Deep Learning Approaches for lncRNA-Mediated Mechanisms: A Comprehensive Review of Recent Developments
Abstract
:1. Introduction
2. Literature Analysis
2.1. Paper Selection Process
2.2. Brief Analysis of Deep Learning Approaches in lncRNA Research
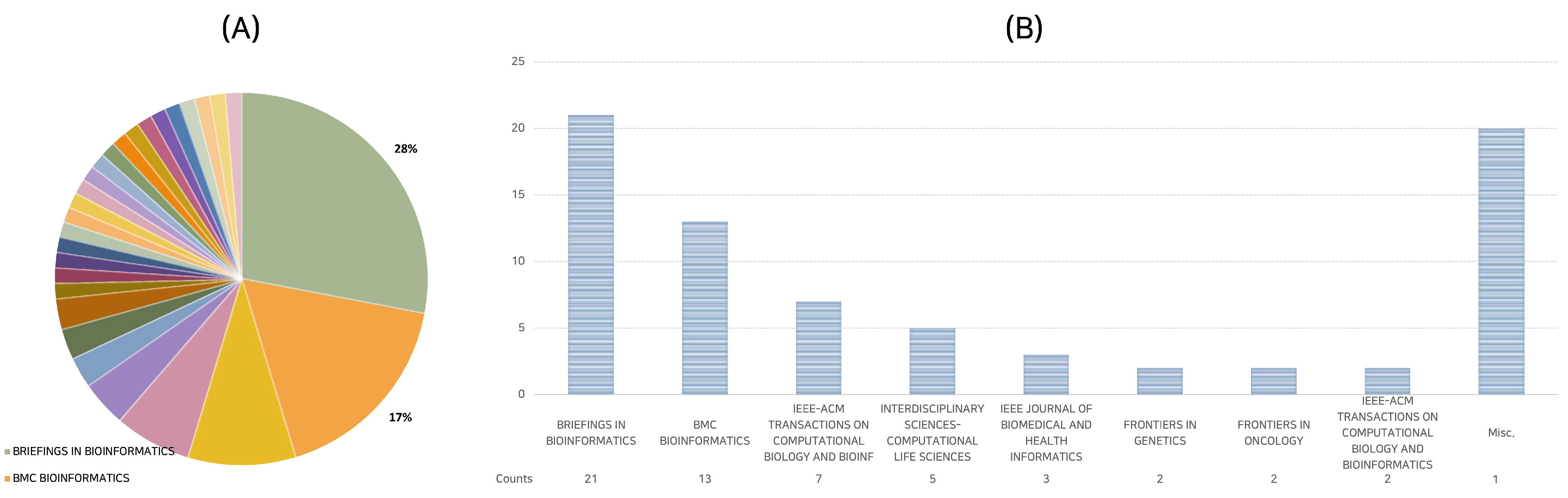
2.3. Distributive Analysis of Publications across Various Journals
3. Deep Learning Approaches in the Prediction of lncRNA–Disease Associations
3.1. Recent Advances from 2021 to 2023
3.2. Emerging Research Trends in Recent Studies
4. Deep Learning Approaches in the Prediction of lncRNA–Protein Interactions
4.1. Recent Advances from 2021 to 2023
4.2. Emerging Research Trends in Recent Studies
5. Deep Learning Approaches in the Prediction of lncRNA–miRNA Interactions
5.1. Recent Advances from 2021 to 2023
5.2. Emerging Research Trends in Recent Studies
6. Deep Learning Approaches in the Classification and Prediction of lncRNA Characteristics
6.1. Recent Advances from 2021 to 2023
6.2. Emerging Research Trends in Recent Studies
7. Other Deep Learning Research Domains and Utilization of lncRNA-Related Data as Deep Learning Inputs
7.1. Recent Advances from 2021 to 2023
7.2. Emerging Research Trends in Recent Studies
8. Challenges and Future Prospects
8.1. Challenges
8.2. Future Prospects and Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greener, J.G.; Kandathil, S.M.; Moffat, L.; Jones, D.T. A guide to machine learning for biologists. Nat. Rev. Mol. Cell Biol. 2022, 23, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Carleo, G.; Cirac, I.; Cranmer, K.; Daudet, L.; Schuld, M.; Tishby, N.; Vogt-Maranto, L.; Zdeborová, L. Machine learning and the physical sciences. Rev. Mod. Phys. 2019, 91, 045002. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Ding, M.; Shaham, S.; Rahayu, W.; Farokhi, F.; Lin, Z. When machine learning meets privacy: A survey and outlook. ACM Comput. Surv. (CSUR) 2021, 54, 1–36. [Google Scholar] [CrossRef]
- Lee, M. The Geometry of Feature Space in Deep Learning Models: A Holistic Perspective and Comprehensive Review. Mathematics 2023, 11, 2375. [Google Scholar] [CrossRef]
- Ko, K.; Yeom, T.; Lee, M. Superstargan: Generative adversarial networks for image-to-image translation in large-scale domains. Neural Netw. 2023, 162, 330–339. [Google Scholar] [CrossRef]
- Ku, H.; Lee, M. TextControlGAN: Text-to-Image Synthesis with Controllable Generative Adversarial Networks. Appl. Sci. 2023, 13, 5098. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M. Class-Continuous Conditional Generative Neural Radiance Field. arXiv 2023, arXiv:2301.00950. [Google Scholar]
- Lee, M. A Mathematical Investigation of Hallucination and Creativity in GPT Models. Mathematics 2023, 11, 2320. [Google Scholar] [CrossRef]
- Brown, T.; Mann, B.; Ryder, N.; Subbiah, M.; Kaplan, J.D.; Dhariwal, P.; Neelakantan, A.; Shyam, P.; Sastry, G.; Askell, A.; et al. Language models are few-shot learners. Adv. Neural Inf. Process. Syst. 2020, 33, 1877–1901. [Google Scholar]
- Radford, A.; Narasimhan, K.; Salimans, T.; Sutskever, I. Improving Language Understanding by Generative Pre-Training; OpenAI Technical Report; OpenAI Inc.: San Francisco, CA, USA, 2018. [Google Scholar]
- Radford, A.; Wu, J.; Child, R.; Luan, D.; Amodei, D.; Sutskever, I. Language Models Are Unsupervised Multitask Learners; OpenAI Technical Report; OpenAI Inc.: San Francisco, CA, USA, 2019. [Google Scholar]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 924–933. [Google Scholar] [CrossRef] [Green Version]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, R.P.; Velmeshev, D.; Faghihi, M.A. De-repressing LncRNA-targeted genes to upregulate gene expression: Focus on small molecule therapeutics. Mol. Ther.-Nucleic Acids 2014, 3, e196. [Google Scholar] [CrossRef]
- Peng, W.X.; Koirala, P.; Mo, Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene 2017, 36, 5661–5667. [Google Scholar] [CrossRef]
- Liu, X.; Xu, M.; Li, P.; Zhang, W.; Zeng, L.h.; Yang, Y.; Yang, G. Roles of lncRNAs in the transcription regulation of HIV-1. Biomed. J. 2022, 45, 580–593. [Google Scholar] [CrossRef]
- Zhou, H.; Simion, V.; Pierce, J.B.; Haemmig, S.; Chen, A.F.; Feinberg, M.W. LncRNA-MAP3K4 regulates vascular inflammation through the p38 MAPK signaling pathway and cis-modulation of MAP3K4. FASEB J. 2021, 35, e21133. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, Y.; Nie, X. Regulatory mechanisms of lncRNAs and their target gene signaling pathways in laryngeal squamous cell carcinoma. Front. Pharmacol. 2020, 11, 1140. [Google Scholar] [CrossRef]
- Farzaneh, M.; Najafi, S.; Anbiyaee, O.; Azizidoost, S.; Khoshnam, S.E. LncRNA MALAT1-related signaling pathways in osteosarcoma. Clin. Transl. Oncol. 2023, 25, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Dykes, I.M.; Emanueli, C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom. Proteom. Bioinform. 2017, 15, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Xi, Y.; McCarthy, R.; Allton, K.; Akdemir, K.C.; Patel, L.R.; Aronow, B.; Lin, C.; Li, W.; Yang, L.; et al. LncPRESS1 is a p53-regulated LncRNA that safeguards pluripotency by disrupting SIRT6-mediated de-acetylation of histone H3K56. Mol. Cell 2016, 64, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Zou, B.; Liang, Y.; Bian, J.; Xu, J.; Zhou, Q.; Zhang, C.; Chen, T.; Yang, M.; Wang, H.; et al. LncRNA RCAT1 promotes tumor progression and metastasis via miR-214-5p/E2F2 axis in renal cell carcinoma. Cell Death Dis. 2021, 12, 689. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Liang, L.; Li, J.; Chen, Y.X. Over-expression of lncRNA DANCR is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11480. [Google Scholar] [PubMed]
- Merry, C.R.; Forrest, M.E.; Sabers, J.N.; Beard, L.; Gao, X.H.; Hatzoglou, M.; Jackson, M.W.; Wang, Z.; Markowitz, S.D.; Khalil, A.M. DNMT1-associated long non-coding RNAs regulate global gene expression and DNA methylation in colon cancer. Hum. Mol. Genet. 2015, 24, 6240–6253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhmdorfer, G.; Wierzbicki, A.T. Control of chromatin structure by long noncoding RNA. Trends Cell Biol. 2015, 25, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Xuan, P.; Gong, Z.; Cui, H.; Li, B.; Zhang, T. Fully connected autoencoder and convolutional neural network with attention-based method for inferring disease-related lncRNAs. Brief. Bioinform. 2022, 23, bbac089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, J.; Wu, Y.; Liu, N.; Wang, Y.; Liang, Y. CapsNet-LDA: Predicting lncRNA-disease associations using attention mechanism and capsule network based on multi-view data. Brief. Bioinform. 2023, 24, bbac531. [Google Scholar] [CrossRef]
