SDS-PAGE-Based Quantitative Assay of Hemolymph Proteins in Honeybees: Progress and Prospects for Field Application
Abstract
:1. Introduction
2. Results and Discussion
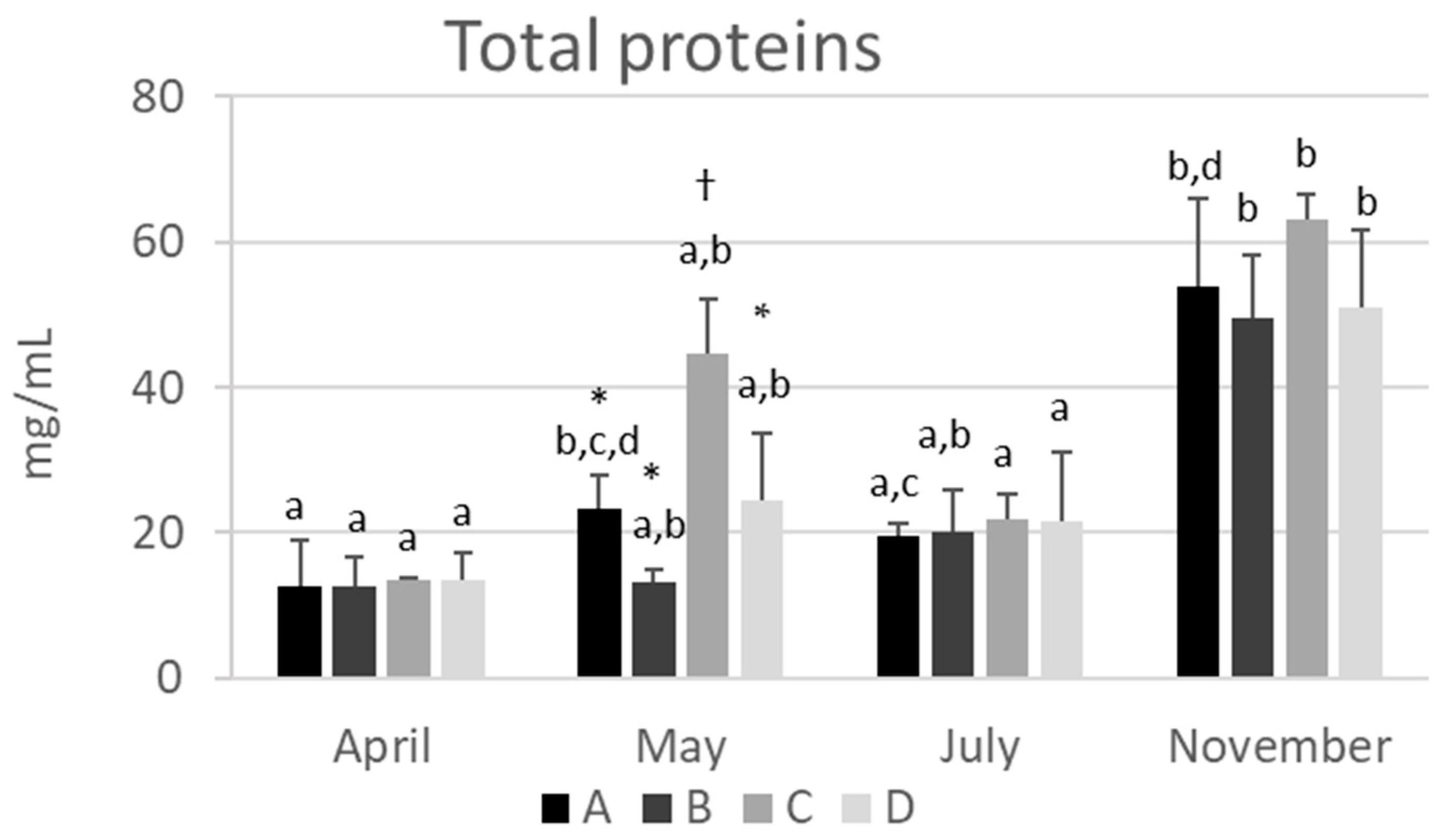
2.1. The Total Protein Concentration in Hemolymph Varies Depending on the Season
2.2. Identification of the Most Abundant Hemolymph Proteins Using Mass Spectrometry
2.3. Quantification of the Most Abundant Hemolymph Proteins
3. Materials and Methods
3.1. Experimental Design and Hemolymph Sampling
3.2. Total Protein Determination
3.3. Protein Separation Using SDS-PAGE
3.4. Protein Identification by Mass Spectrometry
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ararso, Z.; Ma, C.; Qi, Y.; Feng, M.; Han, B.; Hu, H.; Meng, L.; Li, J. Proteome Comparisons between Hemolymph of Two Honeybee Strains (Apis mellifera ligustica) Reveal Divergent Molecular Basis in Driving Hemolymph Function and High Royal Jelly Secretion. J. Proteome Res. 2018, 17, 402–419. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Ramadan, H.; Han, B.; Fang, Y.; Li, J. Hemolymph proteome changes during worker brood development match the biological divergences between western honey bees (Apis mellifera) and eastern honey bees (Apis cerana). BMC Genom. 2014, 15, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunelle, J.L.; Green, R. One-dimensional SDS-polyacrylamide gel electrophoresis (1D SDS-PAGE). Methods Enzymol. 2014, 541, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ferlizza, E.; Campos, A.; Neagu, A.; Cuoghi, A.; Bellei, E.; Monari, E.; Dondi, F.; Almeida, A.M.; Isani, G. The effect of chronic kidney disease on the urine proteome in the domestic cat (Felis catus). Vet. J. 2015, 204, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Isani, G.; Ferlizza, E.; Nally, J.E. Proteomic Research in Urine and other Fluids BT—Proteomics in Domestic Animals: From Farm to Systems Biology; de Almeida, A.M., Eckersall, D., Miller, I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 121–147. ISBN 978-3-319-69682-9. [Google Scholar]
- Erban, T.; Harant, K.; Kamler, M.; Markovic, M.; Titera, D. Detailed proteome mapping of newly emerged honeybee worker hemolymph and comparison with the red-eye pupal stage. Apidologie 2016, 47, 805–817. [Google Scholar] [CrossRef] [Green Version]
- Kurze, C.; Dosselli, R.; Grassl, J.; Le Conte, Y.; Kryger, P.; Baer, B.; Moritz, R.F.A. Differential proteomics reveals novel insights into Nosema-honey bee interactions. Insect Biochem. Mol. Biol. 2016, 79, 42–49. [Google Scholar] [CrossRef]
- Dainat, B.; Dietemann, V.; Imdorf, A.; Charrière, J.-D. A scientific note on the ‘Liebefeld Method’ to estimate honey bee colony strength: Its history, use, and translation. Apidologie 2020, 51, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honey bee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef] [Green Version]
