Utilizing MiSeq Sequencing to Detect Circulating microRNAs in Plasma for Improved Lung Cancer Diagnosis
Abstract
1. Introduction
2. Results
2.1. The Assessment of Hemolysis in Plasma
2.2. Small RNA Sequencing, Quality Control, and Annotation
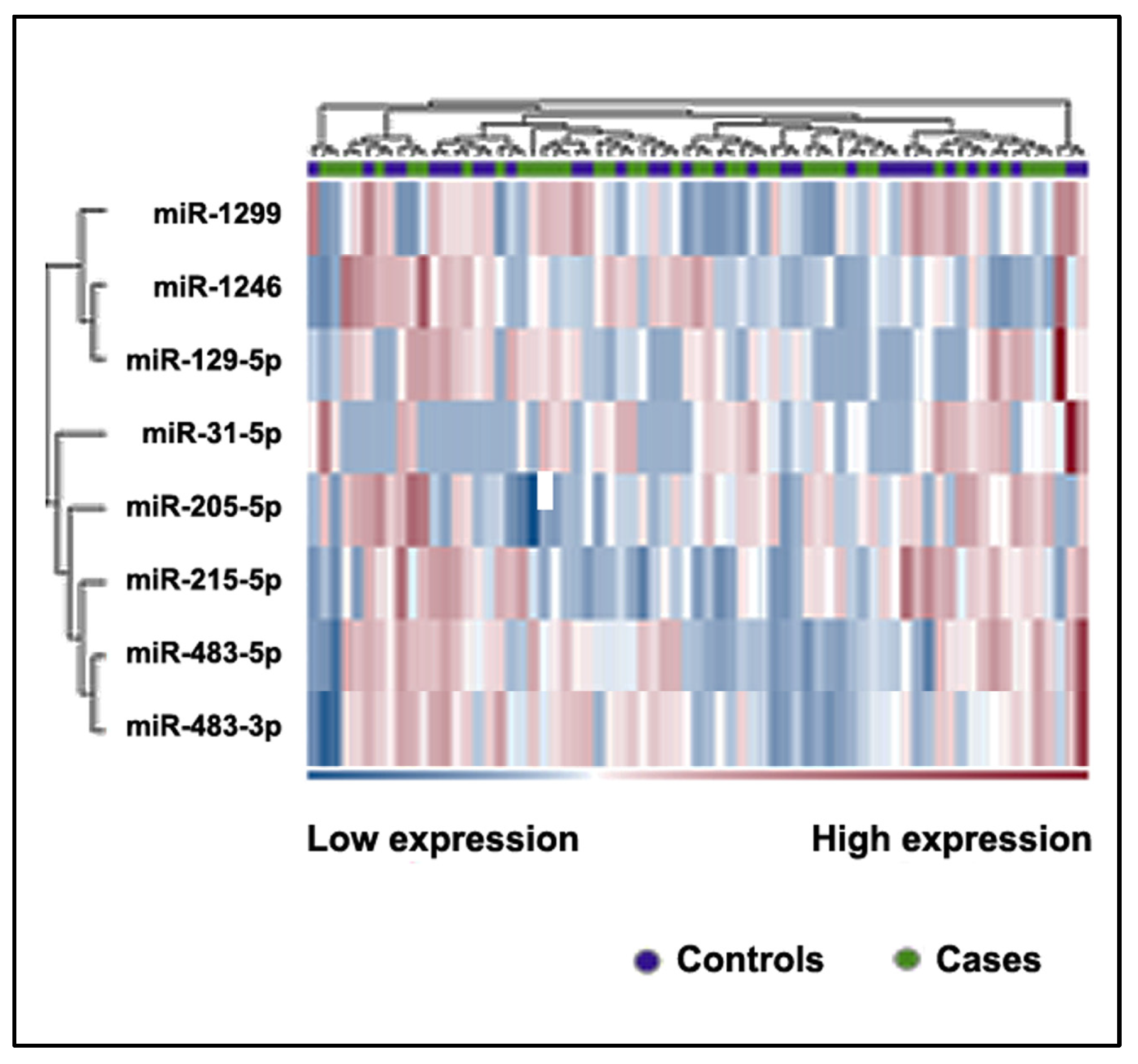