- Madhavan, M.; Gopakumar, G. DBNLDA: Deep Belief Network based representation learning for lncRNA-disease association prediction. Appl. Intell. 2022, 52, 5342–5352. [Google Scholar] [CrossRef]
- Madhavan, M.; Gopalakrishnan, G. Long Non-coding RNAs in Heart Failure: A Deep Belief Network based Cluster Analysis. Curr. Bioinform. 2021, 16, 983–991. [Google Scholar] [CrossRef]
- Ma, Y. DeepMNE: Deep Multi-Network Embedding for lncRNA-Disease Association Prediction. IEEE J. Biomed. Health Inform. 2022, 26, 3539–3549. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yang, Z.; Song, J.; Dai, Q.; Duan, X. DHNLDA: A Novel Deep Hierarchical Network Based Method for Predicting lncRNA-Disease Associations. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 3395–3403. [Google Scholar] [CrossRef]
- Zeng, M.; Lu, C.; Fei, Z.; Wu, F.X.; Li, Y.; Wang, J.; Li, M. DMFLDA: A Deep Learning Framework for Predicting lncRNA-Disease Associations. IEEE-ACM Trans. Comput. Biol. Bioinform. 2021, 18, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Y.; Zhao, S. Dual Attention Mechanisms and Feature Fusion Networks Based Method for Predicting LncRNA-Disease Associations. Interdiscip. Sci.-Comput. Life Sci. 2022, 14, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chen, M.; Pan, X. GCRFLDA: Scoring lncRNA-disease associations using graph convolution matrix completion with conditional random field. Brief. Bioinform. 2022, 23, bbab361. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhong, C. gGATLDA: LncRNA-disease association prediction based on graph-level graph attention network. BMC Bioinform. 2022, 23, 11. [Google Scholar] [CrossRef]
- Xuan, P.; Wang, S.; Cui, H.; Zhao, Y.; Zhang, T.; Wu, P. Learning global dependencies and multi-semantics within heterogeneous graph for predicting disease-related lncRNAs. Brief. Bioinform. 2022, 23, bbac361. [Google Scholar] [CrossRef]
- Xuan, P.; Zhan, L.; Cui, H.; Zhang, T.; Nakaguchi, T.; Zhang, W. Graph Triple-Attention Network for Disease-Related LncRNA Prediction. IEEE J. Biomed. Health Inform. 2022, 26, 2839–2849. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, X.; Yin, M. Heterogeneous graph attention network based on meta-paths for lncRNA-disease association prediction. Brief. Bioinform. 2022, 23, bbab407. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, X.; Tang, L.; Liu, L. Heterogeneous graph neural network for lncRNA-disease association prediction. Sci. Rep. 2022, 12, 17519. [Google Scholar] [CrossRef]
- Jha, A.; Quesnel-Vallieres, M.; Wang, D.; Thomas-Tikhonenko, A.; Lynch, K.W.; Barash, Y. Identifying common transcriptome signatures of cancer by interpreting deep learning models. Genome Biol. 2022, 23, 117. [Google Scholar] [CrossRef]
- Wei, H.; Liao, Q.; Liu, B. iLncRNAdis-FB: Identify lncRNA-Disease Associations by Fusing Biological Feature Blocks Through Deep Neural Network. IEEE-ACM Trans. Comput. Biol. Bioinform. 2021, 18, 1946–1957. [Google Scholar] [CrossRef]
- Guo, Z.H.; Chen, Z.H.; You, Z.H.; Wang, Y.B.; Yi, H.C.; Wang, M.N. A learning-based method to predict LncRNA-disease associations by combining CNN and ELM. BMC Bioinform. 2022, 22, 622. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Lai, D.; Chen, Q.; Wu, X.; Chen, B.; Liu, J.; Wang, J.; Chen, Y.P.P. LDICDL: LncRNA-Disease Association Identification Based on Collaborative Deep Learning. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 1715–1723. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, J.; Sun, T.; Shen, Z. A machine learning framework that integrates multi-omics data predicts cancer-related LncRNAs. BMC Bioinform. 2021, 22, 332. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Zhang, H.; Liu, Z.; Huang, S.; Yin, Y. LR-GNN: A graph neural network based on link representation for predicting molecular associations. Brief. Bioinform. 2022, 23, bbab513. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, Z.Q.; Liu, N.N.; Wu, Y.N.; Gu, C.L.; Wang, Y.L. MAGCNSE: Predicting lncRNA-disease associations using multi-view attention graph convolutional network and stacking ensemble model. BMC Bioinform. 2022, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, F.; Gao, X. MCA-Net: Multi-Feature Coding and Attention Convolutional Neural Network for Predicting lncRNA-Disease Association. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 2907–2919. [Google Scholar] [CrossRef]
- Ai, C.; Yang, H.; Ding, Y.; Tang, J.; Guo, F. A multi-layer multi-kernel neural network for determining associations between non-coding RNAs and diseases. Neurocomputing 2022, 493, 91–105. [Google Scholar] [CrossRef]
- Wu, Q.W.; Cao, R.F.; Xia, J.F.; Ni, J.C.; Zheng, C.H.; Su, Y.S. Extra Trees Method for Predicting LncRNA-Disease Association Based On Multi-Layer Graph Embedding Aggregation. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 3171–3178. [Google Scholar] [CrossRef]
- Al Mamun, A.; Tanvir, R.B.; Sobhan, M.; Mathee, K.; Narasimhan, G.; Holt, G.E.; Mondal, A.M. Multi-Run Concrete Autoencoder to Identify Prognostic lncRNAs for 12 Cancers. Int. J. Mol. Sci. 2021, 22, 11919. [Google Scholar] [CrossRef] [PubMed]
- Wei-Na, L.; Xiao-Nan, F.; Shao-Wu, Z. NELDA: Prediction of LncRNA-disease Associations With Network Embedding. Prog. Biochem. Biophys. 2022, 49, 1369–1380. [Google Scholar]
- Yang, W.; Zheng, X.; Huang, Q.; Liu, Y.; Chen, Y.; Song, Z. Combining BPSO and ELM Models for Inferring Novel lncRNA- Disease Associations. Int. J. Data Warehous. Min. 2023, 19, 1–18. [Google Scholar] [CrossRef]
- Silva, V.A.B.O.; Spinosa, E.J. Graph Convolutional Auto-Encoders for Predicting Novel lncRNA-Disease Associations. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 2264–2271. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Zhang, J.; Chen, J. Potential Prognosis and Diagnostic Value of AKT3, LSM12, MEF2C, and RAB30 in Exosomes in Colorectal Cancer on Spark Framework. J. Healthc. Eng. 2021, 2021, 8218043. [Google Scholar] [CrossRef]
- Sheng, N.; Cui, H.; Zhang, T.; Xuan, P. Attentional multi-level representation encoding based on convolutional and variance autoencoders for lncRNA-disease association prediction. Brief. Bioinform. 2021, 22, bbaa067. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, H.; Jin, C.; Quan, X.; Yin, Y. A representation learning model based on variational inference and graph autoencoder for predicting lncRNA-disease associations. BMC Bioinform. 2021, 22, 136. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Jin, C.; Kang, C. Predicting lncRNA-protein interactions with bipartite graph embedding and deep graph neural networks. Front. Genet. 2023, 14, 1136672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, H.; Feng, S.; Zhang, Q.; Han, S.; Du, W. Capsule-LPI: A LncRNA-protein interaction predicting tool based on a capsule network. BMC Bioinform. 2021, 22, 246. [Google Scholar] [CrossRef]
