Sequencing and Analysis of Complete Chloroplast Genomes Provide Insight into the Evolution and Phylogeny of Chinese Kale (Brassica oleracea var. alboglabra)
Abstract
:1. Introduction
2. Results
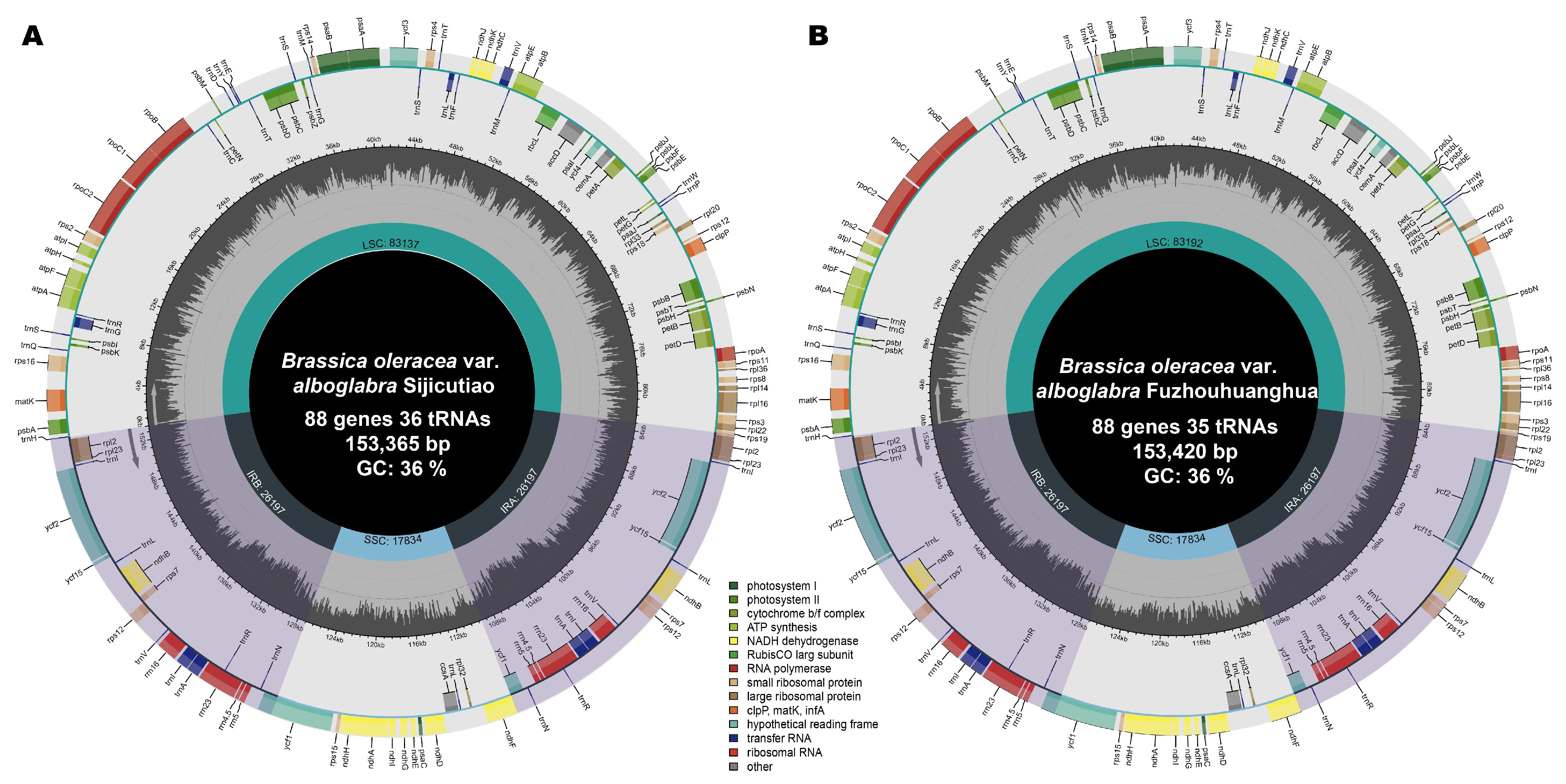
2.1. Chloroplast Genome Characterization
2.2. SSRs and Repeat Sequence Analysis
2.3. RSCU Analysis
2.4. Chloroplast Genome Comparison
2.5. Evolution of the Protein-Coding Genes
2.6. Phylogenetic Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and DNA Extraction and Sequencing
4.2. Gene Assembly and Annotation
4.3. Sequence Analysis and Statistics
4.4. Genome Comparison
4.5. Phylogenetic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sugiura, M. The chloroplast genome. Plant Mol. Biol. 1992, 19, 149–168. [Google Scholar] [CrossRef] [PubMed]
- de Vere, N.; Rich, T.C.; Trinder, S.A.; Long, C. DNA barcoding for plants. Methods Mol. Biol. 2015, 1245, 101–118. [Google Scholar] [PubMed]
- Wickett, N.J.; Forrest, L.L.; Budke, J.M.; Shaw, B.; Goffinet, B. Frequent pseudogenization and loss of the plastid-encoded sulfate-transport gene cysA throughout the evolution of liverworts. Am. J. Bot. 2011, 98, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhu, M.; Su, Y.; Wang, T. A Large Intergenic Spacer Leads to the Increase in Genome Size and Sequential Gene Movement around IR/SC Boundaries in the Chloroplast Genome of Adiantum malesianum (Pteridaceae). Int. J. Mol. Sci. 2022, 23, 15616. [Google Scholar] [CrossRef]
- Bock, R. Structure, function, and inheritance of plastid genomes. Top. Curr. Genet. 2007, 19, 29–63. [Google Scholar]
- Sassenrath-Cole, G.F. Photosynthesis, a comprehensive treatise. Crop Sci. 1998, 39, 282–283. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, L.I.; Bo, S.; Yu, S.; Wang, Q.; Bingliang, A.W. Taxonomic relationship between Chinese kale and other varieties in Brassica oleracea L. Acta Hortic. Sin. 2010, 37, 1161–1168. [Google Scholar]
- Liu, H.; Guan, P. Taxonomic relation between yellow-flower Chinese kale and white-flower Chinese kale. South China Agr. Univ. 1997, 2, 16–19. [Google Scholar]
- Sinskaia, E.N. Geno-systematical investigation of cultivated Brassica. Bull. Appl. Bot. Plant-Breed. 1927, 17, 1–166. [Google Scholar]
- Wang, X.; Luo, P. Studies on the karyotypes and C-banding patterns of Chinese kale (Brassica alboglabra) and cabbage (B. oleracea var. capitata). Acta Bot. Sin. 1987, 26, 362–366. [Google Scholar]
- Wei, Z. Comparison of pollen morphology of Brassica alboglabra and some other species of the genus Brassica. Acta Bot. Yunnanica 1991, 13, 290–292. [Google Scholar]
- Masa, I. Pollen Grahts of Japan Tokyo; Hirokawa Pubilishing: Hilo, HI, USA, 1956. [Google Scholar]