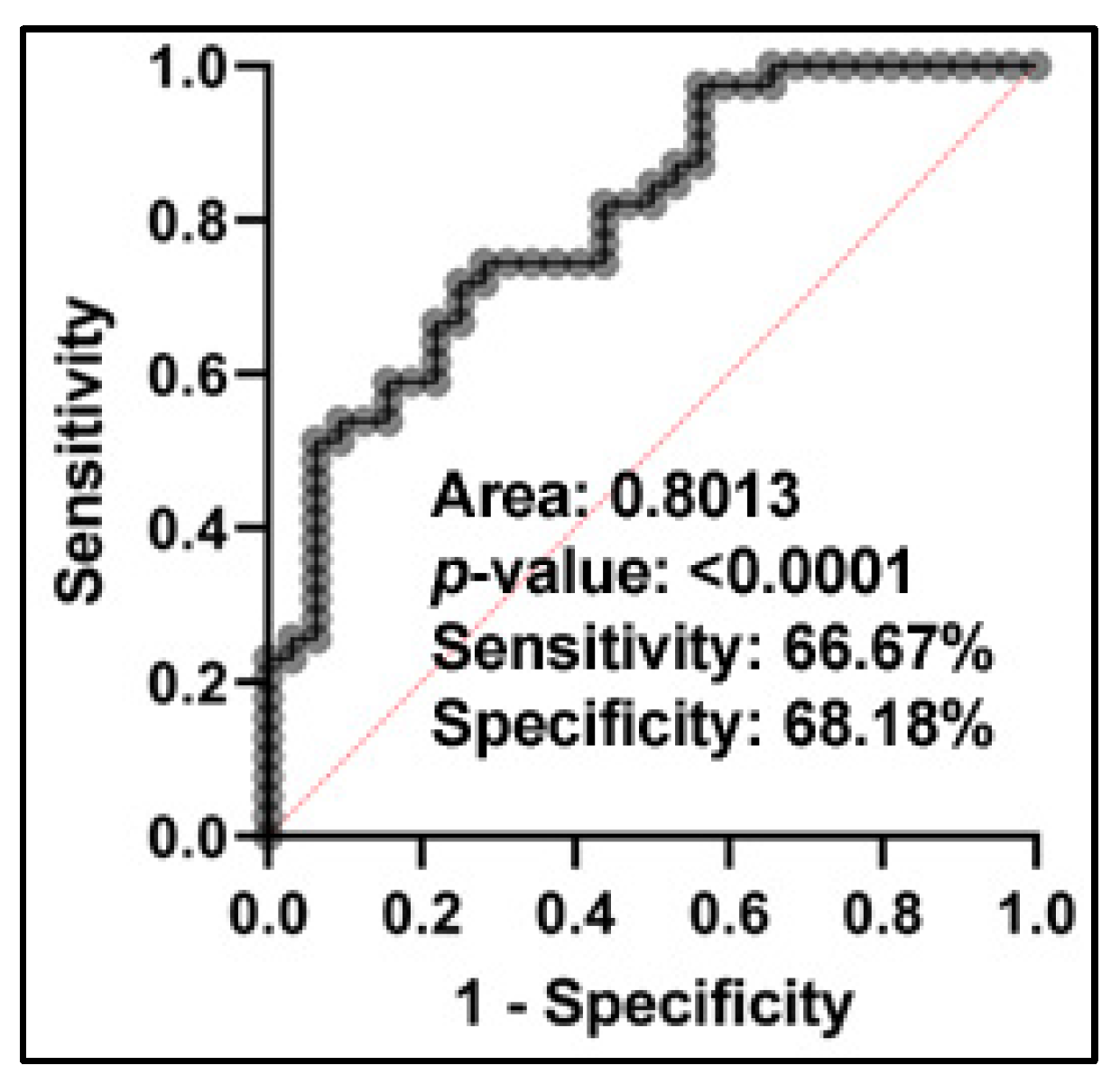
2.3. Differently Expressed Plasma miRNAs between NSCLC Patients and Cancer-Free Smokers
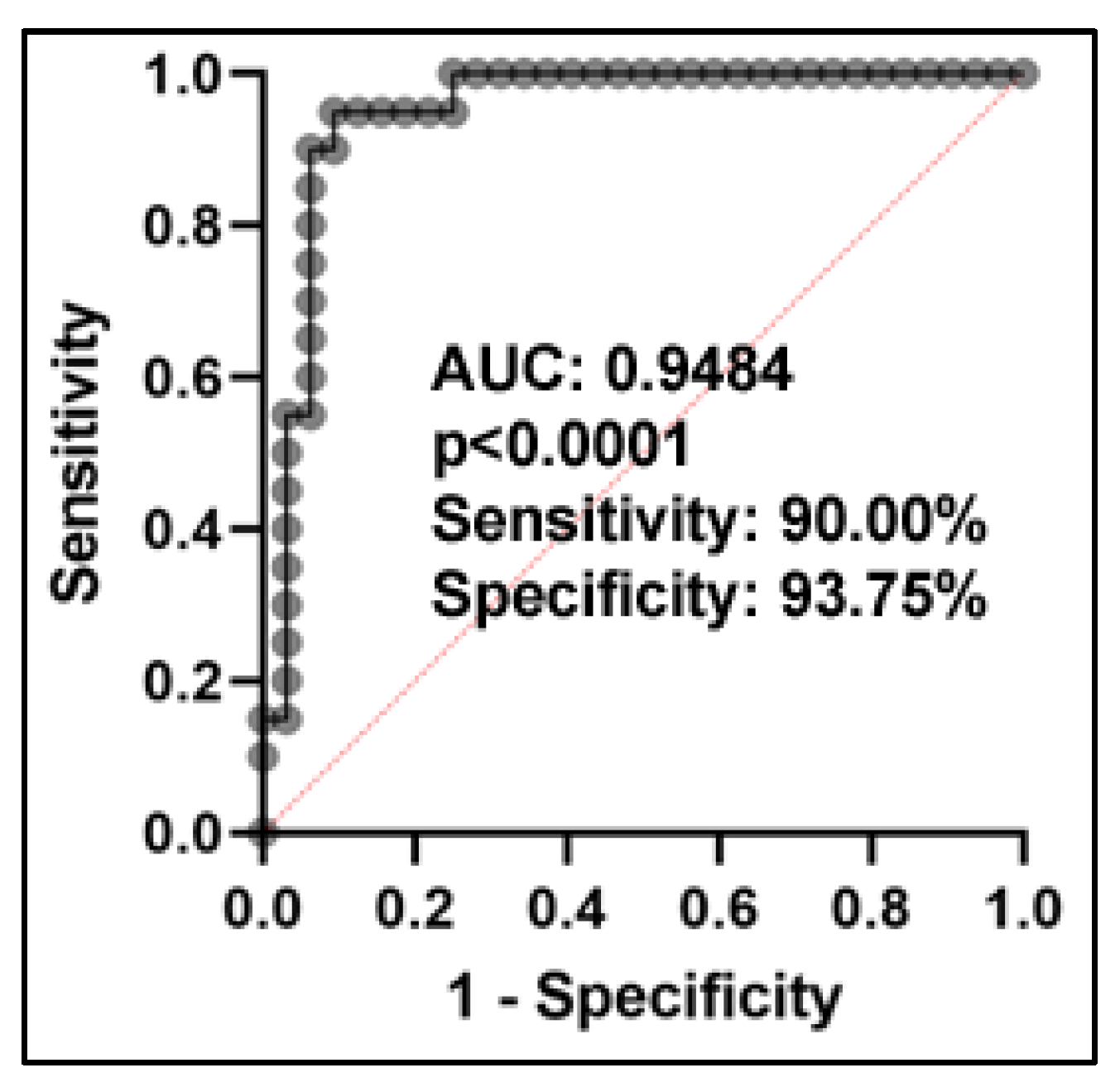
2.4. Differently Expressed Plasma miRNAs between SCC Patients and Cancer-Free Smokers
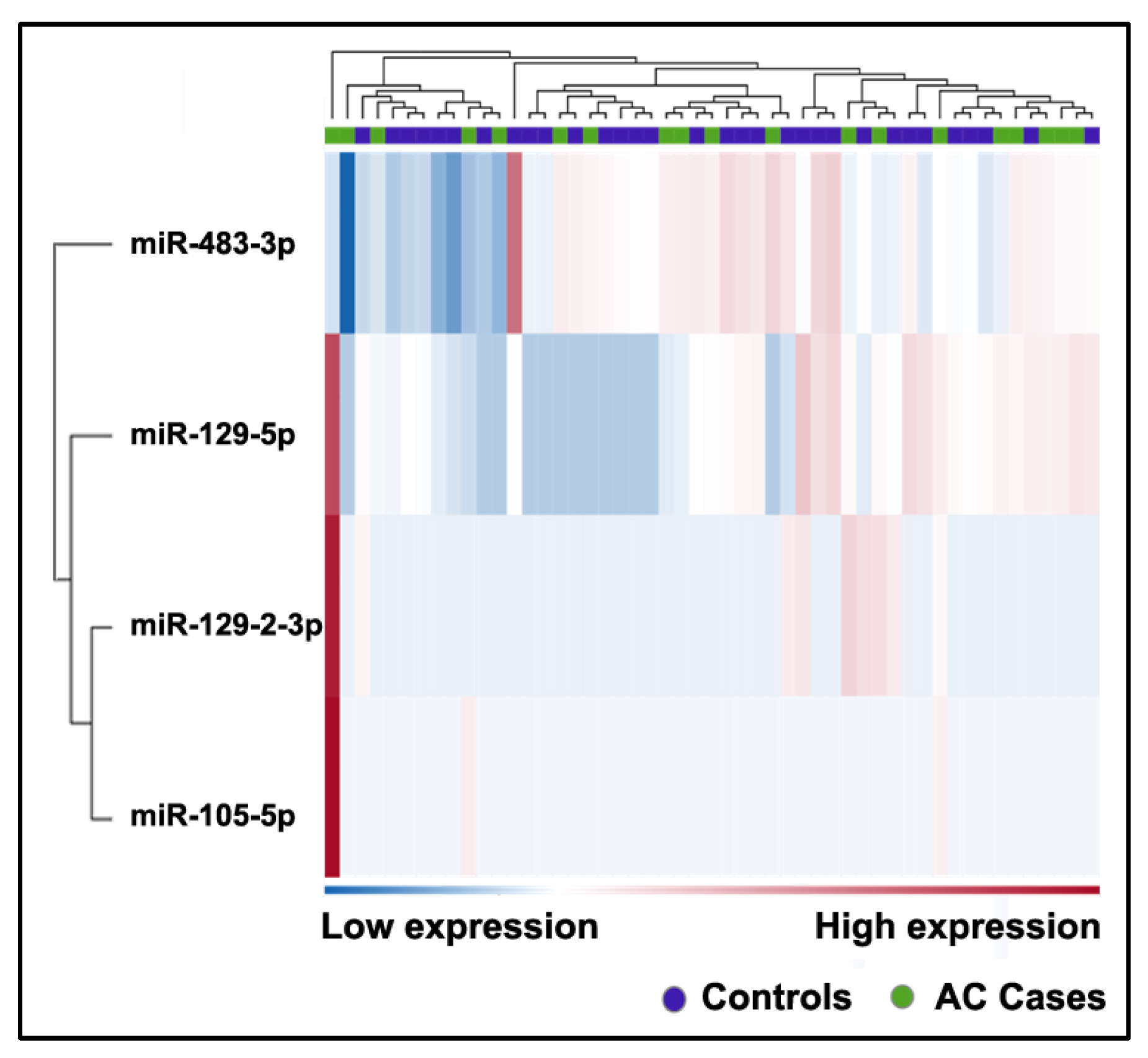
2.5. Differently Expressed Plasma miRNAs between AC Patients and Cancer-Free Smokers
2.6. Differently Expressed Plasma miRNAs between AC Patients and SCC Patients
3. Discussion
4. Materials and Methods
4.1. Participants and Plasma Preparation
4.2. RNA Isolation and cDNA Synthesis
4.3. MiRNA Library Construction, Quality Control, Sequencing, and RNAseq Data Analysis
4.4. Quantitative Real-Time PCR (qRT-PCR) Analysis of Hemolysis in Plasma
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Statistics Center. 2022. Available online: http://cancerstatisticscenter.cancer.org/#!/ (accessed on 10 June 2022).