- Shaw, D.; Chen, H.; Xie, M.; Jiang, T. DeepLPI: A multimodal deep learning method for predicting the interactions between lncRNAs and protein isoforms. BMC Bioinform. 2021, 22, 24. [Google Scholar] [CrossRef]
- Teng, Z.; Zhang, Y.; Dai, Q.; Wu, C.; Li, D. Constructing discriminative feature space for LncRNA-protein interaction based on deep autoencoder and marginal fisher analysis. Comput. Biol. Med. 2023, 157, 106711. [Google Scholar] [CrossRef]
- Peng, L.; Tan, J.; Tian, X.; Zhou, L. EnANNDeep: An Ensemble-based lncRNA-protein Interaction Prediction Framework with Adaptive k-Nearest Neighbor Classifier and Deep Models. Interdiscip. Sci.-Comput. Life Sci. 2022, 14, 209–232. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Liang, D.M.; Du, P.F. iEssLnc: Quantitativeestimation of lncRNA gene essentialities with meta- path-guided random walks on the lncRNA-protein interaction network. Brief. Bioinform. 2023, 24, bbad097. [Google Scholar] [CrossRef]
- Huang, L.; Jiao, S.; Yang, S.; Zhang, S.; Zhu, X.; Guo, R.; Wang, Y. LGFC-CNN: Prediction of lncRNA-Protein Interactions by Using Multiple Types of Features through Deep Learning. Genes 2021, 12, 1689. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shi, Y.; Yan, J.; Qu, W.; Li, X.; Tan, J. LPI-CSFFR: Combining serial fusion with feature reuse for predicting LncRNA-protein interactions. Comput. Biol. Chem. 2022, 99, 107718. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Z.; Tian, X.; Peng, L. LPI-deepGBDT: A multiple-layer deep framework based on gradient boosting decision trees for lncRNA-protein interaction identification. BMC Bioinform. 2021, 22, 479. [Google Scholar] [CrossRef]
- Peng, L.; Wang, C.; Tian, X.; Zhou, L.; Li, K. Finding lncRNA-Protein Interactions Based on Deep Learning With Dual-Net Neural Architecture. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 3456–3468. [Google Scholar]
- Zhou, L.; Duan, Q.; Tian, X.; Xu, H.; Tang, J.; Peng, L. LPI-HyADBS: A hybrid framework for lncRNA-protein interaction prediction integrating feature selection and classification. BMC Bioinform. 2021, 22, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, J.; Shuai, S.C.; Zhao, Q.; Shuai, J. Predicting potential interactions between lncRNAs and proteins via combined graph auto-encoder methods. Brief. Bioinform. 2023, 24, bbac527. [Google Scholar] [CrossRef]
- Zhou, H.; Wekesa, J.S.; Luan, Y.; Meng, J. PRPI-SC: An ensemble deep learning model for predicting plant lncRNA-protein interactions. BMC Bioinform. 2021, 22, 415. [Google Scholar] [CrossRef]
- Song, J.; Tian, S.; Yu, L.; Yang, Q.; Dai, Q.; Wang, Y.; Wu, W.; Duan, X. RLF-LPI: An ensemble learning framework using sequence information for predicting lncRNA-protein interaction based on AE-ResLSTM and fuzzy decision. Math. Biosci. Eng. 2022, 19, 4749–4764. [Google Scholar] [CrossRef]
- Asim, M.N.; Ibrahim, M.A.; Zehe, C.; Trygg, J.; Dengel, A.; Ahmed, S. BoT-Net: A lightweight bag of tricks-based neural network for efficient LncRNA-miRNA interaction prediction. Interdiscip. Sci.-Comput. Life Sci. 2022, 14, 841–862. [Google Scholar] [CrossRef]
- Yang, L.; Li, L.P.; Yi, H.C. DeepWalk based method to predict lncRNA-miRNA associations via lncRNA-miRNA-disease-protein-drug graph. BMC Bioinform. 2022, 22, 621. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Sun, J.; Zhao, Q.; Shuai, J. Predicting the potential human lncRNA-miRNA interactions based on graph convolution network with conditional random field. Brief. Bioinform. 2022, 23, bbac463. [Google Scholar] [CrossRef]
- Song, J.; Tian, S.; Yu, L.; Yang, Q.; Xing, Y.; Zhang, C.; Dai, Q.; Duan, X. MD-MLI: Prediction of miRNA-lncRNA Interaction by Using Multiple Features and Hierarchical Deep Learning. IEEE-ACM Trans. Comput. Biol. Bioinform. 2022, 19, 1724–1733. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Pan, Z.; Sun, X.; Mou, M.; Zhang, B.; Li, Z.; Li, H.; Zhu, F. ncRNAInter: A novel strategy based on graph neural network to discover interactions between lncRNA and miRNA. Brief. Bioinform. 2022, 23, bbac411. [Google Scholar] [CrossRef]
- Hamdy, W.; Ismail, A.; Awad, W.A.; Ibrahim, A.H.; Hassanien, A.E. An Optimized Ensemble Deep Learning Model for Predicting Plant miRNA-IncRNA Based on Artificial Gorilla Troops Algorithm. Sensors 2023, 23, 2219. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, Z.L. PmliHFM: Predicting Plant miRNA-lncRNA Interactions with Hybrid Feature Mining Network. Interdiscip. Sci.-Comput. Life Sci. 2023, 15, 44–54. [Google Scholar] [CrossRef]
- Kang, Q.; Meng, J.; Shi, W.; Luan, Y. Ensemble Deep Learning Based on Multi-level Information Enhancement and Greedy Fuzzy Decision for Plant miRNA-lncRNA Interaction Prediction. Interdiscip. Sci.-Comput. Life Sci. 2021, 13, 603–614. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, L.; Jin, S.; Zeng, X.; Liu, X. preMLI: A pre-trained method to uncover microRNA-lncRNA potential interactions. Brief. Bioinform. 2022, 23, bbab470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Long, Y.; Kwoh, C.K. Class similarity network for coding and long non-coding RNA classification. BMC Bioinform. 2021, 22, 609. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Q.; Nie, F.; Zhao, Q.; Chen, W. DeepLncPro: An interpretable convolutional neural network model for identifying long non-coding RNA promoters. Brief. Bioinform. 2022, 23, bbac447. [Google Scholar] [CrossRef]
- Ritu; Gupta, S.; Sharma, N.K.; Shankar, R. DeepPlnc: Bi-modal deep learning for highly accurate plant lncRNA discovery. Genomics 2022, 114, 110443. [Google Scholar]
- Shi, K.; Liu, T.; Fu, H.; Li, W.; Zheng, X. Genome-wide analysis of lncRNA stability in human. PLoS Comput. Biol. 2021, 17, e1008918. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, P.; Du, H.; Cao, Y.; Peng, Q.; Fu, L. LncDLSM: Identification of Long Non-Coding RNAs With Deep Learning-Based Sequence Model. IEEE J. Biomed. Health Inform. 2023, 27, 2117–2127. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zou, B.; He, M.; Hu, Y.; Dou, Y.; Cui, T.; Tan, P.; Li, S.; Rao, S.; Huang, Y.; et al. LncReader: Identification of dual functional long noncoding RNAs using a multi-head self-attention mechanism. Brief. Bioinform. 2023, 24, bbac579. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, C.; Kwoh, C.K. Predicting the interaction biomolecule types for lncRNA: An ensemble deep learning approach. Brief. Bioinform. 2021, 22, bbaa228. [Google Scholar] [CrossRef]
- AlZubi, A.A.; Alanazi, J.M. An Optimized Technique for RNA Prediction Based on Neural Network. Intell. Autom. Soft Comput. 2023, 35, 3599–3611. [Google Scholar] [CrossRef]
