Strontium Attenuates Hippocampal Damage via Suppressing Neuroinflammation in High-Fat Diet-Induced NAFLD Mice
Abstract
:1. Introduction
2. Results
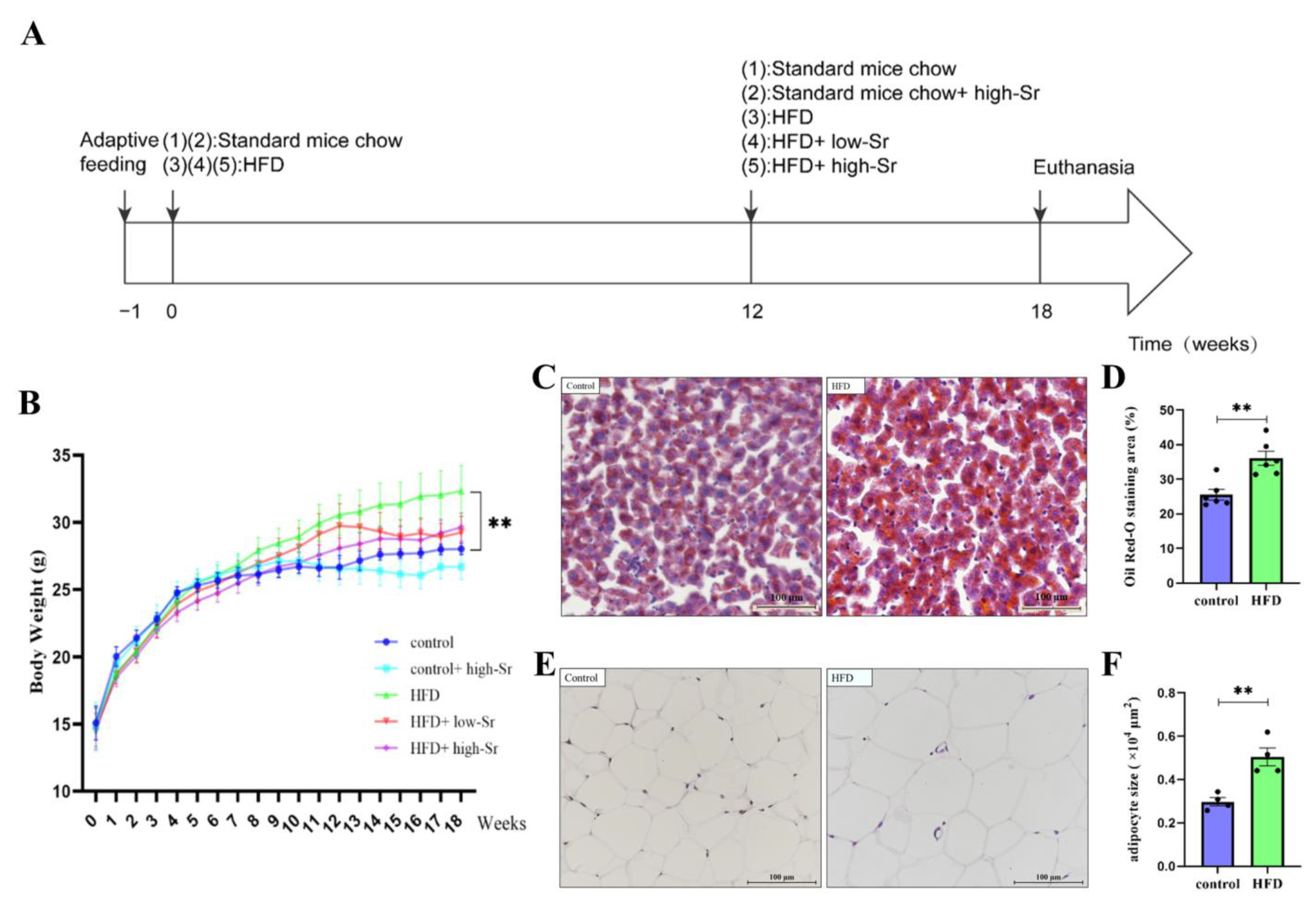
2.1. The NAFLD Mouse Model Induced by an HFD Was Successfully Established
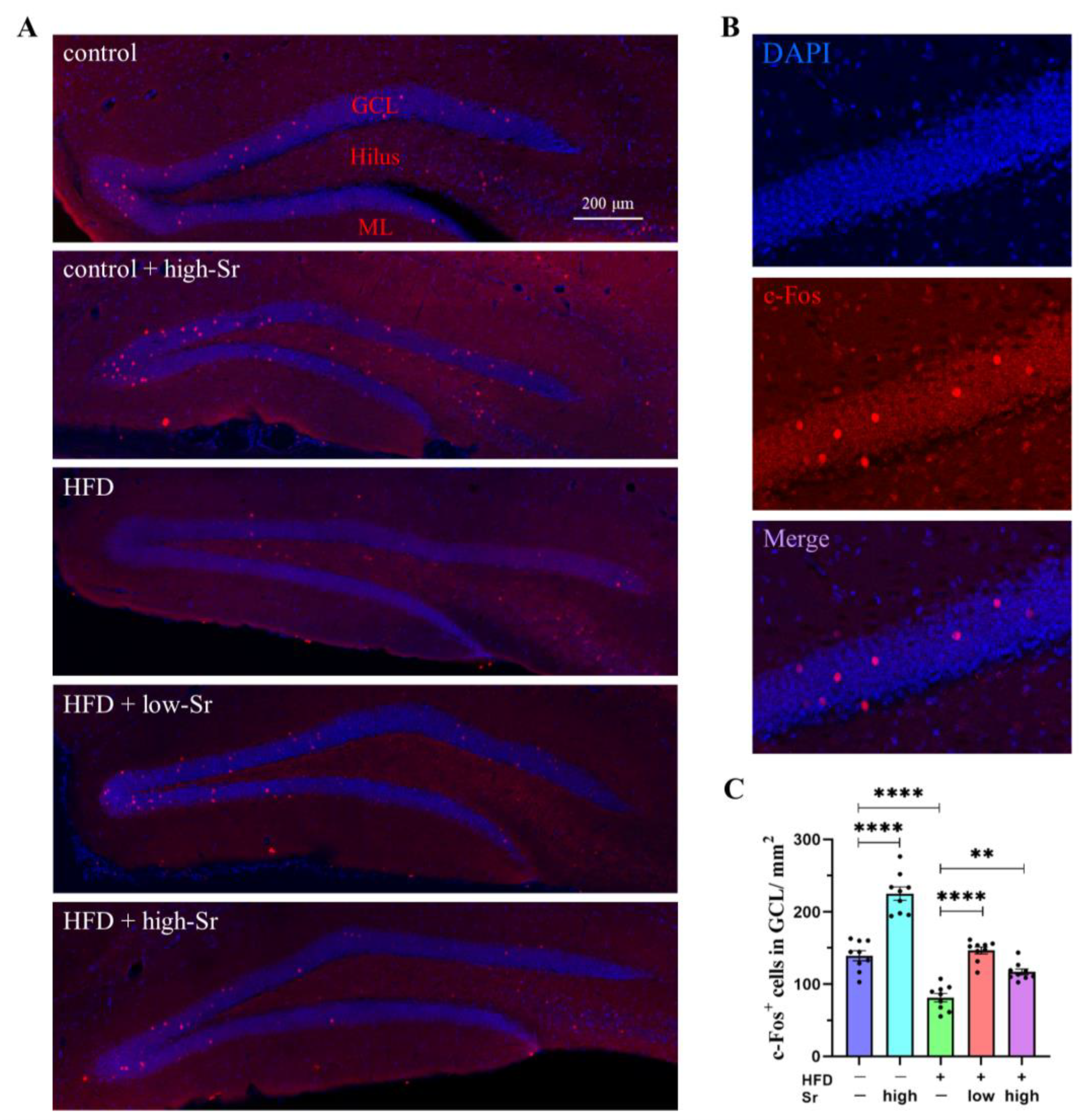
2.2. Sr Increased the Expression of c-Fos in HFD-Fed Mice
2.3. Sr Suppressed HFD-Induced Apoptosis by Inhibiting ERS
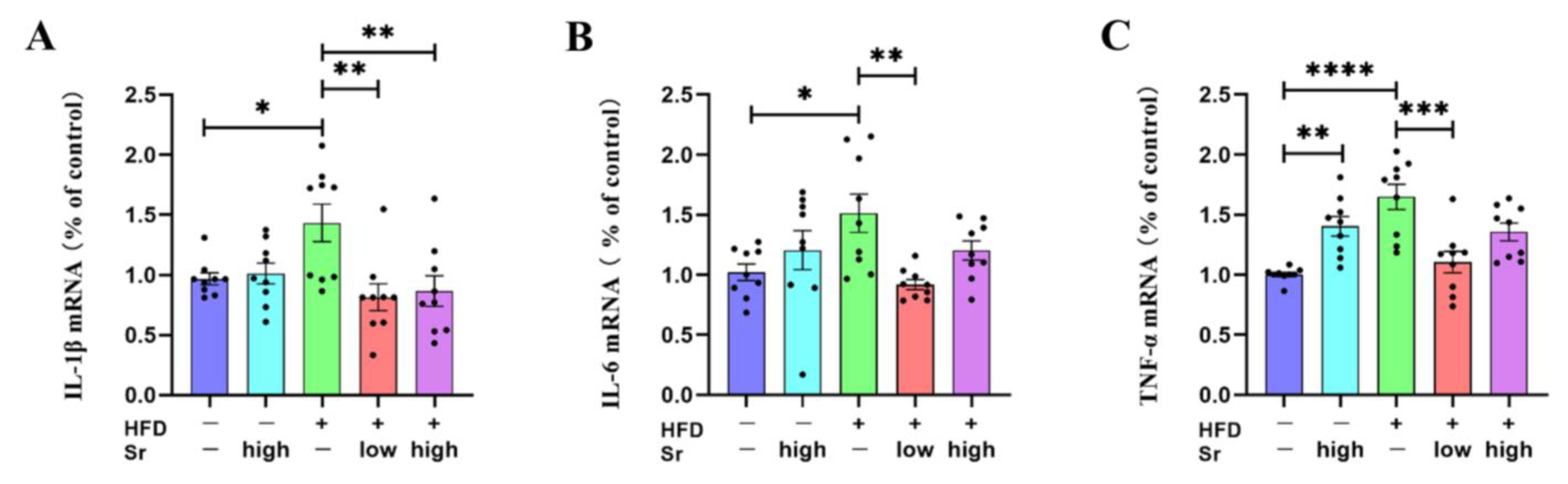
2.4. Sr Inhibited the Production of Inflammatory Cytokines in HFD-Fed Mice
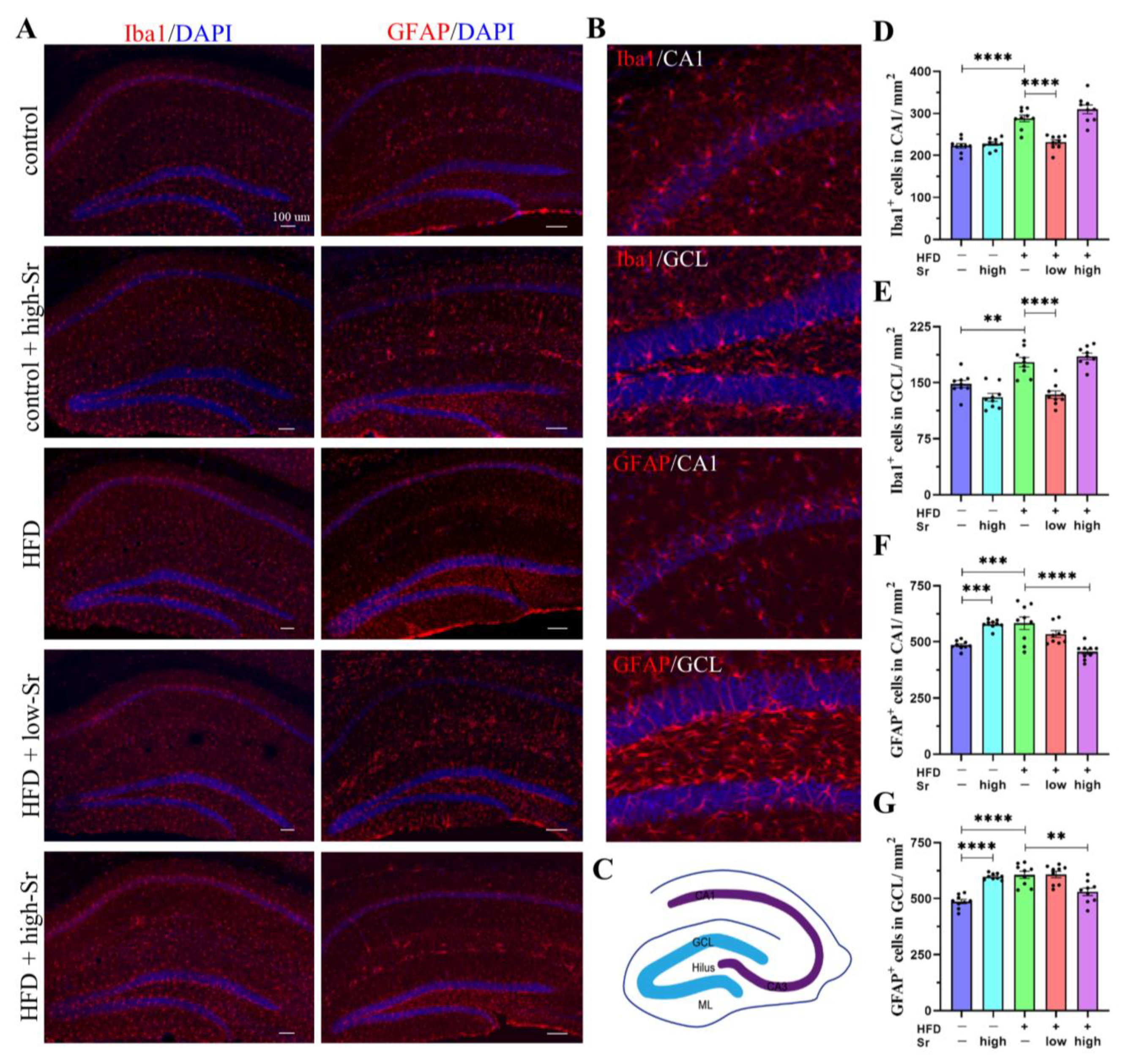
2.5. Sr Suppressed the Activation of Microglia and Astrocytes in the Hippocampus Induced by an HFD
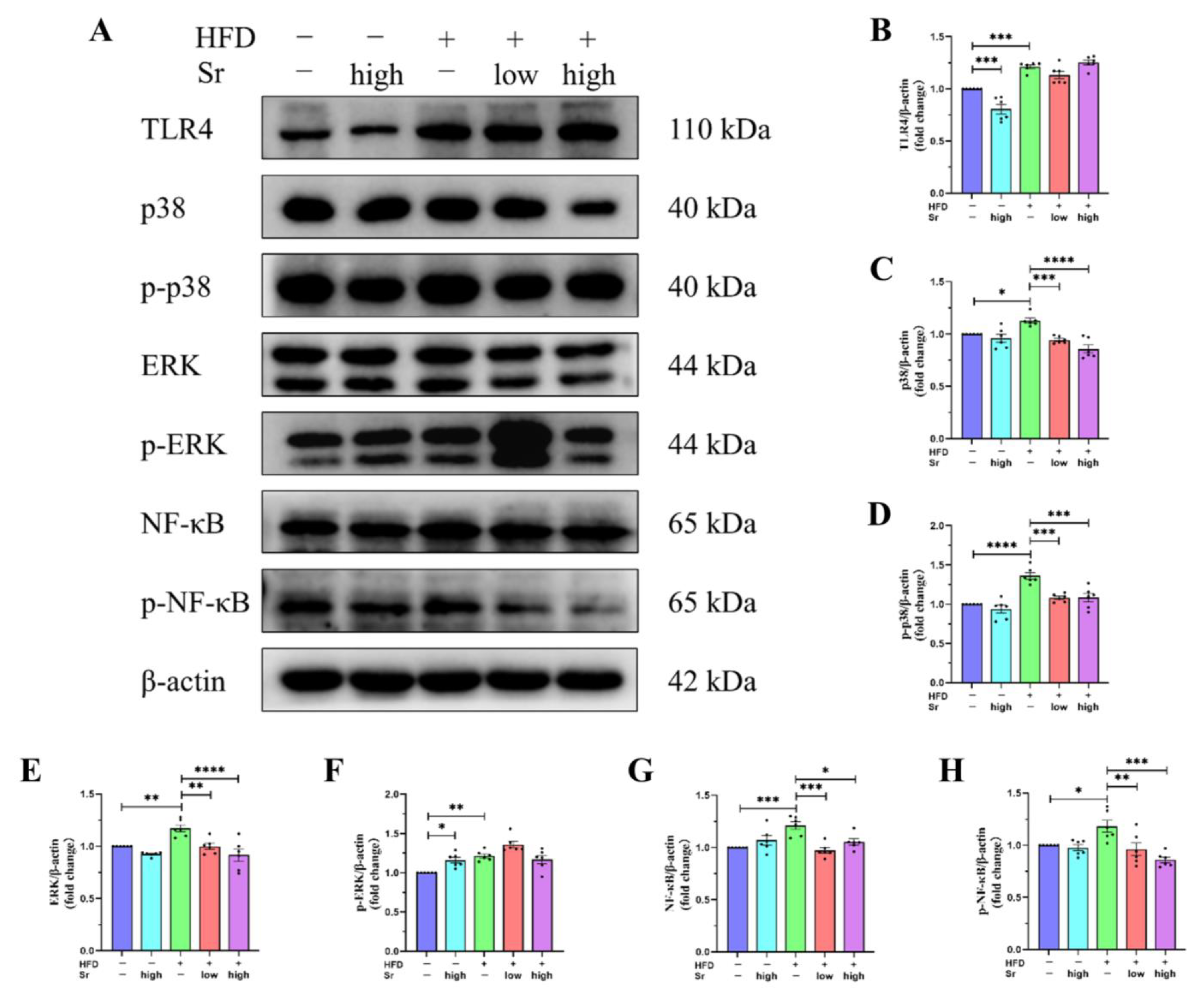
2.6. Sr Inhibited the Activation of the TLR4/p38 MAPK/ERK and NF-κB Pathways Induced by an HFD
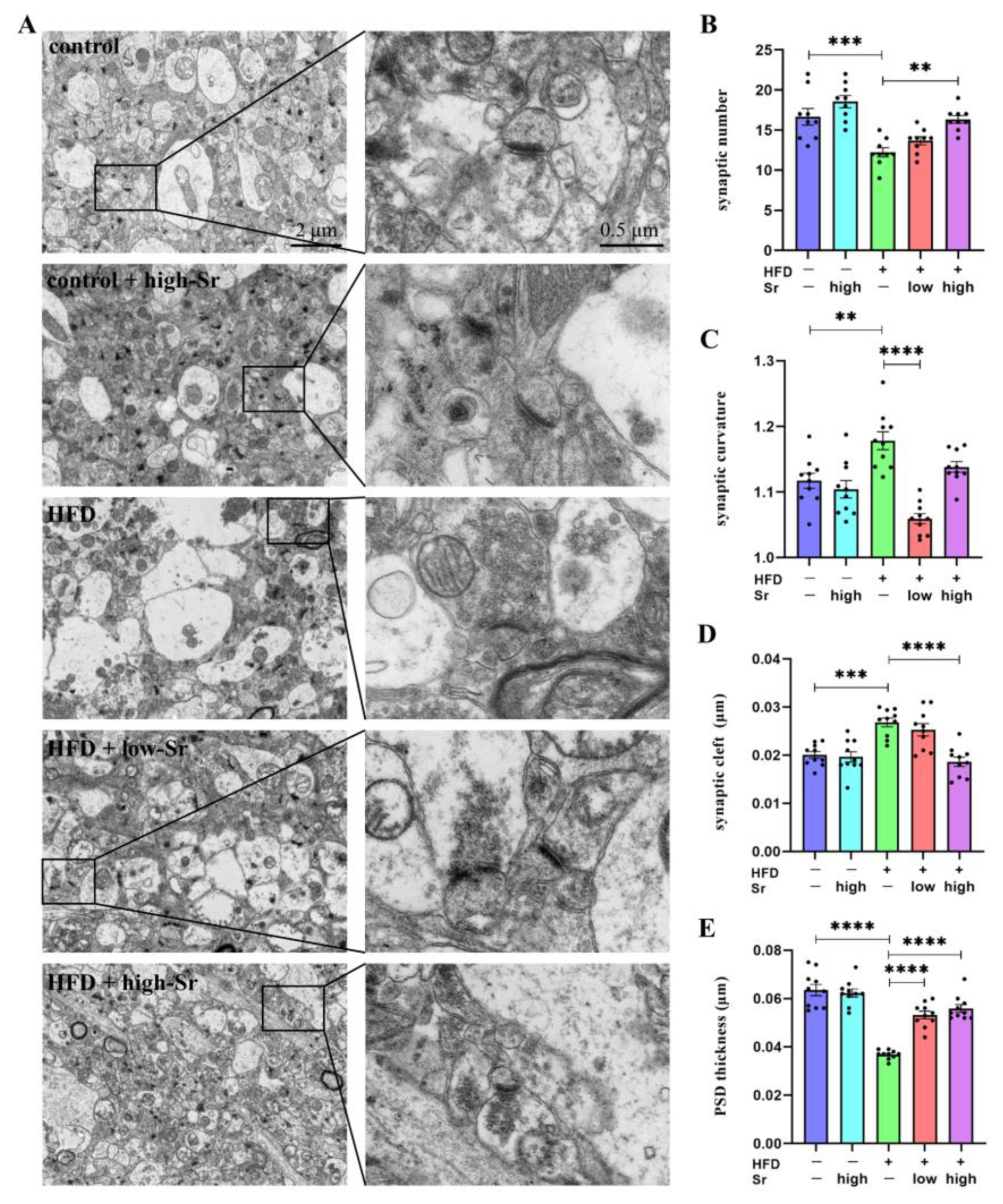
2.7. Sr Suppressed the Alterations in Many Aspects Related to Hippocampal Synaptic Plasticity Induced by an HFD
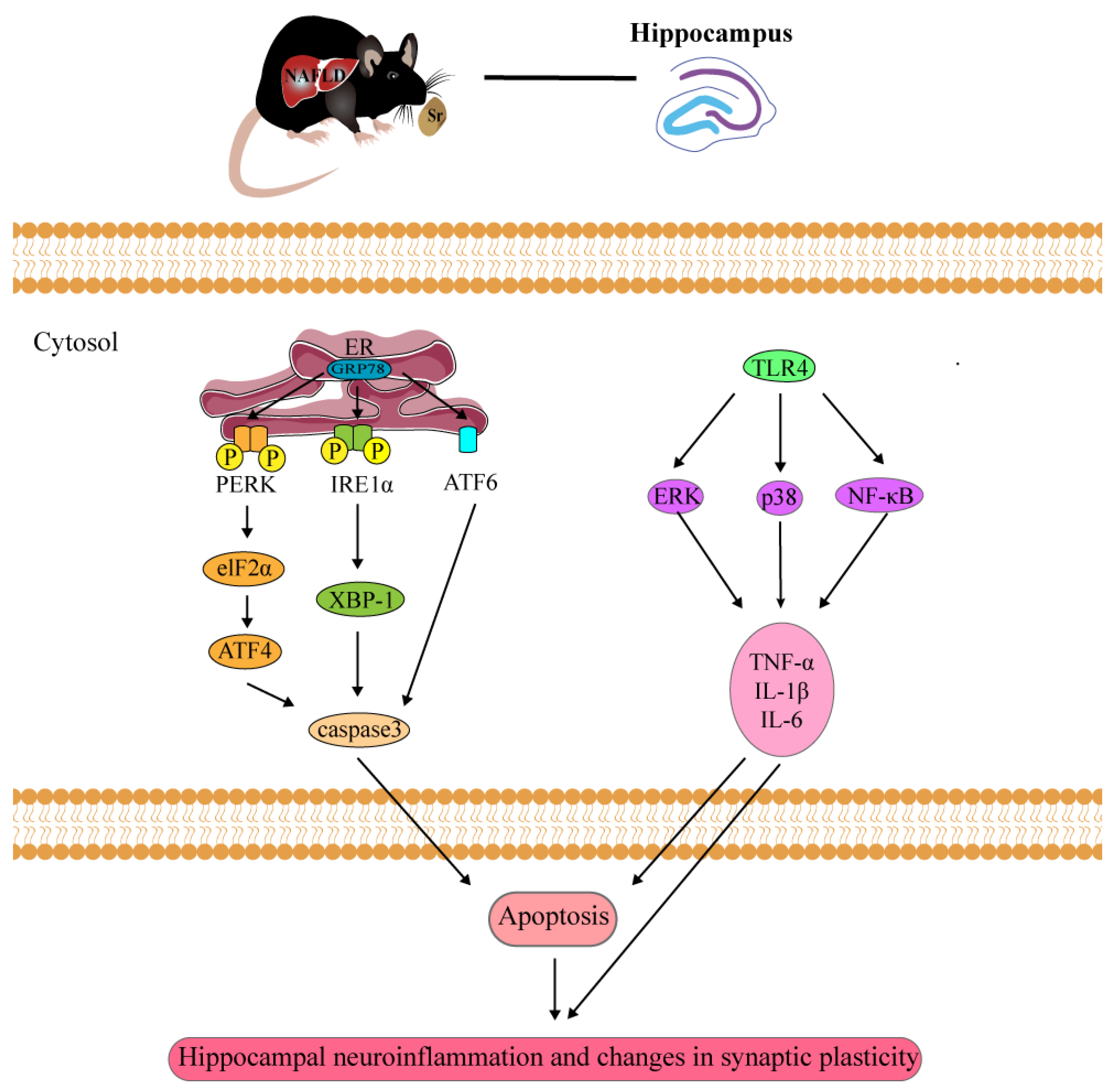
3. Discussion
4. Materials and Methods
4.1. Mice, Diets, and Treatments
4.2. Histology
4.3. Western Blot
4.4. Quantitative RT-PCR
4.5. Immunofluorescence Staining
4.6. Transmission Electron Microscopy (TEM)
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colognesi, M.; Gabbia, D.; Martin, S.D. Depression and Cognitive Impairment—Extrahepatic Manifestations of NAFLD and NASH. Biomedicines 2020, 8, 229. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ye, J.; Sun, Y.; Feng, S.; Chen, Y.; Zhong, B. The Additive Values of the Classification of Higher Serum Uric Acid Levels as a Diagnostic Criteria for Metabolic-Associated Fatty Liver Disease. Nutrients 2022, 14, 3587. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 2020, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, D.; Galizzi, G.; Amato, A.; Terzo, S.; Picone, P.; Cristaldi, L.; Mulè, F.; Di Carlo, M. Regular Intake of Pistachio Mitigates the Deleterious Effects of a High Fat-Diet in the Brain of Obese Mice. Antioxidants 2020, 9, 317. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.; Chen, Y.; Yu, H.; Huang, L.; Tain, Y.; Lin, I.; Sheen, J.; Wang, P.; Tiao, M. Long term N-acetylcysteine administration rescues liver steatosis via endoplasmic reticulum stress with unfolded protein response in mice. Lipids Health Dis. 2020, 19, 105. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E. Effect of High-Fat Diets on Oxidative Stress, Cellular Inflammatory Response and Cognitive Function. Nutrients 2019, 11, 2579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Pemmer, B.; Hofstaetter, J.G.; Meirer, F.; Smolek, S.; Wobrauschek, P.; Simon, R.; Fuchs, R.K.; Allen, M.R.; Condon, K.W.; Reinwald, S.; et al. Increased strontium uptake in trabecular bone of ovariectomized calcium-deficient rats treated with strontium ranelate or strontium chloride. J. Synchrot. Radiat. 2011, 18 Pt 6, 835–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 12, 100273. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Hu, X.; Tao, Y.; Ping, Z.; Wang, L.; Shi, J.; Wu, X.; Zhang, W.; Yang, H.; Nie, Z.; et al. Strontium inhibits titanium particle-induced osteoclast activation and chronic inflammation via suppression of NF-κB pathway. Sci. Rep. 2016, 6, 36251. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Yang, X.; He, J.; Zhong, Q.; Guo, X. The anti-inflammation effect of strontium ranelate on rat chondrocytes with or without IL-1β in vitro. Exp. Ther. Med. 2022, 23, 208. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Q.; Dai, D.; Ying, H.; Wang, Q.; Dai, Y. Effects of strontium fructose 1,6-diphosphate on expression of apoptosis-related genes and oxidative stress in testes of diabetic rats. Int. J. Urol. 2008, 15, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.; Gunaratnam, K.; Tong, J.; Duque, G. Biochemical changes induced by strontium ranelate in differentiating adipocytes. Biochimie 2013, 95, 793–798. [Google Scholar] [CrossRef]
- Gladding, J.M.; Abbott, K.N.; Antoniadis, C.P.; Stuart, A.; Begg, D.P. The Effect of Intrahippocampal Insulin Infusion on Spatial Cognitive Function and Markers of Neuroinflammation in Diet-induced Obesity. Front. Endocrinol. 2018, 9, 752. [Google Scholar] [CrossRef] [Green Version]
- Winocur, G.; Greenwood, C.E. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol. Aging 2005, 26 (Suppl. S1), 46–49. [Google Scholar] [CrossRef]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullins, C.A.; Gannaban, R.B.; Khan, M.S.; Shah, H.; Siddik, M.A.B.; Hegde, V.K.; Reddy, P.H.; Shin, A.C. Neural Underpinnings of Obesity: The Role of Oxidative Stress and Inflammation in the Brain. Antioxidants 2020, 9, 1018. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shen, Y.; Pan, T.; Zhu, T.; Li, X.; Xu, F.; Betancor, M.B.; Jiao, L.; Tocher, D.R.; Zhou, Q. Dietary Betaine Mitigates Hepatic Steatosis and Inflammation Induced by a High-Fat-Diet by Modulating the Sirt1/Srebp-1/Pparα Pathway in Juvenile Black Seabream (Acanthopagrus schlegelii). Front. Immunol. 2021, 12, 694720. [Google Scholar] [CrossRef]