- Cabbri, R.; Ferlizza, E.; Nanetti, A.; Monari, E.; Andreani, G.; Galuppi, R.; Isani, G. Biomarkers of nutritional status in honeybee haemolymph: Effects of different biotechnical approaches for Varroa destructor treatment and wintering phase. Apidologie 2018, 49, 606–618. [Google Scholar] [CrossRef] [Green Version]
- Kunc, M.; Dobeš, P.; Hurychová, J.; Vojtek, L.; Poiani, S.B.; Danihlík, J.; Havlík, J.; Titěra, D.; Hyršl, P. The Year of the Honey Bee (Apis mellifera L.) with Respect to Its Physiology and Immunity: A Search for Biochemical Markers of Longevity. Insects 2019, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Dostálková, S.; Dobeš, P.; Kunc, M.; Hurychová, J.; Škrabišová, M.; Petřivalský, M.; Titěra, D.; Havlík, J.; Hyršl, P.; Danihlík, J. Winter honeybee (Apis mellifera) populations show greater potential to induce immune responses than summer populations after immune stimuli. J. Exp. Biol. 2021, 224, jeb232595. [Google Scholar] [CrossRef]
- Basualdo, M.; Barragán, S.; Vanagas, L.; García, C.; Solana, H.; Rodríguez, E.; Bedascarrasbure, E. Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera: Apidae). J. Econ. Entomol. 2013, 106, 1553–1558. [Google Scholar] [CrossRef]
- Bitondi, M.M.G.; Simões, Z.L.P. The relationship between level of pollen in the diet, vitellogenin and juvenile hormone titres in Africanized Apis mellifera workers. J. Apic. Res. 1996, 35, 27–36. [Google Scholar] [CrossRef]
- Basualdo, M.; Barragán, S.; Antúnez, K. Bee bread increases honeybee haemolymph protein and promote better survival despite of causing higher Nosema ceranae abundance in honeybees. Environ. Microbiol. Rep. 2014, 6, 396–400. [Google Scholar] [CrossRef]
- Van Der Horst, D.J.; Rodenburg, K.W. Lipoprotein assembly and function in an evolutionary perspective. Biomol. Concepts 2010, 1, 165–183. [Google Scholar] [CrossRef]
- Leipart, V.; Halskau, Ø.; Amdam, G. V How Honey Bee Vitellogenin Holds Lipid Cargo: A Role for the C-Terminal. Front. Mol. Biosci. 2022, 9, 865194. [Google Scholar] [CrossRef]
- Amdam, G.V.; Norberg, K.; Hagen, A.; Omholt, S.W. Social exploitation of vitellogenin. Proc. Natl. Acad. Sci. USA 2003, 100, 1799–1802. [Google Scholar] [CrossRef] [Green Version]
- Haydak, M.H. Honey Bee Nutrition. Annu. Rev. Entomol. 1970, 15, 143–156. [Google Scholar] [CrossRef]
- Havukainen, H.; Halskau, Ø.; Skjaerven, L.; Smedal, B.; Amdam, G. V Deconstructing honeybee vitellogenin: Novel 40 kDa fragment assigned to its N terminus. J. Exp. Biol. 2011, 214, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.A.; Boyd, A.; House, M.; Cowin, G.; Baer, B. Multi-modal imaging and analysis in the search for iron-based magnetoreceptors in the honeybee Apis mellifera. R. Soc. Open Sci. 2018, 5, 181163. [Google Scholar] [CrossRef] [Green Version]