- Lin, R.; Wichadakul, D. Interpretable Deep Learning Model Reveals Subsequences of Various Functions for Long Non-Coding RNA Identification. Front. Genet. 2022, 13, 876721. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wu, Y.; Lu, C.; Zhang, F.; Wu, F.X.; Li, M. DeepLncLoc: A deep learning framework for long non-coding RNA subcellular localization prediction based on subsequence embedding. Brief. Bioinform. 2022, 23, bbab360. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ding, M.; Feng, J.; Ji, B.; Huang, P.; Zhang, J.; Yu, X.; Cao, Z.; Yang, Y.; Zhou, Y.; et al. EVlncRNA-Dpred: Improved prediction of experimentally validated lncRNAs by deep learning. Brief. Bioinform. 2023, 24, bbac583. [Google Scholar] [CrossRef]
- Cai, J.; Wang, T.; Deng, X.; Tang, L.; Liu, L. GM-lncLoc: LncRNAs subcellular localization prediction based on graph neural network with meta-learning. BMC Genom. 2023, 24, 52. [Google Scholar] [CrossRef]
- Li, M.; Zhao, B.; Yin, R.; Lu, C.; Guo, F.; Zeng, M. GraphLncLoc: Long non-coding RNA subcellular localization prediction using graph convolutional networks based on sequence to graph transformation. Brief. Bioinform. 2023, 24, bbac565. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Kang, Q.; Chang, Z.; Luan, Y. PlncRNA-HDeep: Plant long noncoding RNA prediction using hybrid deep learning based on two encoding styles. BMC Bioinform. 2021, 22, 242. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Feng, R.; Kahlert, U.D.; Chen, Z.; Torres-dela Roche, L.A.; Soliman, A.; Miao, C.; De Wilde, R.L.; Shi, W. Construction of ceRNA Networks Associated With CD8 T Cells in Breast Cancer. Front. Oncol. 2022, 12, 883197. [Google Scholar] [CrossRef]
- Marete, A.; Ariel, O.; Ibeagha-Awemu, E.; Bissonnette, N. Identification of Long Non-coding RNA Isolated From Naturally Infected Macrophages and Associated With Bovine Johne’s Disease in Canadian Holstein Using a Combination of Neural Networks and Logistic Regression. Front. Vet. Sci. 2021, 8, 639053. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Chan, K.C.C.; You, Z.H.; Hu, P.; Wang, L.; Huang, Z.A. Predicting microRNA-disease associations from lncRNA-microRNA interactions via Multiview Multitask Learning. Brief. Bioinform. 2021, 22, bbaa133. [Google Scholar] [CrossRef]
- Qiu, W.; Yang, J.; Wang, B.; Yang, M.; Tian, G.; Wang, P.; Yang, J. Evaluating the Microsatellite Instability of Colorectal Cancer Based on Multimodal Deep Learning Integrating Histopathological and Molecular Data. Front. Oncol. 2022, 12, 3011. [Google Scholar] [CrossRef]
- Wang, W.; Chen, H. Predicting miRNA-disease associations based on lncRNA-miRNA interactions and graph convolution networks. Brief. Bioinform. 2023, 24, bbac495. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S. Prediction of Tumor Lymph Node Metastasis Using Wasserstein Distance-Based Generative Adversarial Networks Combing with Neural Architecture Search for Predicting. Mathematics 2023, 11, 729. [Google Scholar] [CrossRef]
- Gao, M.; Liu, S.; Qi, Y.; Guo, X.; Shang, X. GAE-LGA: Integration of multi-omics data with graph autoencoders to identify lncRNA-PCG associations. Brief. Bioinform. 2022, 23, bbac452. [Google Scholar] [CrossRef]
- Baek, J.; Lee, B.; Kwon, S.; Yoon, S. LncRNAnet: Long non-coding RNA identification using deep learning. Bioinformatics 2018, 34, 3889–3897. [Google Scholar] [CrossRef]
- Fan, X.N.; Zhang, S.W. lncRNA-MFDL: Identification of human long non-coding RNAs by fusing multiple features and using deep learning. Mol. Biosyst. 2015, 11, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Q.; Li, B.X.; Zeng, G.R.; Liu, Q.Y.; Ai, D.M. Prediction of long non-coding RNAs based on deep learning. Genes 2019, 10, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaath, H.; Vishnubalaji, R.; Elango, R.; Kardousha, A.; Islam, Z.; Qureshi, R.; Alam, T.; Kolatkar, P.R.; Alajez, N.M. Long non-coding RNA and RNA-binding protein interactions in cancer: Experimental and machine learning approaches. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Z.Y.; Ruan, S.M.; Mo, S.; Qin, J.J.; Cheng, X.D. Long non-coding RNAs towards precision medicine in gastric cancer: Early diagnosis, treatment, and drug resistance. Mol. Cancer 2020, 19, 1–22. [Google Scholar] [CrossRef]
- Alipanahi, B.; Delong, A.; Weirauch, M.T.; Frey, B.J. Predicting the sequence specificities of DNA-and RNA-binding proteins by deep learning. Nat. Biotechnol. 2015, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lopez-del Rio, A.; Martin, M.; Perera-Lluna, A.; Saidi, R. Effect of sequence padding on the performance of deep learning models in archaeal protein functional prediction. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Heward, J.A.; Lindsay, M.A. Long non-coding RNAs in the regulation of the immune response. Trends Immunol. 2014, 35, 408–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percha, B.; Altman, R.B. Learning the structure of biomedical relationships from unstructured text. PLoS Comput. Biol. 2015, 11, e1004216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Zhang, Y.; Williams, J.; Antoniou, E.; McCombie, W.R.; Wu, S.; Zhu, W.; Davidson, N.O.; Denoya, P.; Li, E. Parallel comparison of Illumina RNA-Seq and Affymetrix microarray platforms on transcriptomic profiles generated from 5-aza-deoxy-cytidine treated HT-29 colon cancer cells and simulated datasets. BMC Bioinform. 2013, 14, S1. [Google Scholar] [CrossRef] [Green Version]
- Rudin, C. Stop explaining black box machine learning models for high stakes decisions and use interpretable models instead. Nat. Mach. Intell. 2019, 1, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Zeiler, M.D.; Fergus, R. Visualizing and understanding convolutional networks. In Proceedings of the Computer Vision–ECCV 2014: 13th European Conference, Zurich, Switzerland, 6–12 September 2014; Proceedings, Part I 13. Springer: Cham, Switzerland, 2014; pp. 818–833. [Google Scholar]
- Choi, S.R.; Lee, M. Estimating the Prognosis of Low-Grade Glioma with Gene Attention Using Multi-Omics and Multi-Modal Schemes. Biology 2022, 11, 1462. [Google Scholar] [CrossRef] [PubMed]
| Research Topics | Deep Learning Approaches |
|---|---|