- Buchs, M.; Dreifuss, J.J.; Grau, J.D.; Nordmann, J.J. Strontium as a substitute for calcium in the process leading to neurohypophysial hormone secretion. J. Physiol. 1972, 222, 168–169. [Google Scholar]
- Miledi, R. Strontium as a Substitute for Calcium in the Process of Transmitter Release at the Neuromuscular Junction. Nature 1966, 212, 1233–1234. [Google Scholar] [CrossRef]
- Moser, S.; van der Eerden, B. Osteocalcin-A Versatile Bone-Derived Hormone. Front. Endocrinol. 2019, 9, 794. [Google Scholar] [CrossRef] [Green Version]
- Kesarwani, M.; Kincaid, Z.; Gomaa, A.; Huber, E.; Rohrabaugh, S.; Siddiqui, Z.; Bouso, M.F.; Latif, T.; Xu, M.; Komurov, K.; et al. Targeting c-FOS and DUSP1 abrogates intrinsic resistance to tyrosine-kinase inhibitor therapy in BCR-ABL-induced leukemia. Nat. Med. 2017, 23, 472–482. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, T.; Yamada, K.; Iwasaki, K.; Tsujimoto, T.; Higashi, H.; Kimura, T.; Iwasaki, N.; Sudo, H. Caspase-3 knockout inhibits intervertebral disc degeneration related to injury but accelerates degeneration related to aging. Sci. Rep. 2019, 9, 19324. [Google Scholar] [CrossRef] [Green Version]
- Marc Francaux, C.P.; Philp, A.; Raymackers, J.M.; Meakin, P.J.; Ashford, M.L.; Delzenne, N.M.; Francaux, M.; Baar, K. The unfolded protein response is activated in skeletal muscle by high-fat feeding: Potential role in the downregulation of protein synthesis. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E695–E705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Kaufman, R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature 2008, 454, 455–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.H.; Cai, M.; Deng, J.; Yu, P.; Liang, H.; Yang, F. Anticancer Function and ROS-Mediated Multi-Targeting Anticancer Mechanisms of Copper (II) 2-hydroxy-1-naphthaldehyde Complexes. Molecules 2019, 24, 2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.; Xu, W.; Reed, J.C. Cell death and endoplasmic reticulum stress: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008, 7, 1013–1030. [Google Scholar] [CrossRef]
