systemic lupus erythematosus. Lupus 2013, 22, 333–335. [Google Scholar] [CrossRef]
- Fugmann, T.; Borgia, B.; Révész, C.; Godó, M.; Forsblom, C.; Hamar, P.; Holthöfer, H.; Neri, D.; Roesli, C. Proteomic identification of vanin-1 as a marker of kidney damage in a rat model of type 1 diabetic nephropathy. Kidney Int. 2011, 80, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.; Qin, W.; Buya, M.; Dong, X.; Zheng, W.; Lu, W.; Chen, J.; Guo, Q.; Wu, Y. VNN1, a potential biomarker for pancreatic cancer-associated new-onset diabetes, aggravates paraneoplastic islet dysfunction by increasing oxidative stress. Cancer Lett. 2016, 373, 241–250. [Google Scholar] [CrossRef]
- Huang, H.; Dong, X.; Kang, M.X.; Xu, B.; Chen, Y.; Zhang, B.; Chen, J.; Xie, Q.P.; Wu, Y.L. Novel Blood Biomarkers of Pancreatic Cancer–Associated Diabetes Mellitus Identified by Peripheral Blood–Based Gene Expression Profiles. Am. J. Gastroenterol. 2010, 105, 1661–1669. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Tenenbaum, A. Adiponectin: A manifold therapeutic target for metabolic syndrome, diabetes, and coronary disease? Cardiovasc. Diabetol. 2014, 13, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldfield, L.; Evans, A.; Rao, R.G.; Jenkinson, C.; Purewal, T.; Psarelli, E.E.; Menon, U.; Timms, J.F.; Pereira, S.P.; Ghaneh, P.; et al. Blood levels of adiponectin and IL-1Ra distinguish type 3c from type 2 diabetes: Implications for earlier pancreatic cancer detection in new-onset diabetes. Ebiomedicine 2022, 75, 103802. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.J.; Wickramasinghe, V.O. RNA in cancer. Nat. Rev. Cancer 2021, 21, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Binang, H.B.; Wang, Y.; Tewara, M.A.; Du, L.; Shi, S.; Li, N.; Nsenga, A.G.A.; Wang, C. Expression levels and associations of five long non-coding RNAs in gastric cancer and their clinical significance. Oncol. Lett. 2020, 19, 2431–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Le, P.; Romano, G.; Nana-Sinkam, P.; Acunzo, M. Non-Coding RNAs in Cancer Diagnosis and Therapy: Focus on Lung Cancer. Cancers 2021, 13, 1372. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Shi, L.; Luo, Z. Long Non-coding RNAs in Cancer: Implications for Diagnosis, Prognosis, and Therapy. Front. Med. 2020, 7, 902. [Google Scholar] [CrossRef]
- Chen, F.; Wang, N.; Tan, H.-Y.; Guo, W.; Zhang, C.; Feng, Y. The functional roles of exosomes-derived long non-coding RNA in human cancer. Cancer Biol. Ther. 2019, 20, 583–592. [Google Scholar] [CrossRef]
- Li, C.; Ni, Y.-Q.; Xu, H.; Xiang, Q.-Y.; Zhao, Y.; Zhan, J.-K.; He, J.-Y.; Li, S.; Liu, Y.-S. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 2021, 6, 383. [Google Scholar] [CrossRef] [PubMed]
- Verhoeckx, K.; Cotter, P.; López-Expósito, I.; Kleiveland, C.; Lea, T.; Mackie, A.; Requena, T.; Swiatecka, D.; Wichers, H. (Eds.) The Impact of Food Bioactives on Health; Springer International Publishing: Cham, Switzerland, 2015; Available online: http://link.springer.com/10.1007/978-3-319-16104-4 (accessed on 24 April 2023).