| Prediction of lncRNA–disease associations | ACLDA, combining autoencoders, CNN, and attention mechanism [27]; CapsNet–LDA, predicting lncRNA–disease associations using capsule network and attention [28]; DBNLDA, deep belief network-based lncRNA–disease association prediction [29]; Deep learning cluster analysis of lncRNAs in heart failure [30]; DeepMNE, deep multi-network embedding for lncRNA–disease prediction [31]; DHNLDA, deep hierarchical network with stacked autoencoder and ResNet [32]; DMFLDA, deep matrix factorization for predicting lncRNA–disease associations [33]; Dual attention network, enhances the learning of lncRNA–disease feature sets [34]; GCRFLDA, graph convolutional matrix completion-based lncRNA–disease prediction [35]; gGATLDA, lncRNA–disease associations prediction via graph-level attention networks [36]; GSMV, learning of global dependencies and multi-semantics within heterogeneous graphs [37]; GTAN, graph neural network for predicting lncRNA–disease associations [38]; HGATLDA, heterogeneous graph attention network for lncRNA–disease associations [39]; HGNNLDA, heterogeneous graph neural network for lncRNA–disease association [40]; Identifying cancer transcriptome signatures via deep learning interpretation [41]; iLncRNAdis–FB, CNN with fusing biological feature blocks [42]; LDACE, combining extreme learning machine with CNN [43]; LDICDL, identifying lncRNA–disease associations using collaborative deep learning [44]; LGDLDA, predicting disease-related lncRNAs via multiomics data and machine learning [45]; LR–GNN, graph neural network-based prediction of molecular associations [46]; MAGCNSE, lncRNA–disease association prediction via multi-view graph convolutional network [47]; MCA–Net, predicting lncRNA–disease associations using attention CNN [48]; MLMKDNN, predicting ncRNA–disease associations via deep multiple kernel learning [49]; MLGCNET, predicting lncRNA–disease associations using multi-layer graph embedding [50]; Multi-run concrete autoencoder identifying prognostic lncRNAs for cancers [51]; NELDA, predicting lncRNA–disease associations via deep autoencoder models [52]; Novel computational approach, lncRNA–disease prediction via BPSO and ML–ELM [53]; PANDA, graph convolutional autoencoders predicting novel lncRNA–disease associations [54]; Prognostic and diagnostic value of lncRNA in colorectal cancer [55]; VADLP, predicting lncRNA–disease associations with attentional multi-level encoding [56]; VGAELDA, predicting lncRNA–disease associations using variational inference and autoencoders [57]. |
| Prediction of lncRNA–protein interactions | BiHo–GNN, using bipartite graph embedding [58]; Capsule–LPI, a multichannel capsule network for lncRNA–protein interaction prediction [59]; DeepLPI, a multimodal deep learning method for lncRNA–protein isoform interactions [60]; DFRPI, deep autoencoder and marginal Fisher analysis [61]; EnANNDeep, ensemble-based framework with adaptive k-nearest neighbor for the lncRNA–protein interaction [62]; iEssLnc, graph neural network-based estimation of lncRNA gene essentiality [63]; LGFC–CNN, using deep learning with feature combination [64]; LPI–CSFFR, CNN-based lncRNA–protein interaction prediction with serial fusion and feature reuse [65]; LPI–deepGBDT, gradient boosting decision trees-based lncRNA–protein interaction identification [66]; LPI–DLDN, dual-net neural architecture for lncRNA–protein interactions prediction [67]; LPI–HyADBS, hybrid framework with DNN, XGBoost, SVM for lncRNA–protein interaction [68]; LPICGAE, predicting lncRNA–protein interactions using combined graph autoencoders [69]; PRPI–SC, ensemble deep learning for plant lncRNA–protein interactions prediction [70]; RLF–LPI, ensemble learning framework with residual LSTM and fusion attention [71]. |
| Prediction of lncRNA–miRNA interactions | BoT–Net, efficient lncRNA–miRNA interaction prediction using the bag of tricks-based neural network [72]; DeepWalk–LMI, inferring lncRNA–miRNA associations via comprehensive graph [73]; GCNCRF, predicting lncRNA–miRNA interactions using graph convolution and conditional random field [74]; MD–MLI, predicting lncRNA–miRNA interactions using multiple features and hierarchical deep learning [75]; ncRNAInter, a novel strategy using a graph neural network to discover lncRNA–miRNA interactions [76]; Optimized ensemble deep learning, predicting plant lncRNA–miRNA based on artificial gorilla troops algorithm [77]; PmliHFM, plant lncRNA–miRNA interaction prediction via hybrid feature mining network [78]; PmliPEMG, multi-level information enhancement and greedy fuzzy decision for plant lncRNA–miRNA interaction prediction [79]; preMLI, uncovering potential lncRNA–miRNA interactions through pre-training and deep feature mining [80]. |
| Classification and Prediction of lncRNA characteristics | Class similarity network, identifying lncRNAs using relationships among samples [81]; DeepLncPro, CNN for identifying lncRNA promoters [82]; DeepPlnc, high accuracy plant lncRNA identification using bimodal CNN [83]; Genome-wide analysis, exploring features related to human lncRNA stability [84]; LncDLSM, lncRNA identification using the deep learning-based sequence model [85]; LncReader, identifying dual-functional lncRNAs using multi-head self-attention [86]; lncIBTP, predicting interaction biomolecule type for a given lncRNA using ensemble deep learning [87]; RNA prediction based on neural network integration of CNN and Bi-LSTM [88]; Xlnc1DCNN, interpretable deep learning model, lncRNA identification using 1D CNN [89]. |
| Prediction of lncRNA subcellular localization | DeepLncLoc, a deep learning framework for lncRNA subcellular localization using subsequence embedding [90]; EVlncRNA–Dpred, an improved prediction method of experimentally validated lncRNAs using deep learning [91]; GM–lncLoc, lncRNA subcellular localization prediction based on graph neural network with meta-learning [92]; GraphLncLoc, predicting lncRNA subcellular localization using graph convolutional networks and sequence-to-graph transformation [93]; PlncRNA–HDeep, a plant long non-coding RNA prediction method that utilizes hybrid deep learning with two encoding styles [94]. |
| Prediction of functional roles of lncRNAs in immune response pathways | CD8–Net, constructing ceRNA networks for CD8 T cells in breast cancer [95]; JD–lncRNA–ID, identifying lncRNA associated with bovine Johne’s disease using neural networks and logistic regression [96]; |
| Deep learning applications through the utilization of lncRNA input data | MVMTMDA, predicting miRNA–disease associations through lncRNA–miRNA interactions [97]; Predicting microsatellite instability in colorectal cancer using multimodal deep learning [98]; Predicting miRNA–disease associations, a method based on lncRNA–miRNA interactions and graph convolution networks [99]; WGAN–psoNN, tumor lymph node metastasis prediction using WGAN and psoNN [100]. |
| Identification of lncRNA–protein-coding gene (PCG) associations | GAE-LGA, deep learning prediction of lncRNA–PCG associations with cross-omics correlation learning [101]. |