- American Cancer Society. Cancer Facts & Figures 2022. 2022. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf (accessed on 10 June 2022).
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Valencia-Sanchez, M.A.; Liu, J.; Hannon, G.J.; Parker, R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006, 20, 515–524. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: microRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Ambros, V. The functions of animal microRNAs. Nature 2004, 431, 350–355. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Zhang, C.; Jing, Y.; Wang, C.; Liu, C.; Zhang, R.; Wang, J.; Zhang, J.; Zen, K.; et al. Investigation of microRNA expression in human serum during the aging process. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 102–109. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Nishida, N.; Calin, G.A.; Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 2014, 11, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Croce, C.M. microRNA involvement in human cancer. Carcinogenesis 2012, 33, 1126–1133. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, P.H. Are circulating microRNAs peripheral biomarkers for Alzheimer’s disease? Biochim. Biophys. Acta 2016, 1862, 1617–1627. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Leng, Q.; Lin, Y.; Jiang, F.; Lee, C.J.; Zhan, M.; Fang, H.; Wang, Y. A plasma miRNA signature for lung cancer early detection. Oncotarget 2017, 8, 111902–111911. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Leng, Q.; Jiang, Z.; Guarnera, M.A.; Zhou, Y.; Chen, X.; Wang, H.; Zhou, W.; Cai, L.; Fang, H.; et al. A classifier integrating plasma biomarkers and radiological characteristics for distinguishing malignant from benign pulmonary nodules. Int. J. Cancer 2017, 141, 1240–1248. [Google Scholar] [CrossRef]
- Ma, J.; Li, N.; Guarnera, M.; Jiang, F. Quantification of Plasma miRNAs by Digital PCR for Cancer Diagnosis. Biomark Insights 2013, 8, 127–136. [Google Scholar] [CrossRef]
- Shen, J.; Liu, Z.; Todd, N.W.; Zhang, H.; Liao, J.; Yu, L.; Guarnera, M.A.; Li, R.; Cai, L.; Zhan, M.; et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer 2011, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Todd, N.W.; Zhang, H.; Yu, L.; Lingxiao, X.; Mei, Y.; Guarnera, M.; Liao, J.; Chou, A.; Lu, C.L.; et al. Plasma microRNAs as potential biomarkers for non-small-cell lung cancer. Lab. Investig. 2011, 91, 579–587. [Google Scholar] [CrossRef]
- Li, J.; Fang, H.; Jiang, F.; Ning, Y. External validation of a panel of plasma microRNA biomarkers for lung cancer. Biomark Med. 2019, 13, 1557–1564. [Google Scholar] [CrossRef]
- Lin, Y.; Leng, Q.; Zhan, M.; Jiang, F. A Plasma Long Noncoding RNA Signature for Early Detection of Lung Cancer. Transl. Oncol. 2018, 11, 1225–1231. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.; Wang, Y.; Jiang, F. A Direct Plasma miRNA Assay for Early Detection and Histological Classification of Lung Cancer. Transl. Oncol. 2018, 11, 883–889. [Google Scholar] [CrossRef]