- Zhang, Y.; Huang, S.; Li, P.; Chen, Q.; Li, Y.; Zhou, Y.; Wang, L.; Kang, M.; Zhang, B.; Yang, B.; et al. Pancreatic cancer-derived exosomes suppress the production of GIP and GLP-1 from STC-1 cells in vitro by down-regulating the PCSK1/3. Cancer Lett. 2018, 431, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Pang, W.; Zhang, A.; Li, L.; Yao, W.; Dai, X. Exosomal miR-19a decreases insulin production by targeting Neurod1 in pancreatic cancer associated diabetes. Mol. Biol. Rep. 2021, 49, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kitaguchi, T.; Oya, M.; Wada, Y.; Tsuboi, T.; Miyawaki, A. Extracellular calcium influx activates adenylate cyclase 1 and potentiates insulin secretion in MIN6 cells. Biochem. J. 2013, 450, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, B.; Zheng, W.; Kang, M.; Chen, Q.; Qin, W.; Li, C.; Zhang, Y.; Shao, Y.; Wu, Y. Exosomes derived from pancreatic cancer cells induce insulin resistance in C2C12 myotube cells through the PI3K/Akt/FoxO1 pathway. Sci. Rep. 2017, 7, 538. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal Muscle FOXO1 (FKHR) Transgenic Mice Have Less Skeletal Muscle Mass, Down-regulated Type I (Slow Twitch/Red Muscle) Fiber Genes, and Impaired Glycemic Control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [Green Version]
- Wang, L. Exosomal microRNA-let-7b-5p Derived from Pancreatic Cancer Cells Possibly Promotes Insulin Resistance in C2C12 Myotube Cells by Targeting SLC6A15. In Review. 2021. Available online: https://www.researchsquare.com/article/rs-689703/v1 (accessed on 5 August 2022).
- Dai, X.; Pang, W.; Zhou, Y.; Yao, W.; Xia, L.; Wang, C.; Chen, X.; Zen, K.; Zhang, C.-Y.; Yuan, Y. Altered profile of serum microRNAs in pancreatic cancer-associated new-onset diabetes mellitus. J. Diabetes 2016, 8, 422–433. [Google Scholar] [CrossRef]
- Yao, K.; Wang, Q.; Jia, J.; Zhao, H. A competing endogenous RNA network identifies novel mRNA, miRNA and lncRNA markers for the prognosis of diabetic pancreatic cancer. Tumor Biol. 2017, 39, 1010428317707882. [Google Scholar] [CrossRef] [Green Version]
- Lan, B.; Zeng, S.; Grützmann, R.; Pilarsky, C. The Role of Exosomes in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4332. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Liu, P.; Wu, Y.; Meng, X.; Wu, M.; Han, J.; Tan, X. Exosomal zinc transporter ZIP4 promotes cancer growth and is a novel diagnostic biomarker for pancreatic cancer. Cancer Sci. 2018, 109, 2946–2956. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Wei, Q.; Koay, E.J.; Liu, Y.; Ning, B.; Bernard, P.W.; Zhang, N.; Han, H.; Katz, M.H.; Zhao, Z.; et al. Chemoresistance Transmission via Exosome-Mediated EphA2 Transfer in Pancreatic Cancer. Theranostics 2018, 8, 5986–5994. [Google Scholar] [CrossRef]
- Wang, X.; Luo, G.; Zhang, K.; Cao, J.; Huang, C.; Jiang, T.; Liu, B.; Su, L.; Qiu, Z. Hypoxic Tumor-Derived Exosomal miR-301a Mediates M2 Macrophage Polarization via PTEN/PI3Kγ to Promote Pancreatic Cancer Metastasis. Cancer Res. 2018, 78, 4586–4598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Tang, T.; Yang, X.; Qin, P.; Wang, P.; Zhang, H.; Bai, M.; Wu, R.; Li, F. Tumor-derived exosomal long noncoding RNA LINC01133, regulated by Periostin, contributes to pancreatic ductal adenocarcinoma epithelial-mesenchymal transition through the Wntβ-catenin pathway by silencing AXIN2. Oncogene 2021, 40, 3164–3179. [Google Scholar] [CrossRef] [PubMed]
- Robless, E.E.; Howard, J.A.; Casari, I.; Falasca, M. Exosomal long non-coding RNAs in the diagnosis and oncogenesis of pancreatic cancer. Cancer Lett. 2021, 501, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhao, G.; Yang, S.; Zhu, S.; Zhang, S.; Li, P. The significance of exosomal RNAs in the development, diagnosis, and treatment of pancreatic cancer. Cancer Cell Int. 2021, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Reese, M.; Dhayat, S.A. Small extracellular vesicle non-coding RNAs in pancreatic cancer: Molecular mechanisms and clinical implications. J. Hematol. Oncol. 2021, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, P.; Dong, W.; Liu, H.; Sun, J.; Zhao, L. LncRNA PVT1 promotes exosome secretion through YKT6, RAB7, and VAMP3 in pancreatic cancer. Aging 2020, 12, 10427–10440. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.J.; Falasca, M.; Chari, S.T.; Greenfield, J.R.; Xu, Z.; Pirola, R.C.; Wilson, J.S.; Apte, M.V. Role of Pancreatic Stellate Cell-Derived Exosomes in Pancreatic Cancer-Related Diabetes: A Novel Hypothesis. Cancers 2021, 13, 5224. [Google Scholar] [CrossRef]