- Liao, K.; Guo, M.; Niu, F.; Yang, L.; Callen, S.E.; Buch, S. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J. Neuroinflamm. 2016, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Henkel, A.S.; Lecuyer, B.; Olivares, S.; Green, R.M. Endoplasmic Reticulum Stress Regulates Hepatic Bile Acid Metabolism in Mice. Cell. Mol. Gastroenterol. Hepatol. 2016, 3, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.; Lin, C.; Lu, C.; Martel, J.; Ko, Y.; Ojcius, D.M.; Tseng, S.; Wu, T.; Chen, Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Tu, J.; Li, X.; Hua, Q.; Liu, W.; Liu, Y.; Pan, B.; Hu, P.; Zhang, W. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef]
- Conti, P.; Lauritano, D.; Caraffa, A.; Gallenga, C.E.; Kritas, S.K.; Ronconi, G.; Martinotti, S. Microglia and mast cells generate proinflammatory cytokines in the brain and worsen inflammatory state: Suppressor effect of IL-37. Eur. J. Pharmacol. 2020, 875, 173035. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, D.; Wang, Y.; Niu, L.; Jiang, H. Short-Chain Fatty Acids Reduce Oligodendrocyte Precursor Cells Loss by Inhibiting the Activation of Astrocytes via the SGK1/IL-6 Signalling Pathway. Neurochem. Res. 2022, 47, 3476–3489. [Google Scholar] [CrossRef]
- Vodret, S.; Bortolussi, G.; Jašprová, J.; Vitek, L.; Muro, A.F. Inflammatory signature of cerebellar neurodegeneration during neonatal hyperbilirubinemia in Ugt1 -/- mouse model. J. Neuroinflamm. 2017, 14, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, Y.; Ohsawa, K.; Kanazawa, H.; Kohsaka, S.; Imai, Y. Iba1 Is an Actin-Cross-Linking Protein in Macrophages/Microglia. Biochem. Biophys. Res. Commun. 2001, 286, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara-Espinosa, J.V.; Santana-Martínez, R.A.; Maldonado, P.D.; Zetter, M.; Becerril-Villanueva, E.; Pérez-Sánchez, G.; Pavón, L.; Mata-Espinosa, D.; Barrios-Payán, J.; López-Torres, M.O.; et al. Experimental Pulmonary Tuberculosis in the Absence of Detectable Brain Infection Induces Neuroinflammation and Behavioural Abnormalities in Male BALB/c Mice. Int. J. Mol. Sci. 2020, 21, 9483. [Google Scholar] [CrossRef]
- Shi, H.; Ge, X.; Ma, X.; Zheng, M.; Cui, X.; Pan, W.; Zheng, P.; Yang, X.; Zhang, P.; Hu, M.; et al. A fiber-deprived diet causes cognitive impairment and hippocampal microglia-mediated synaptic loss through the gut microbiota and metabolites. Microbiome 2021, 9, 223. [Google Scholar] [CrossRef]