| Ref. | Methods | Accuracy | Merits | Disadvantages |
|---|---|---|---|---|
| [27] | ACLDA, fully connected autoencoder and CNN with attention mechanisms | AUC: 0.956 AUPR: 0.393 | Improved prediction performance; potential for disease exploration | Failure to deeply integrate topology information |
| [28] | CapsNet–LDA, attention mechanism, stacked autoencoder, adaptive allocation, CapsNet architecture | AUC: ≈0.97 | Superior performance and robustness, good generalization | Complexity due to the use of vector neurons |
| [29] | DBNLDA, node embedding, DBN, and neural network regression model | AUC: 0.96 AUPR: 0.968 | Better prediction performance, potential in disease therapy | Complexity due to diverse network structured data |
| [30] | Deep belief network for HF lncRNAs, topic model-based network cluster analysis | AUC: 0.92 | Identification of key lncRNAs, potential diagnostic biomarkers | Focused only on HF, needs wider disease scope |
| [31] | DeepMNE, deep multi-network embedding, network fusion based on deep learning | AUC: 0.9462 AUPR: 0.9505 | Superior performance in identifying new associations between lncRNAs and diseases | Complexity due to multiomics data integration |
| [32] | DHNLDA, deep hierarchical network, stacked autoencoder, ResNet, stacked ensemble module | AUC: 0.975 | High predictive performance, potential for identifying disease associations | The complexity of the hierarchical network structure |
| [33] | DMFLDA, deep matrix factorization, non-linear hidden layers | AUC: 0.8393 | Better than SIMCLDA, TPGLDA, MFLDA, LDAP; capable of complex relationship representation | More experimentation needed |
| [34] | Dual attention network method, feature fusion networks | AUC: 0.914 AUPR: 0.339 | Superior performance in recognizing potential lncRNA-disease associations across 10 categories of diseases | Not specified |
| [35] | GCRFLDA, scoring lncRNA–disease associations, graph convolution matrix completion, conditional random field | AUC: 0.9630 | Outperforms DMFLDA and LDASR; confirmed associations in case studies | Not specified |
| [36] | gGATLDA, GNN model with enclosing subgraphs and integrated feature vectors | AUC: 0.9888 AUPR: 0.9890 | High accuracy, F1-Score, stable prediction performance | Sensitivity to different datasets, possible lower accuracy and precision for some data |
| [37] | GSMV, global dependencies, semantic information, multi-view features, self-attention mechanism, dilated convolution-based learning module | AUC: 0.983 AUPR: 0.589 | Superior performance, rich semantic information extraction | Not specified |
| [38] | GTAN, GNN with three attention mechanisms, multi-layer CNN | AUC: 0.983 AUPR: 0.454 | High accuracy; potential for discovering new disease-related lncRNA candidates | Not specified |
| [39] | HGATLDA, heterogeneous graph attention network, meta-paths | AUC: 0.9424 AUPR: 0.4701 | Efficient in fusing node features, topological structures and semantic info; handles imbalance in prediction | Not specified |
| [40] | HGNNLDA, GNN, heterogeneous network, restart random walk, type-based neighbor aggregation | AUC: 0.9786 AUPR: 0.8891 | Exploits network topology for better predictions; potential for predicting new diseases | Not specified |
| [41] | Feedforward neural networks based on gene expression | Acc.: 0.9862 AUPR: 0.9988 | High prediction accuracy, useful for identifying commonly deregulated features across cancer types | Model performance can vary with fewer samples |
| [42] | iLncRNAdis–FB, fusing biological feature blocks using CNN | AUC: 0.909 AUPR: 0.363 | Better performance compared to other predictors; web server for potentially associated disease detection | Fails to remove noise and irrelevant information |
| [43] | LDACE, CNN for feature mining, ELM for prediction tasks | Acc: 0.8252 AUC: 0.8995 | Remarkable performance in cross-validation, case studies for robustness | Adequate feasibility in bioinformatics not verified |
| [44] | LDICDL, collaborative deep learning, autoencoder for feature denoising, matrix decomposition for potential association prediction | AUC: 0.8651 AUPR: 0.0306 | Outperforms other methods, handles new lncRNA or diseases | Limited matrix decomposition for prediction |
| [45] | LGDLDA, non-linear feature learning of neural networks and node representation approximation | AUC: 0.926 | Better stability and performance in cancer-related lncRNA prediction, utilized diverse data | Complexity due to diverse data integration |
| [46] | LR–GNN, GNN based on link representation for predicting molecular associations; GCN encoder for node embedding and layer-wise fusing rule for the output | AUC: 0.9474 AUPR: 0.9497 | Outperforms state-of-the-art methods in molecular association predictions, versatile in different association types | May need an optimal layer-fusing rule design for performance |
| [47] | MAGCNSE, multi-view attention graph convolutional network and stacking ensemble model | Acc.: 0.9395 | Enhanced performance in lncRNA–disease associations predictions, effective utilization of multi-view data | Complexity of the stacking ensemble model |
| [48] | MCA–Net, multi-feature coding, six similarity features, attention convolutional neural network | Acc.: 0.967 AUC: 0.994 | Outperforms state-of-the-art methods on three datasets | Requires careful tuning of model parameters |
| [49] | MLMKDNN, multi-layer multi-kernel DNN, feature matrices, kernel space mapping, DNN | AUC: 0.976 AUPR: 0.92 | High AUPR value on three types of datasets | Complexity due to multiple-feature integration |
| [50] | MLGCNET, graph convolutional network, reconstructed similarity networks, latent feature representations of nodes, extra trees method | AUC: 0.982 AUPR: 0.408 | Superior prediction performance, effective for specific diseases | Complexity due to multi-layer graph convolutional network |
| [51] | mrCAE, multi-run concrete autoencoder, lncRNA expression profiles, multiple runs | Acc.: 0.95 | Better feature selection, identified 128 lncRNAs related to 12 cancers | Stochastic nature of CAE may affect reproducibility |
| [52] | NELDA, SVM classifier-based model, deep autoencoder models, weighted average strategy | AUC: 0.9827 | Superior AUC result, high potential in disease diagnosis and treatment | Relies on quality negative samples selection |
| [53] | Novel method using BPSO and ELM models, wrapper feature extraction method | Acc.: ≈0.93 | Highest accuracy, effective in predicting key lncRNA–disease relationships | Necessity of optimal lncRNA subset selection |