- Li, M.; Guo, H.; Wang, Q.; Chen, K.; Marko, K.; Tian, X.; Yang, Y. Pancreatic stellate cells derived exosomal miR-5703 promotes pancreatic cancer by downregulating CMTM4 and activating PI3K/Akt pathway. Cancer Lett. 2020, 490, 20–30. [Google Scholar] [CrossRef]
- Ma, Q.; Wu, H.; Xiao, Y.; Liang, Z.; Liu, T. Upregulation of exosomal microRNA-21 in pancreatic stellate cells promotes pancreatic cancer cell migration and enhances Ras/ERK pathway activity. Int. J. Oncol. 2020, 56, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zeng, Z.; He, Z.; Lei, S. Hypoxic pancreatic stellate cell-derived exosomal mirnas promote proliferation and invasion of pancreatic cancer through the PTEN/AKT pathway. Aging 2021, 13, 7120–7132. [Google Scholar] [CrossRef] [PubMed]
- Perera, C.; Xu, Z.; Mekapogu, A.R.; Hosen, S.Z.; Pothula, S.; Greenfield, J.; Chari, S.; Goldstein, D.; Pirola, R.; Wilson, J.; et al. 1133 Pancreatic Stellate Cell and Cancer Cell Derived Exosomes Impair Beta Cell Function: Implications for Pancreatic Cancer Related Diabetes. Gastroenterology 2020, 158, S-221. [Google Scholar] [CrossRef]
- Valerio, A.; Basso, D.; Fogar, P.; Falconi, M.; Greco, E.; Bassi, C.; Seraglia, R.; Abu-Hilal, M.; Navaglia, F.; Zambon, C.-F.; et al. Maldi-TOF analysis of portal sera of pancreatic cancer patients: Identification of diabetogenic and antidiabetogenic peptides. Clin. Chim. Acta 2004, 343, 119–127. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Y.; Gao, H.; Jia, Y.; Xu, Y.; Wan, X.; Zhang, Z.; Yu, H.; Yan, S. Identification of Key Genes Involved in Pancreatic Ductal Adenocarcinoma with Diabetes Mellitus Based on Gene Expression Profiling Analysis. Pathol. Oncol. Res. 2021, 27, 604730. [Google Scholar] [CrossRef]

| S/N | PCRD | T2DM |
|---|---|---|
| 1. | Patients develop DM despite preceding weight loss | Patients gain weight at the time of DM onset |
| 2. | Occurs many months before the occurrence of cachexia (with greater than a 5% weight loss over 6 months) | Not associated with cachexia |
| 3. | Occurs within 3–5 years before the clinical diagnosis of PC | Occurs with or without PC development |
| 4. | Insulin levels are normal or low | Insulin levels are high and there is a marked insulin resistance |
| 5. | Usually improves or is resolved following PC treatments, including the surgical resection of the tumour | Persistent DM accompanies PDAC patients with long-standing T2DM even after surgical resection |
| 6. | Low levels of the glucose-dependent insulinotropic polypeptide (GIP) are evident | Variable levels of GIP |
| 7. | Pancreatic polypeptide (PP) levels are low or absent | PP levels are high or normal |
| 8. | Low glucagon levels | High glucagon levels |
| 9. | Occurs at any age | Occurs mainly in adulthood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binang, H.B.; Perera, C.J.; Apte, M.V. Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes. Int. J. Mol. Sci. 2023, 24, 10203. https://doi.org/10.3390/ijms241210203
Binang HB, Perera CJ, Apte MV. Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes. International Journal of Molecular Sciences. 2023; 24(12):10203. https://doi.org/10.3390/ijms241210203
Chicago/Turabian StyleBinang, Helen B., Chamini J. Perera, and Minoti V. Apte. 2023. "Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes" International Journal of Molecular Sciences 24, no. 12: 10203. https://doi.org/10.3390/ijms241210203
APA StyleBinang, H. B., Perera, C. J., & Apte, M. V. (2023). Role of Pancreatic Tumour-Derived Exosomes and Their Cargo in Pancreatic Cancer-Related Diabetes. International Journal of Molecular Sciences, 24(12), 10203. https://doi.org/10.3390/ijms241210203