- Kim, D.; Krenz, A.; Toussaint, L.E.; Maurer, K.J.; Robinson, S.; Yan, A.; Torres, L.; Bynoe, M.S. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J. Neuroinflamm. 2016, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhigang, L.; Patil, I.Y.; Tianyi, J.; Harsh, S.; Walsh, J.P.; Stiles, B.L.; Fei, Y.; Enrique, C.; Luque, R.M. High-Fat Diet Induces Hepatic Insulin Resistance and Impairment of Synaptic Plasticity. PLoS ONE 2015, 10, e0128274. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qiao, Q.; Zhao, T.; Zhang, W.; Ren, B.; Liu, Q.; Liu, X. Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system. Food Funct. 2017, 8, 710–719. [Google Scholar] [CrossRef]
- Estrada, L.D.; Ahumada, P.; Cabrera, D.; Arab, J.P. Liver Dysfunction as a Novel Player in Alzheimer’s Progression: Looking Outside the Brain. Front. Aging Neurosci. 2019, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.C.; Chung, C.Y.; Chiu, C.H.; Lin, H.C.; Yang, J.T. The Effect of Polybutylcyanoacrylate Nanoparticles as a Protos Delivery Vehicle on Dental Bone Formation. Int. J. Mol. Sci. 2021, 22, 4873. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, A.H.; Torres, A.L.; Vasconcelos, D.P.; Ribeiro-Machado, C.; Barbosa, J.N.; Barbosa, M.A.; Barrias, C.C.; Ribeiro, C.C. Osteogenic, anti-osteoclastogenic and immunomodulatory properties of a strontium-releasing hybrid scaffold for bone repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1289–1303. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C 2017, 78, 1222–1230. [Google Scholar] [CrossRef]
- Sun, W.; Hua, S.; Li, X.; Shen, L.; Wu, H.; Ji, H. Microbially produced vitamin B12 contributes to the lipid-lowering effect of silymarin. Nat. Commun. 2023, 14, 477. [Google Scholar] [CrossRef]
- Xu, Z.; Fan, J.; Ding, X.; Qiao, L.; Wang, G. Characterization of high-fat, diet-induced, non-alcoholic steatohepatitis with fibrosis in rats. Dig. Dis. Sci. 2010, 55, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhao, J.; Gui, W.; Sun, D.; Dai, H.; Xiao, L.; Chu, H.; Du, F.; Zhu, Q.; Schnabl, B.; et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br. J. Pharmacol. 2018, 175, 469–484. [Google Scholar] [CrossRef] [Green Version]