- Chan, Q.W.T.; Howes, C.G.; Foster, L.J. Quantitative comparison of caste differences in honeybee hemolymph. Mol. Cell. Proteom. 2006, 5, 2252–2262. [Google Scholar] [CrossRef] [Green Version]
- Burmester, T. Origin and evolution of arthropod hemocyanins and related proteins. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2002, 172, 95–107. [Google Scholar] [CrossRef]
- Martins, J.R.; Nunes, F.M.F.; Cristino, A.S.; Simões, Z.L.P.; Bitondi, M.M.G. The four hexamerin genes in the honey bee: Structure, molecular evolution and function deduced from expression patterns in queens, workers and drones. BMC Mol. Biol. 2010, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Danty, E.; Arnold, G.; Burmester, T.; Huet, J.C.; Huet, D.; Pernollet, J.C.; Masson, C. Identification and developmental profiles of hexamerins in antenna and hemolymph of the honeybee, Apis mellifera. Insect Biochem. Mol. Biol. 1998, 28, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; So, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Zurovcova, M.; Benes, V.; Zurovec, M.; Kucerova, L. Expansion of imaginal disc growth factor gene family in diptera reflects the evolution of novel functions. Insects 2019, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Buttstedt, A.; Moritz, R.F.A.; Erler, S. Origin and function of the major royal jelly proteins of the honeybee (Apis mellifera) as members of the yellow gene family. Biol. Rev. 2014, 89, 255–269. [Google Scholar] [CrossRef]
- Pelosi, P.; Zhou, J.J.; Ban, L.P.; Calvello, M. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. 2006, 63, 1658–1676. [Google Scholar] [CrossRef]
- Sun, J.S.; Xiao, S.; Carlson, J.R. The diverse small proteins called odorant-binding proteins. Open Biol. 2018, 8, 180208. [Google Scholar] [CrossRef] [Green Version]
- Amdam, G.V.; Aase, A.L.T.O.; Seehuus, S.-C.; Kim Fondrk, M.; Norberg, K.; Hartfelder, K. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 2005, 40, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Cremonz, T.M.; De Jong, D.; Bitondi, M.M.G. Quantification of Hemolymph Proteins as a Fast Method for Testing Protein Diets for Honey Bees (Hymenoptera: Apidae). J. Econ. Entomol. 1998, 91, 1284–1289. [Google Scholar] [CrossRef]
- Amdam, G.V.; Simões, Z.L.P.; Hagen, A.; Norberg, K.; Schrøder, K.; Mikkelsen, Ø.; Kirkwood, T.B.L.; Omholt, S.W. Hormonal control of the yolk precursor vitellogenin regulates immune function and longevity in honeybees. Exp. Gerontol. 2004, 39, 767–773. [Google Scholar] [CrossRef]
- Pinto, L.Z.; Bitondi, M.M.G.; Simões, Z.L.P. Inhibition of vitellogenin synthesis in Apis mellifera workers by a juvenile hormone analogue, pyriproxyfen. J. Insect Physiol. 2000, 46, 153–160. [Google Scholar] [CrossRef]
- van der Steen, J.J.M.; Martel, A.-C.; Hendrickx, P. The fraction haemolymph vitellogenin of a honey bee colony, derived from a pooled haemolymph sample, a colony vitality parameter. J. Apic. Res. 2015, 54, 55–58. [Google Scholar] [CrossRef]