- Khodakov, D.; Wang, C.; Zhang, D.Y. Diagnostics based on nucleic acid sequence variant profiling: PCR, hybridization, and NGS approaches. Adv. Drug Deliv. Rev. 2016, 105, 3–19. [Google Scholar] [CrossRef]
- Lefterova, M.I.; Shen, P.; Odegaard, J.I.; Fung, E.; Chiang, T.; Peng, G.; Davis, R.W.; Wang, W.; Kharrazi, M.; Schrijver, I.; et al. Next-Generation Molecular Testing of Newborn Dried Blood Spots for Cystic Fibrosis. J. Mol. Diagn. 2016, 18, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Udar, N.; Lofton-Day, C.; Dong, J.; Vavrek, D.; Jung, A.S.; Meier, K.; Iyer, A.; Slaughter, R.; Gutekunst, K.; Bach, B.A.; et al. Clinical validation of the next-generation sequencing-based Extended RAS Panel assay using metastatic colorectal cancer patient samples from the phase 3 PRIME study. J. Cancer Res. Clin. Oncol. 2018, 144, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Zhelankin, A.V.; Vasiliev, S.V.; Stonogina, D.A.; Babalyan, K.A.; Sharova, E.I.; Doludin, Y.V.; Shchekochikhin, D.Y.; Generozov, E.V.; Akselrod, A.S. Elevated Plasma Levels of Circulating Extracellular miR-320a-3p in Patients with Paroxysmal Atrial Fibrillation. Int. J. Mol. Sci. 2020, 21, 3485. [Google Scholar] [CrossRef]
- Wen, C.; Wu, L.; Qin, Y.; Van Nostrand, J.D.; Ning, D.; Sun, B.; Xue, K.; Liu, F.; Deng, Y.; Liang, Y.; et al. Evaluation of the reproducibility of amplicon sequencing with Illumina MiSeq platform. PLoS ONE 2017, 12, e0176716. [Google Scholar] [CrossRef]
- Zhou, S.W.; Su, B.B.; Zhou, Y.; Feng, Y.Q.; Guo, Y.; Wang, Y.X.; Qi, P.; Xu, S. Aberrant miR-215 expression is associated with clinical outcome in breast cancer patients. Med. Oncol. 2014, 31, 259. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Fan, W.X. MiRNA-1246 suppresses the proliferation and migration of renal cell carcinoma through targeting CXCR4. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5979–5987. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Mannoor, K.; Gao, L.; Tan, A.; Guarnera, M.A.; Zhan, M.; Shetty, A.; Stass, S.A.; Xing, L.; Jiang, F. Characterization of microRNA transcriptome in lung cancer by next-generation deep sequencing. Mol. Oncol. 2014, 8, 1208–1219. [Google Scholar] [CrossRef]
- Xing, L.; Todd, N.W.; Yu, L.; Fang, H.; Jiang, F. Early detection of squamous cell lung cancer in sputum by a panel of microRNA markers. Mod. Pathol. 2010, 23, 1157–1164. [Google Scholar] [CrossRef]
- Li, H.; Jiang, Z.; Leng, Q.; Bai, F.; Wang, J.; Ding, X.; Li, Y.; Zhang, X.; Fang, H.; Yfantis, H.G.; et al. A prediction model for distinguishing lung squamous cell carcinoma from adenocarcinoma. Oncotarget 2017, 8, 50704–50714. [Google Scholar] [CrossRef]
- Gao, J.B.; Zhu, M.N.; Zhu, X.L. miRNA-215-5p suppresses the aggressiveness of breast cancer cells by targeting Sox9. FEBS Open Bio. 2019, 9, 1957–1967. [Google Scholar] [CrossRef]