- Lan, Y.; Gheo, T.; Qian, W. Studies on the Pollen Morphology of the Genus Brassica (Cruciferae) in China. J. Univ. Chin. Acad. Sci. 1989, 27, 386–394. [Google Scholar]
- Treccarichi, S.; Ben Ammar, H.; Amari, M.; Cali, R.; Tribulato, A.; Branca, F. Molecular Markers for Detecting Inflorescence Size of Brassica oleracea L. Crops and B. oleracea Complex Species (n = 9) Useful for Breeding of Broccoli (B. oleracea var. italica) and Cauliflower (B. oleracea var. botrytis). Plants 2023, 12, 407. [Google Scholar] [CrossRef]
- Nagaharu, U. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Jpn. J. Bot. 1935, 7, 389–452. [Google Scholar]
- Katepa-Mupondwa, F.; Gugel, R.K.; Raney, J.P. Genetic diversity for agronomic, morphological and seed quality traits in Sinapis alba L. (yellow mustard). Can. J. Plant Sci. 2005, 86, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.Y.; Wang, Y.; Chen, M.; Dong, S.; Shao, Z.Q.; Liu, Y. Maternal Inheritance of U’s Triangle and Evolutionary Process of Brassica Mitochondrial Genomes. Front. Plant Sci. 2020, 11, 805. [Google Scholar] [CrossRef]
- Song, X.; Wei, Y.; Xiao, D.; Gong, K.; Sun, P.; Ren, Y.; Yuan, J.; Wu, T.; Yang, Q.; Li, X.; et al. Brassica carinata genome characterization clarifies U’s triangle model of evolution and polyploidy in Brassica. Plant Physiol. 2021, 186, 388–406. [Google Scholar] [CrossRef]
- Yamagishi, H.; Tanaka, Y.; Terachi, T. Complete mitochondrial genome sequence of black mustard (Brassica nigra; BB) and comparison with Brassica oleracea (CC) and Brassica carinata (BBCC). Genome 2014, 57, 577–582. [Google Scholar] [CrossRef]
- Yang, T.; Cai, B.; Jia, Z.; Wang, Y.; Wang, J.; King, G.J.; Ge, X.; Li, Z. Sinapis genomes provide insights into whole-genome triplication and divergence patterns within tribe Brassiceae. Plant J. 2023, 113, 246–261. [Google Scholar] [CrossRef]
- Ebert, D.; Peakall, R. Chloroplast simple sequence repeats (cpSSRs): Technical resources and recommendations for expanding cpSSR discovery and applications to a wide array of plant species. Mol. Ecol. Resour. 2009, 9, 673–690. [Google Scholar] [CrossRef]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2011, 16, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahima, K.; Sunil Kumar, K.N.; Rakhesh, K.V.; Rajeswaran, P.S.; Sharma, A.; Sathishkumar, R. Advancements and future prospective of DNA barcodes in the herbal drug industry. Front. Pharmacol. 2022, 13, 947512. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Lee, H.L. Complete chloroplast genome sequences from Korean ginseng (Panax schinseng Nees) and comparative analysis of sequence evolution among 17 vascular plants. DNA Res. 2004, 11, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Dugas, D.V.; Hernandez, D.; Koenen, E.J.; Schwarz, E.; Straub, S.; Hughes, C.E.; Jansen, R.K.; Nageswara-Rao, M.; Staats, M.; Trujillo, J.T.; et al. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions, and accelerated rate of evolution in clpP. Sci. Rep. 2015, 5, 16958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Tao, M.; Shan, X.; Pan, Y.; Sun, C.; Song, L.; Pei, X.; Jing, Z.; Dai, Z. Characterization of the complete chloroplast genome of Brassica oleracea var. italica and phylogenetic relationships in Brassicaceae. PLoS ONE 2022, 17, e0263310. [Google Scholar] [CrossRef]
- Du, X.; Zeng, T.; Feng, Q.; Hu, L.; Luo, X.; Weng, Q.; He, J.; Zhu, B. The complete chloroplast genome sequence of yellow mustard (Sinapis alba L.) and its phylogenetic relationship to other Brassicaceae species. Gene 2020, 731, 144340. [Google Scholar] [CrossRef]
- Yang, Z.; Nielsen, R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Mol. Biol. Evol. 2000, 17, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gan, C.; Luo, X.; Dong, C.; Zhou, S.; Xiong, Q.; Weng, Q.; Hu, X.; Du, X.; Zhu, B. Complete chloroplast genome features of the model heavy metal hyperaccumulator Arabis paniculata Franch and its phylogenetic relationships with other Brassicaceae species. Physiol. Mol. Biol. Plants 2022, 28, 775–789. [Google Scholar] [CrossRef]
- Wu, Z.; Liao, R.; Yang, T.; Dong, X.; Lan, D.; Qin, R.; Liu, H. Analysis of six chloroplast genomes provides insight into the evolution of Chrysosplenium (Saxifragaceae). BMC Genom. 2020, 21, 621. [Google Scholar] [CrossRef]
- Heyno, E.; Ermakova, M.; Lopez-Calcagno, P.E.; Woodford, R.; Brown, K.L.; Matthews, J.S.A.; Osmond, B.; Raines, C.A.; von Caemmerer, S. Rieske FeS overexpression in tobacco provides increased abundance and activity of cytochrome b6 f. Physiol. Plant. 2022, 174, e13803. [Google Scholar] [CrossRef]
- Pradhan, A.K.; Prakash, S.; Mukhopadhyay, A.; Pental, D. Phytogeny of Brassica and allied genera based on variation in chloroplast and mitochondrial DNA patterns: Molecular and taxonomic classifications are incongruous. Theor. Appl. Genet. 1992, 85, 331–340. [Google Scholar] [CrossRef]
- Yim, W.C.; Swain, M.L.; Ma, D.; An, H.; Bird, K.A.; Curdie, D.D.; Wang, S.; Ham, H.D.; Luzuriaga-Neira, A.; Kirkwood, J.S.; et al. The final piece of the Triangle of U: Evolution of the tetraploid Brassica carinata genome. Plant Cell 2022, 34, 4143–4172. [Google Scholar] [CrossRef]
- Gawel, N.J.; Jarret, R.L. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Mol. Biol. Rep. 1991, 9, 262–266. [Google Scholar] [CrossRef]