| [54] | PANDA, graph-based method, heterogeneous graph, graph autoencoder, neural network for prediction | AUC: 0.976 AUPR: 0.956 | Impressive AUC-ROC, promising for predicting novel lncRNA–disease associations | Depends on the quality of graph-based information |
| [55] | Prognostic and diagnostic value of lncRNA in colorectal cancer with the classification of mRNA, lncRNA, and circRNA in exosomes | ND | Potential for exploring immune infiltration levels in CRC, diagnosis, therapy, and prognosis | No quantitative classification performance evaluation |
| [56] | VADLP, deeply embedded node attributes, weighted inter-layer and intra-layer edges, convolutional autoencoder, variance autoencoder | AUC: 0.956 AUPR: 0.449 | Improved recall rates, powerful in discovering true disease-related lncRNAs | Complex due to multiple representations |
| [57] | VGAELDA, integrates variational inference and graph autoencoders, alternate training via variational inference | AUC: 0.968 AUPR: 0.838 | Robustness and preciseness for predicting unknown lncRNA–disease associations | Complex due to the alternate training approach |
| Ref. | Methods | Accuracy | Merits | Disadvantages |
|---|---|---|---|---|
| [58] | BiHo–GNN, bipartite graph embedding based on GNN | AUC: 0.950 AUPR: 0.899 | High AUC and recall, outperforms existing methods | Not specified |
| [59] | Capsule–LPI, multimodal features, multichannel capsule network framework | AUC: 0.951 AUPR: 0.932 | Superior performance, integration of multimodal features | Absence of detailed evaluation for each feature |
| [60] | DeepLPI, interactions between lncRNAs and protein isoforms with the hybrid framework of deep neural networks | AUC: 0.866 AUPR: 0.703 | Use of isoforms, application of multiple instance learning | Lower performance metrics compared to other methods |
| [61] | DFRPI, deep autoencoder and marginal Fisher analysis, random forest-based predictor | AUC: 0.906 | Constructing a discriminative feature space, high precision | Necessity to generate a reasonable and effective feature space |
| [62] | EnANNDeep, an ensemble-based framework with an adaptive k-nearest neighbor classifier and deep models | AUC: 0.916 AUPR: 0.905 | Incorporates multiple source features, performs well in cross-validations | May produce prediction bias with single dataset evaluation |
| [63] | iEssLnc, graph neural network with meta-path-guided random walks on the lncRNA–protein interaction network | AUC: 0.912 AUPR: 0.921 | Provides quantitative essentiality scores for lncRNA genes | Specific to essential lncRNA genes, not general lncRNA–protein interactions |
| [64] | LGFC–CNN, deep learning-based prediction combining raw sequence composition, hand-designed, and structure features | AUC: 0.976 AUPR: 0.970 | Multiple-feature integration, highly accurate performance | Not specified |
| [65] | LPI–CSFFR, a feature fusion method based on CNN with feature reuse and serial fusion | AUC: 0.879 | Integrates diverse features of lncRNAs and proteins, high accuracy | Requires complex feature fusion |
| [66] | LPI–deepGBDT, multiple-layer deep framework based on gradient boosting decision trees | AUC: 0.9073 AUPR: 0.8849 | Uses diverse biological information of lncRNAs and proteins | Limited application for new lncRNAs or proteins |
| [67] | Deep learning framework with dual-net neural architecture, LPI–DLDN | AUC: 0.911 AUPR: 0.898 | Best average AUC and AUPR, outperforms six other LPI prediction methods | Requires dimension reduction for feature concatenation |
| [68] | LPI–HyADBS, AdaBoost-based feature selection, combined with DNN, XGBoost, C-SVM | AUC: 0.851 AUPR: 0.841 | Hybrid approach integrates multiple classifiers, surpasses six other models | Requires complex integration of classifiers |
| [69] | LPICGAE, combined graph autoencoders | AUC: 0.974 Acc.: 0.985 | Low-dimensional representations, outperforms six other computational methods | May need alternate loss minimization for optimal results |
| [70] | PRPI–SC, ensemble deep learning model using stacked denoising autoencoder and CNN | Acc.: 0.889 AUC: 0.950 | Predicts plant LPIs, generalizes well beyond plant data | Only reports accuracy for plant data |
| [71] | RLF–LPI, AE–ResLSTM with fuzzy decision | Acc.: 0.921 AUC: 0.980 | Potential for high performance due to the use of AE–ResLSTM and fuzzy decision | Not specified |
| Ref. | Methods | Accuracy | Merits | Disadvantages |
|---|---|---|---|---|
| [72] | BoT–Net, LSTM with DropConnect, feature pooling | Acc.: 0.8738 AUC: 0.9449 | Optimized lncRNA sequence length, improved specificity | Not specified |
| [73] | DWLMI, DeepWalk on lncRNA–miRNA–disease–protein–drug graph | Acc.: 0.9522 AUC: 0.9856 | High accuracy, incorporation of multi-dimensional data | Evaluation of each feature’s influence not discussed |
| [74] | GCNCRF, GCN with Conditional random field and attention mechanism | AUC: 0.947 | High AUC, inclusion of lncRNA/miRNA similarity network | Not specified |
| [75] | MD–MLI, hierarchical deep learning with multiple features | Acc.: 0.9859 | High accuracy, uses multiple sequence-derived features | Not specified |
| [76] | ncRNAInter, graph neural network-based RNA representation | AUC: 0.973 AUPR: 0.975 | Robust performance, universal applicability for diverse species | Not specified |
| [77] | Optimized ensemble deep learning model, leverages independent recurrent neural networks and convolutional neural networks | Acc.: 0.977 | Improved accuracy via optimal hyperparameter tuning, works with large-scale data | Not specified |
| [78] | PmliHFM, hybrid feature mining network for predicting plant miRNA–lncRNA interactions | Acc.: 0.938 AUC: 0.963 | Different encodings for miRNA and lncRNA, ensemble module integration | Not specified |
| [79] | PmliPEMG, ensemble deep learning model with multi-level information enhancement and greedy fuzzy decision | Acc.: 0.888 AUC: 0.971 | Incorporates the fusion of complex features and multi-scale convolutional long short-term memory networks | Not specified |
| [80] | preMLI, deep learning model based on rna2vec pre-training and deep feature mining mechanism | Acc.: 0.924 AUC: 0.977 | Uses rna2vec for RNA word vector representation, excellent cross-species prediction capabilities | Not specified |
| Ref. | Methods | Accuracy | Merits | Disadvantages |
|---|---|---|---|---|
| [81] | Class similarity network, Siamese neural network-inspired model | Acc: 0.9843 | Directly explores relationships among input samples, achieving high-level features | Insufficient exploration of relationship among samples |
| [82] | DeepLncPro, convolutional neural network model for identifying lncRNA promoters | Acc: 0.8622 | Superior to existing methods, can extract and analyze transcription factor binding motifs | Not specified |