- Zhao, G.; Jing, X.; Li, Z.; Wu, X.; Gao, Z.; Ma, R. The diagnostic and prognostic values of circulating miRNA-1246 in multiple myeloma. Hematology 2022, 27, 778–784. [Google Scholar] [CrossRef]
- Kaiyuan, D.; Lijuan, H.; Xueyuan, S.; Yunhui, Z. The role and underlying mechanism of miR-1299 in cancer. Future Sci. OA 2021, 7, FSO693. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, J.H.; Gong, F.S.; Xiao, J. MiR-141-3p suppresses gastric cancer induced transition of normal fibroblast and BMSC to cancer-associated fibroblasts via targeting STAT4. Exp. Mol. Pathol. 2019, 107, 85–94. [Google Scholar] [CrossRef]
- Yuan, L.; Bing, Z.; Yan, P.; Li, R.; Wang, C.; Sun, X.; Yang, J.; Shi, X.; Zhang, Y.; Yang, K. Integrative data mining and meta-analysis to investigate the prognostic role of microRNA-200 family in various human malignant neoplasms: A consideration on heterogeneity. Gene 2019, 716, 144025. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; He, Q.; Lu, Y.; Tao, F.; Zhao, L.; Ou, R. MicroRNA-218-5p inhibits cell growth and metastasis in cervical cancer via LYN/NF-kappaB signaling pathway. Cancer Cell Int. 2018, 18, 198. [Google Scholar] [CrossRef] [PubMed]
- Lun, W.; Wu, X.; Deng, Q.; Zhi, F. MiR-218 regulates epithelial-mesenchymal transition and angiogenesis in colorectal cancer via targeting CTGF. Cancer Cell Int. 2018, 18, 83. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Q.; Wang, K. MicroRNA-218-5p affects lung adenocarcinoma progression through targeting endoplasmic reticulum oxidoreductase 1 alpha. Bioengineered 2022, 13, 10061–10070. [Google Scholar] [CrossRef] [PubMed]
- Pepe, M.S.; Etzioni, R.; Feng, Z.; Potter, J.D.; Thompson, M.L.; Thornquist, M.; Winget, M.; Yasui, Y. Phases of biomarker development for early detection of cancer. J. Natl. Cancer Inst. 2001, 93, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Jia, Y.; Zhang, X. Cell-free circulating tumor DNA in plasma/serum of non-small cell lung cancer. Tumour. Biol. 2015, 36, 7–19. [Google Scholar] [CrossRef]
- Balgkouranidou, I.; Chimonidou, M.; Milaki, G.; Tsaroucha, E.; Kakolyris, S.; Georgoulias, V.; Lianidou, E. SOX17 promoter methylation in plasma circulating tumor DNA of patients with non-small cell lung cancer. Clin. Chem. Lab Med. 2016, 54, 1385–1393. [Google Scholar] [CrossRef]
- Normanno, N.; Denis, M.G.; Thress, K.S.; Ratcliffe, M.; Reck, M. Guide to detecting epidermal growth factor receptor (EGFR) mutations in ctDNA of patients with advanced non-small-cell lung cancer. Oncotarget 2017, 8, 12501–12516. [Google Scholar] [CrossRef]
- Ohori, M.; Wheeler, T.M.; Scardino, P.T. The New American Joint Committee on Cancer and International Union Against Cancer TNM classification of prostate cancer. Clinicopathologic correlations. Cancer 1994, 74, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Qiagen, Albertslund, Denmark. QIAGEN RNA-seq Analysis Portal 2.5.1. Available online: https://resources.qiagenbioinformatics.com (accessed on 2 April 2022).