- Dragunow, M.; Robertson, H.A. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature 1987, 329, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Knauf, C.; Cani, P.D.; Kim, D.H.; Iglesias, M.A.; Chabo, C.; Waget, A.; Colom, A.; Rastrelli, S.; Delzenne, N.M.; Drucker, D.J. Role of Central Nervous System Glucagon-Like Peptide-1 Receptors in Enteric Glucose Sensing. Diabetes 2008, 57, 2603–2612. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Ton, H.; Zhao, R.; Geron, E.; Li, M.; Dong, Y.; Zhang, Y.; Yu, B.; Yang, G.; Xie, Z. Sevoflurane induces neuronal activation and behavioral hyperactivity in young mice. Sci. Rep. 2020, 10, 11226. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, N.; Xu, Y.; Ti, Y.; Chen, J.; Chen, J.; Zhang, J.; Zhao, J. Endoplasmic reticulum stress-mediated inflammatory signaling pathways within the osteolytic periosteum and interface membrane in particle-induced osteolysis. Cell Tissue Res. 2015, 363, 427–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, S.; Buel, G.R.; Wolgamott, L.; Plas, D.R.; Asara, J.M.; Blenis, J.; Yoon, S. ERK2 Mediates Metabolic Stress Response to Regulate Cell Fate. Mol. Cell. 2015, 59, 382–398. [Google Scholar] [CrossRef] [Green Version]
- Salvadó, L.; Xavier, P.; Barroso, E.; Vazquez-Carrera, M. Targeting endoplasmic reticulum stress in insulin resistance. Trends Endocrinol. Metab. 2015, 26, 438–448. [Google Scholar] [CrossRef]
- Sprenkle, N.T.; Sims, S.G.; Sánchez, C.L.; Meares, G.P. Endoplasmic reticulum stress and inflammation in the central nervous system. Mol. Neurodegener. 2017, 12, 42. [Google Scholar] [CrossRef] [Green Version]
- Rashid, K.; Sil, P.C. Curcumin ameliorates testicular damage in diabetic rats by suppressing cellular stress-mediated mitochondria and endoplasmic reticulum-dependent apoptotic death. Biochim. Biophys. Acta 2015, 1852, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Menzie-Suderam, J.M.; Modi, J.; Xu, H.; Bent, A.; Trujillo, P.; Medley, K.; Jimenez, E.; Shen, J.; Marshall, M.; Tao, R.; et al. Granulocyte-colony stimulating factor gene therapy as a novel therapeutics for stroke in a mouse model. J. Biomed. Sci. 2020, 27, 99. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Minamino, T.; Komuro, I.; Kitakaze, M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 2010, 107, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Hall, G.; Lane, B.M.; Khan, K.; Pediaditakis, I.; Xiao, J.; Wu, G.; Wang, L.; Kovalik, M.E.; Chryst-Stangl, M.; Davis, E.E.; et al. The Human FSGS-Causing ANLN R431C Mutation Induces Dysregulated PI3K/AKT/mTOR/Rac1 Signaling in Podocytes. J. Am. Soc. Nephrol. 