- Zhang, T.; Zeng, C.X.; Yang, J.B.; Li, H.T.; Li, D.Z. Fifteen novel universal primer pairs for sequencing whole chloroplast genomes and a primer pair for nuclear ribosomal DNAs. J. Syst. Evol. 2016, 54, 219–227. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Wang, X.R.; Tang, H.R. The complete chloroplast genome sequence of Rubus lambertianus var. glaber: Sequence divergence and phylogenetic application. Mitochondrial DNA B Resour. 2020, 5, 1261–1262. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Chen, H.; Jiang, M.; Wang, L.; Wu, X.; Huang, L.; Liu, C. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 2019, 47, W65–W73. [Google Scholar] [CrossRef]
- Zheng, S.; Poczai, P.; Hyvönen, J.; Tang, J.; Amiryousefi, A. Chloroplot: An Online Program for the Versatile Plotting of Organelle Genomes. Front. Genet. 2020, 11, 576124. [Google Scholar] [CrossRef] [PubMed]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Zhang, R.; Li, J.; Zheng, K.; Xiao, J.; Zheng, Y. Analyses of chloroplast genome of Eutrema japonicum provide new insights into the evolution of Eutrema species. Agronomy 2021, 11, 2546. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef] [Green Version]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Bi, G.; Mao, Y.; Xing, Q.; Cao, M. HomBlocks: A multiple-alignment construction pipeline for organelle phylogenomics based on locally collinear block searching. Genomics 2018, 110, 18–22. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liang, Q.; Zhang, C.; Huang, H.; He, H.; Wang, M.; Li, M.; Huang, Z.; Tang, Y.; Chen, Q.; et al. Sequencing and Analysis of Complete Chloroplast Genomes Provide Insight into the Evolution and Phylogeny of Chinese Kale (Brassica oleracea var. alboglabra). Int. J. Mol. Sci. 2023, 24, 10287. https://doi.org/10.3390/ijms241210287
Wang Y, Liang Q, Zhang C, Huang H, He H, Wang M, Li M, Huang Z, Tang Y, Chen Q, et al. Sequencing and Analysis of Complete Chloroplast Genomes Provide Insight into the Evolution and Phylogeny of Chinese Kale (Brassica oleracea var. alboglabra). International Journal of Molecular Sciences. 2023; 24(12):10287. https://doi.org/10.3390/ijms241210287
Chicago/Turabian StyleWang, Yilin, Qiannan Liang, Chenlu Zhang, Huanhuan Huang, Hao He, Mengyu Wang, Mengyao Li, Zhi Huang, Yi Tang, Qing Chen, and et al. 2023. "Sequencing and Analysis of Complete Chloroplast Genomes Provide Insight into the Evolution and Phylogeny of Chinese Kale (Brassica oleracea var. alboglabra)" International Journal of Molecular Sciences 24, no. 12: 10287. https://doi.org/10.3390/ijms241210287