- Blondal, T.; Jensby Nielsen, S.; Baker, A.; Andreasen, D.; Mouritzen, P.; Wrang Teilum, M.; Dahlsveen, I.K. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods 2013, 59, S1–S6. [Google Scholar] [CrossRef] [PubMed]



| miRNAs | FC | p-Value |
|---|---|---|
| miR-1246 | 2.32 | 3.62 × 10−4 |
| miR-129-5p | 8.93 | 1.34 × 10−7 |
| miR-1299 | −3.19 | 3.06 × 10−4 |
| miR-205-5p | 2.55 | 2.30 × 10−4 |
| miR-215-5p | −2.33 | 2.64 × 10−5 |
| miR-31-5p | −4.17 | 8.08 × 10−5 |
| miR-483-3p | −5.2 | 5.28 × 10−9 |
| miR-483-5p | −3 | 2.56 × 10−5 |
| miRNAs | FC | p-Value |
|---|---|---|
| miR-1299 | −7.37 | 1.55 × 10−7 |
| miR-141-3p | −2.39 | 1.27 × 10−3 |
| miR-200a-3p | −4.1 | 2.66 × 10−6 |
| miR-200b-3p | −2.95 | 4.52 × 10−4 |
| miR-200b-5p | −3.81 | 9.06 × 10−4 |
| miR-205-5p | 3.69 | 8.16 × 10−6 |
| miR-215-5p | −2.75 | 2.99 × 10−5 |
| miR-31-5p | −6.44 | 3.85 × 10−5 |
| miR-429 | −3.47 | 1.69 × 10−4 |
| miR-483-3p | −6.03 | 1.08 × 10−7 |
| miR-483-5p | −3.68 | 2.89 × 10−5 |
| miRNAs | FC | p-Value |
|---|---|---|
| miR-105-5p | 15.79 | 4.93 × 10−5 |
| miR-129-2-3p | 8.79 | 7.12 × 10−5 |
| miR-129-5p | 17.4 | 3.82 × 10−11 |
| miR-483-3p | −4.54 | 1.05 × 10−5 |
| Name | FC | p-Value |
|---|---|---|
| miR-105-5p | 18.95 | 2.18 × 10−4 |
| miR-129-2-3p | 7.72 | 4.96 × 10−4 |
| miR-129-5p | 21.39 | 2.27 × 10−10 |
| miR-141-3p | 4.1 | 3.08 × 10−6 |
| miR-200a-3p | 3.36 | 3.14 × 10−4 |
| miR-200b-3p | 3.63 | 1.66 × 10−4 |
| miR-200c-3p | 2.64 | 5.66 × 10−5 |
| miR-218-5p | 5.9 | 1.52 × 10−4 |
| miR-375-3p | 4.29 | 2.04 × 10−6 |
| AC Cases (n = 19) | SCC (n = 20) | Controls (n = 32) | p-Value | |
|---|---|---|---|---|
| Age | 65.5 (SD, 7.8) | 67.0 (SD, 6.4) | 60.9 (SD, 11.2) | 0.0877 |
| Sex | 0.2872 | |||
| Female | 9 | 5 | 14 | |
| Male | 10 | 15 | 18 | |
| Race | 0.8261 | |||
| African Americans | 6 | 7 | 12 | |
| White Americans | 13 | 13 | 19 | |
| Hispanic | 0 | 0 | 1 | |
| Smoking pack years (median) | 30 | 50 | 14 | 0.0051 |
| Stage | ||||
| Stage I | 2 | 3 | ||
| Stage II | 1 | 1 | ||
| Stage III | 5 | 2 | ||
| Stage IV | 1 | 3 | ||
| Unknown | 10 | 11 | ||
| Histological type |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Tsou, J.-H.; Stass, S.A.; Jiang, F. Utilizing MiSeq Sequencing to Detect Circulating microRNAs in Plasma for Improved Lung Cancer Diagnosis. Int. J. Mol. Sci. 2023, 24, 10277. https://doi.org/10.3390/ijms241210277
Geng X, Tsou J-H, Stass SA, Jiang F. Utilizing MiSeq Sequencing to Detect Circulating microRNAs in Plasma for Improved Lung Cancer Diagnosis. International Journal of Molecular Sciences. 2023; 24(12):10277. https://doi.org/10.3390/ijms241210277
Chicago/Turabian StyleGeng, Xinyan, Jen-Hui Tsou, Sanford A. Stass, and Feng Jiang. 2023. "Utilizing MiSeq Sequencing to Detect Circulating microRNAs in Plasma for Improved Lung Cancer Diagnosis" International Journal of Molecular Sciences 24, no. 12: 10277. https://doi.org/10.3390/ijms241210277
APA StyleGeng, X., Tsou, J.-H., Stass, S. A., & Jiang, F. (2023). Utilizing MiSeq Sequencing to Detect Circulating microRNAs in Plasma for Improved Lung Cancer Diagnosis. International Journal of Molecular Sciences, 24(12), 10277. https://doi.org/10.3390/ijms241210277