2018, 29, 2110–2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Ma, R.; Zhang, G.; Wang, S.; Yin, J.; Wang, E.; Xiong, E.; Zhang, Q.; Li, Y. Estrogen and propofol combination therapy inhibits endoplasmic reticulum stress and remarkably attenuates cerebral ischemia-reperfusion injury and OGD injury in hippocampus. Biomed. Pharmacother. 2018, 108, 1596–1606. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Choi, S.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Visfatin Induces Inflammation and Insulin Resistance via the NF-κB and STAT3 Signaling Pathways in Hepatocytes. J. Diabetes Res. 2019, 2019, 4021623. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Y.; Liu, X.; Li, S.; Cheng, C.; Chen, S.; Le, W. Dynamic changes of CX3CL1/CX3CR1 axis during microglial activation and motor neuron loss in the spinal cord of ALS mouse model. Transl. Neurodegener. 2018, 7, 35. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Brown, M.A. Innate immunity in the central nervous system. J. Clin. Investig. 2012, 122, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Borah, A. Global loss of acetylcholinesterase activity with mitochondrial complexes inhibition and inflammation in brain of hypercholesterolemic mice. Sci. Rep. 2017, 7, 17922. [Google Scholar] [CrossRef] [Green Version]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Halleskog, C.; Dijksterhuis, J.P.; Kilander, M.B.C.; Becerril-Ortega, J.; Villaescusa, J.C.; Lindgren, E.; Arenas, E.; Schulte, G. Heterotrimeric G protein-dependent WNT-5A signaling to ERK1/2 mediates distinct aspects of microglia proinflammatory transformation. J. Neuroinflamm. 2012, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Gan, P.; Ding, L.; Hang, G.; Xia, Q.; Huang, Z.; Ye, X.; Qian, X. Oxymatrine Attenuates Dopaminergic Neuronal Damage and Microglia-Mediated Neuroinflammation Through Cathepsin D-Dependent HMGB1/TLR4/NF-κB Pathway in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 776. [Google Scholar] [CrossRef]
- Liu, L.; Dong, Y.; Shan, X.; Li, L.; Xia, B.; Wang, H. Anti-Depressive Effectiveness of Baicalin In Vitro and In Vivo. Molecules 2019, 24, 326. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Hu, C.; Yin, L.; Tao, X.; Xu, L.; Qi, Y.; Han, X.; Xu, Y.; Zhao, Y.; Wang, C. Dioscin reduces lipopolysaccharide-induced inflammatory liver injury via regulating TLR4/MyD88 signal pathway. Int. Immunopharmacol. 2016, 36, 132–141. [Google Scholar] [CrossRef]