- Aurori, C.M.; Buttstedt, A.; Dezmirean, D.S.; Mărghitaş, L.A.; Moritz, R.F.A.; Erler, S. What is the main driver of ageing in long-lived winter honeybees: Antioxidant enzymes, innate immunity, or vitellogenin? J. Gerontol. A. Biol. Sci. Med. Sci. 2014, 69, 633–639. [Google Scholar] [CrossRef] [Green Version]
- Robbs, S.L.; Ryan, R.O.; Schmidt, J.O.; Keim, P.S.; Law, J.H. Lipophorin of the larval honeybee, Apis mellifera L. J. Lipid Res. 1985, 26, 241–247. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [Green Version]
- Münch, D.; Kreibich, C.D.; Amdam, G. V Aging and its modulation in a long-lived worker caste of the honey bee. J. Exp. Biol. 2013, 216, 1638–1649. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Heerman, M.C.; Cook, S.C.; Evans, J.D.; DeGrandi-Hoffman, G.; Banmeke, O.; Zhang, Y.; Huang, S.; Hamilton, M.; Chen, Y.P. Transferrin-mediated iron sequestration suggests a novel therapeutic strategy for controlling Nosema disease in the honey bee, Apis mellifera. PLoS Pathog. 2021, 17, e1009270. [Google Scholar] [CrossRef]
- Geiser, D.L.; Winzerling, J.J. Insect transferrins: Multifunctional proteins. Biochim. Biophys. Acta—Gen. Subj. 2012, 1820, 437–451. [Google Scholar] [CrossRef]
- Iatsenko, I.; Marra, A.; Boquete, J.-P.; Peña, J.; Lemaitre, B. Iron sequestration by transferrin 1 mediates nutritional immunity in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2020, 117, 7317–7325. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smart, M.D.; Otto, C.R.V.; Lundgren, J.G. Nutritional status of honey bee (Apis mellifera L.) workers across an agricultural land-use gradient. Sci. Rep. 2019, 9, 16252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlizza, E.; Isani, G.; Dondi, F.; Andreani, G.; Vasylyeva, K.; Bellei, E.; Almeida, A.M.; Matzapetakis, M. Urinary proteome and metabolome in dogs (Canis lupus familiaris): The effect of chronic kidney disease. J. Proteom. 2020, 222, 103795. [Google Scholar] [CrossRef] [PubMed]
- Andreani, G.; Ferlizza, E.; Cabbri, R.; Fabbri, M.; Bellei, E.; Isani, G. Essential (Mg, Fe, Zn and Cu) and Non-Essential (Cd and Pb) Elements in Predatory Insects (Vespa crabro and Vespa velutina): A Molecular Perspective. Int. J. Mol. Sci. 2021, 22, 228. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Brown, M.B.; Forsythe, A.B. Robust Tests for the Equality of Variances. J. Am. Stat. Assoc. 1974, 69, 364–367. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Friedman, M. A Comparison of Alternative Tests of Significance for the Problem of m Rankings. Ann. Math. Stat. 1940, 11, 86–92. [Google Scholar] [CrossRef]
- Nemenyi, P. Distribution-Free Multiple Comparisons. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 1963. [Google Scholar]