| [83] | DeepPlnc, bimodal CNN-based system for plant lncRNA discovery | Acc: 0.9806 AUC: 0.9955 | High accuracy, can handle ambiguous boundaries and incomplete sequences | Not specified |
| [84] | Deep learning-based regression for genome-wide analysis of lncRNA stability | ND (Mainly focused on genome-wide analysis) | Comprehensive understanding of lncRNA stability | Absence of a detailed quantitative prediction model for half-lives |
| [85] | LncDLSM, deep learning-based sequence model for lncRNA identification | Acc: 0.9652 AUC: 0.9962 | No dependency on prior biological knowledge, can be applied to other species | Not specified |
| [86] | LncReader, multi-head self-attention mechanism | Acc.: 0.969 AUC: 0.803 AUPR: 0.265 | Excels in dual-functional lncRNA identification; superior performance compared to classical machine learning methods | Not specified |
| [87] | lncIBTP, ensemble deep learning approach | Acc.: 0.704 AUC: 0.790 AUPR: 0.642 | Novel approach to predicting interaction biomolecule type for lncRNA, performs well on 5-fold cross-validation | Does not specifically predict lncRNA functions |
| [88] | CNN and Bi–LSTM combined model for RNA prediction | Acc.: 0.977 | Superior classification effect compared to a single model, demonstrates strong generalization capacity | Not specified |
| [89] | Xlnc1DCNN, 1D convolutional neural network | Acc.: 0.945 AUC: 0.983 | Outperforms other tools in accuracy and the F1-score on the human test set, provides prediction explanations | Inconsistent annotations among public databases |
| Ref. | Methods | Merits | Disadvantages |
|---|---|---|---|
| [90] | DeepLncLoc: Uses a subsequence embedding method that keeps the order information of lncRNA sequences. Utilizes a text convolutional neural network for feature extraction and prediction. | Effective for lncRNA subcellular localization prediction. Preserves sequence order information. | Depends on the quality of subsequence embedding. Might miss some complex patterns. |
| [91] | EVlncRNA–Dpred: Uses deep learning algorithms to distinguish experimentally validated lncRNAs from mRNAs and high-throughput lncRNAs. Utilizes a three-layer deep learning neural network with a small convolutional neural network. | Provides a method for prioritizing potentially functional lncRNAs for experimental validations. | Accuracy can be limited by the small dataset sizes. |
| [92] | GM–lncLoc: Uses a graph neural network with meta-learning to predict lncRNA subcellular localization. Combines the initial sequence information with graph structure information to extract features. | Shows high accuracy, holds the potential to solve the problem of limited samples in lncRNA subcellular localization. | Performance heavily depends on the quality of the graph’s structure information. |
| [93] | GraphLncLoc: Uses graph convolutional networks to predict lncRNA subcellular localization. Transforms lncRNA sequences into de Bruijn graphs. | Can reveal sequence patterns and motifs. Demonstrates robustness against k-mer frequency features. | Transforming sequences into graphs might lead to the loss of certain information. |
| [94] | PlncRNA–HDeep: Hybrid deep learning model that uses LSTM and CNN trained on RNA sequences encoded by p-nucleotide and one-hot encodings. | Achieves high accuracy on the Zea mays dataset. More effective than traditional machine learning methods and some existing tools. | Model complexity could lead to overfitting. |
| [95] | Uses lncRNAs to develop a prognostic model that predicts the survival rates of BC patients. Constructs ceRNA networks correlated with the infiltration of CD8 T cells. | Can help understand the role of lncRNA in BC. Useful for predicting patient prognosis. | Relies on the bioinformatic prediction of CD8 T cell abundances, which might not always be accurate. |
| [96] | A combined approach using logistic regression and multilayered neural networks to identify lncRNAs related to Bovine Johne’s Disease. | Identifies potential lncRNA targets in host immunity against Mycobacterium avium infection. | Not specified |
| [97] | Multi-view multitask learning method that predicts microRNA–disease associations from lncRNA–microRNA interactions. | Developed the MVMTMDA model for predicting miRNA–disease associations, achieving an average AUC of 0.8410 ± 0.018. | Requires comprehensive lncRNA–miRNA interaction data. |
| [98] | Multimodal deep learning integrating histopathological and molecular data to evaluate the microsatellite instability of colorectal cancer. | The developed model achieves a high AUC of 0.952 when combining an H&E image with DNA methylation data. | Accuracy decreases when combining an H&E image with all types of molecular data. |
| [99] | Uses graph convolution networks with multichannel attention mechanism to predict miRNA–disease associations based on lncRNA–miRNA interactions. | Achieves average AUC values of 0.8994, 0.9032, and 0.9044 in different cross-validation setups. | Lacks comparative analysis with non-deep learning models. |
| [100] | WGAN–psoNN: Combines the Wasserstein distance-based generative adversarial network (WGAN) and particle swarm optimization neural network (psoNN) to predict lymph node metastasis events using lncRNA expression profiles. | Reduces the requirement for deep learning data quantity and architecture selection. | Uses the novel neural network architecture search (NAS) method, which is untested in other studies. |
| [101] | GAE–LGA: Uses graph autoencoders to integrate multiomics data and identify lncRNA–PCG associations. | Shows strong robustness in capturing lncRNA–PCG associations and outperforms other machine learning-based methods. | Not specified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Lee, M. Deep Learning Approaches for lncRNA-Mediated Mechanisms: A Comprehensive Review of Recent Developments. Int. J. Mol. Sci. 2023, 24, 10299. https://doi.org/10.3390/ijms241210299
Kim Y, Lee M. Deep Learning Approaches for lncRNA-Mediated Mechanisms: A Comprehensive Review of Recent Developments. International Journal of Molecular Sciences. 2023; 24(12):10299. https://doi.org/10.3390/ijms241210299
Chicago/Turabian StyleKim, Yoojoong, and Minhyeok Lee. 2023. "Deep Learning Approaches for lncRNA-Mediated Mechanisms: A Comprehensive Review of Recent Developments" International Journal of Molecular Sciences 24, no. 12: 10299. https://doi.org/10.3390/ijms241210299
APA StyleKim, Y., & Lee, M. (2023). Deep Learning Approaches for lncRNA-Mediated Mechanisms: A Comprehensive Review of Recent Developments. International Journal of Molecular Sciences, 24(12), 10299. https://doi.org/10.3390/ijms241210299