- Ma, L.; Gong, X.; Kuang, G.; Jiang, R.; Chen, R.; Wan, J. Sesamin ameliorates lipopolysaccharide/d-galactosamine-induced fulminant hepatic failure by suppression of Toll-like receptor 4 signaling in mice. Biochem. Biophys. Res. Commun. 2015, 461, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Z.; Liu, X.; Hu, J.; Liu, R.; Zhu, N.; Li, Y. The Antioxidant Effects of Whey Protein Peptide on Learning and Memory Improvement in Aging Mice Models. Nutrients 2021, 13, 2100. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Ohya, R.; Kondo, K.; Ano, Y. Iso-α-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 2018, 8, 4760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcleod, F.; Bossio, A.; Marzo, A.; Ciani, L.; Sibilla, S.; Hannan, S.; Wilson, G.A.; Palomer, E.; Smart, T.G.; Gibb, A.; et al. Wnt Signaling Mediates LTP-Dependent Spine Plasticity and AMPAR Localization through Frizzled-7 Receptors. Cell Rep. 2018, 23, 1060–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandel, E.R. The Molecular Biology of Memory Storage: A Dialogue Between Genes and Synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Fréchou, M.; Liere, P.; Zhang, S.; Pianos, A.; Fernandez, N.; Denier, C.; Mattern, C.; Schumacher, M.; Guennoun, R. A Role of Endogenous Progesterone in Stroke Cerebroprotection Revealed by the Neural-Specific Deletion of Its Intracellular Receptors. J. Neurosci. 2017, 37, 10998–11020. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zeng, F.; Ma, Y.; Yu, J.; Xiang, C.; Feng, X.; Wang, S.; Wang, J.; Zhao, S.; Zhu, X. Strontium Attenuates Hippocampal Damage via Suppressing Neuroinflammation in High-Fat Diet-Induced NAFLD Mice. Int. J. Mol. Sci. 2023, 24, 10248. https://doi.org/10.3390/ijms241210248
Wang S, Zeng F, Ma Y, Yu J, Xiang C, Feng X, Wang S, Wang J, Zhao S, Zhu X. Strontium Attenuates Hippocampal Damage via Suppressing Neuroinflammation in High-Fat Diet-Induced NAFLD Mice. International Journal of Molecular Sciences. 2023; 24(12):10248. https://doi.org/10.3390/ijms241210248
Chicago/Turabian StyleWang, Shuai, Fangyuan Zeng, Yue Ma, Jiaojiao Yu, Chenyao Xiang, Xiao Feng, Songlin Wang, Jianguo Wang, Shanting Zhao, and Xiaoyan Zhu. 2023. "Strontium Attenuates Hippocampal Damage via Suppressing Neuroinflammation in High-Fat Diet-Induced NAFLD Mice" International Journal of Molecular Sciences 24, no. 12: 10248. https://doi.org/10.3390/ijms241210248