- Mauchly, J.W. Significance Test for Sphericity of a Normal n-Variate Distribution. Ann. Math. Stat. 1940, 11, 204–209. [Google Scholar] [CrossRef]



| Band 1 | Entry Name 2 | Protein Full Name | MM (Da) 3 | Score 4 | Sign. Pept 5 | Sign. Seq 6 | emPAI 7 | Organism |
|---|---|---|---|---|---|---|---|---|
| 1 | A0A088AS56 | Apolipophorin (I) | 369,557 | 10,642 | 579 | 116 | 3.07 | Apis cerana |
| 2 | A0A088ADL8 | Vitellogenin | 202,025 | 3244 | 232 | 54 | 2.38 | Apis mellifera |
| 3 | A0A088ADL8 | Vitellogenin | 202,025 | 34 | 7 | 3 | 0.05 | Apis mellifera |
| 4 | A0A088AS56 | Apolipophorin (fragment) (II) | 369,557 | 3848 | 224 | 35 | 2.59 | Apis cerana |
| A0A088AQB0 | Leucin-rich repeat-containing protein | 76,627 | 1001 | 59 | 13 | 0.96 | Apis cerana | |
| 5 | A0A088AFH7 | Transferrin | 80,005 | 1790 | 103 | 25 | 2.92 | |
| 6 | A5YVK7 | Hexamerin 70a | 81,522 | 1429 | 101 | 19 | 2.14 | Apis mellifera |
| 7 | Q86MV4 | Prophenoloxidase | 80,443 | 2644 | 149 | 20 | 2.45 | Apis mellifera |
| A0A088AS36 | Uncharacterized protein | 49,054 | 94 | 7 | 3 | 0.22 | Apis mellifera | |
| 8 | A0A088AMK2 | Chitinase-like protein Idgf4 | 50,442 | 2708 | 149 | 16 | 4.19 | Apis cerana |
| MRJP1_APIME | Major royal jelly protein 1 | 49,311 | 511 | 16 | 14 | 1.48 | Apis mellifera | |
| 9 | B0LUE8 | Apoliphorin-III like protein | 21,335 | 1723 | 44 | 5 | 3.32 | Apis mellifera |
| 10 | Q1W640 | OBP14 | 15,216 | 1000 | 54 | 6 | 8.19 | Apis mellifera |
| 11 | Q1W641 | OBP13 | 15,494 | 77 | 10 | 5 | 1.69 | Apis mellifera |
| Q1W633 | OBP21 | 15,536 | 112 | 4 | 2 | 0.48 | Apis mellifera |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isani, G.; Bellei, E.; Rudelli, C.; Cabbri, R.; Ferlizza, E.; Andreani, G. SDS-PAGE-Based Quantitative Assay of Hemolymph Proteins in Honeybees: Progress and Prospects for Field Application. Int. J. Mol. Sci. 2023, 24, 10216. https://doi.org/10.3390/ijms241210216
Isani G, Bellei E, Rudelli C, Cabbri R, Ferlizza E, Andreani G. SDS-PAGE-Based Quantitative Assay of Hemolymph Proteins in Honeybees: Progress and Prospects for Field Application. International Journal of Molecular Sciences. 2023; 24(12):10216. https://doi.org/10.3390/ijms241210216
Chicago/Turabian StyleIsani, Gloria, Elisa Bellei, Cecilia Rudelli, Riccardo Cabbri, Enea Ferlizza, and Giulia Andreani. 2023. "SDS-PAGE-Based Quantitative Assay of Hemolymph Proteins in Honeybees: Progress and Prospects for Field Application" International Journal of Molecular Sciences 24, no. 12: 10216. https://doi.org/10.3390/ijms241210216
APA StyleIsani, G., Bellei, E., Rudelli, C., Cabbri, R., Ferlizza, E., & Andreani, G. (2023). SDS-PAGE-Based Quantitative Assay of Hemolymph Proteins in Honeybees: Progress and Prospects for Field Application. International Journal of Molecular Sciences, 24(12), 10216. https://doi.org/10.3390/ijms241210216





