Target and Cell Therapy for Atherosclerosis and CVD
Abstract
1. Introduction
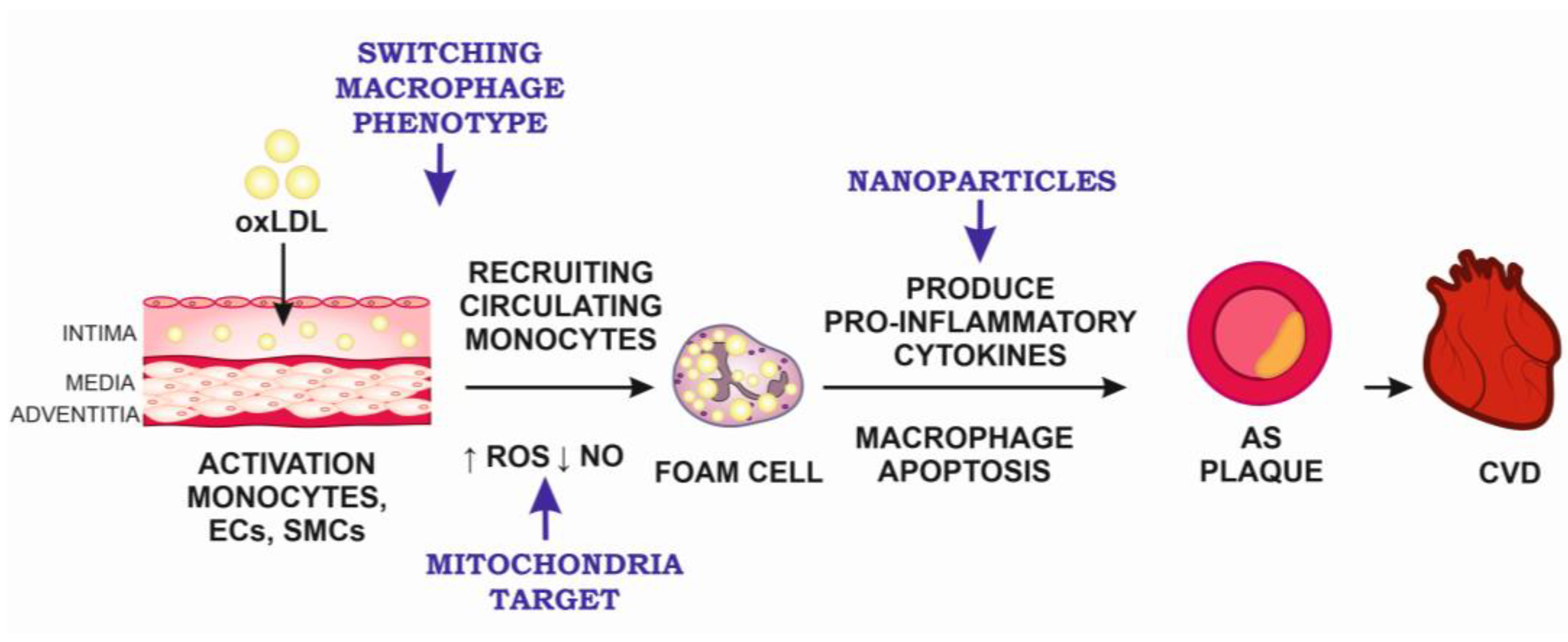
2. Target Therapy for Atherosclerosis
2.1. The Pathogenesis of Atherosclerosis at the Cellular Level
2.2. Monocyte Activation As a Target for Atherosclerosis Therapy
2.3. Mitochondrial Therapy for Atherosclerosis
2.4. Other Targets for Atherosclerosis Therapy
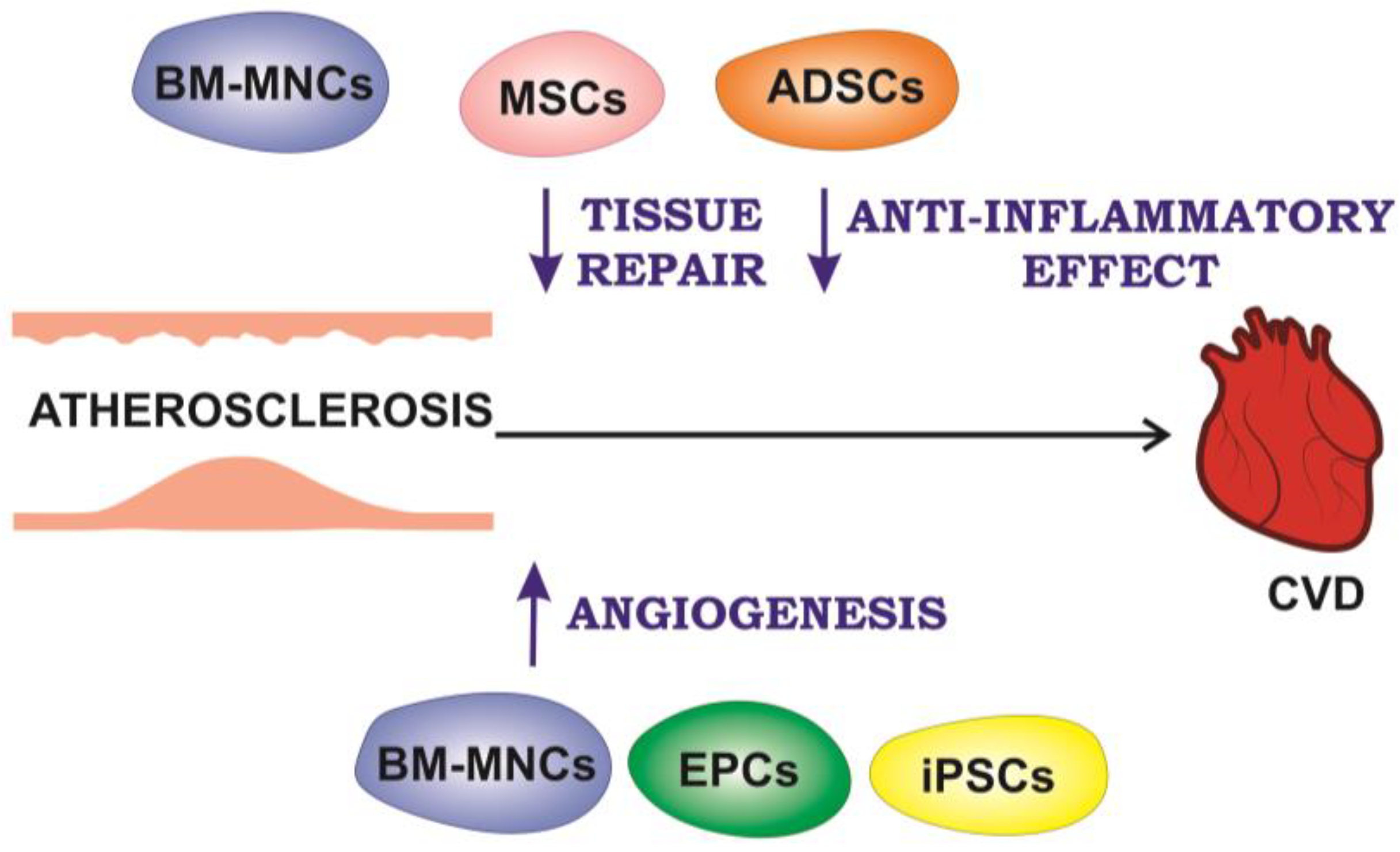
3. Cell Therapy for Atherosclerotic CVDs
3.1. Cell Therapy for Myocardial Infarction
3.2. Cell Therapy for Peripheral Arterial Disease
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and Challenges in Translating the Biology of Atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef]
- Van Camp, G. Cardiovascular disease prevention. Acta Clin. Belg. 2014, 69, 407–411. [Google Scholar] [CrossRef]
- Wei, J.; Xu, H.; Liese, A.D.; Merchant, A.T.; Wang, L.; Yang, C.; Lohman, M.C.; Brown, M.J.; Wang, T.; Friedman, D.B. Ten-Year Cardiovascular Disease Risk Score and Cognitive Function Among Older Adults: The National Health and Nutrition Examination Survey 2011 to 2014. J. Am. Heart Assoc. 2023, 12, e028527. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cell Therapy for Peripheral Artery Disease. Curr. Opin. Pharmacol. 2018, 39, 27–34. [Google Scholar] [CrossRef]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell. Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Wong, S.P.; Rowley, J.E.; Redpath, A.N.; Tilman, J.D.; Fellous, T.G.; Johnson, J.R. Pericytes, Mesenchymal Stem Cells and Their Contributions to Tissue Repair. Pharmacol Ther 2015, 151, 107–120. [Google Scholar] [CrossRef]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-Based Therapy Approaches: The Hope for Incurable Diseases. Regen. Med. 2014, 9, 649–672. [Google Scholar] [CrossRef]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell Therapy to Improve Regeneration of Skeletal Muscle Injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Zarei, F.; Abbaszadeh, A. Application of Cell Therapy for Anti-Aging Facial Skin. Curr. Stem. Cell Res. Ther. 2019, 14, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Wysoczynski, M.; Khan, A.; Bolli, R. New Paradigms in Cell Therapy: Repeated Dosing, Intravenous Delivery, Immunomodulatory Actions, and New Cell Types. Circ. Res. 2018, 123, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.H.; Smith, L.; Owens, D.; Quelch, R.; Przyborski, S. Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells. Bioengineering 2022, 9, 185. [Google Scholar] [CrossRef]
- Wolpert, L.; Tickle, C.; Martinez Arias, A. Principles of Development, 6th ed.; Oxford University Press: Oxford, UK, 2019; p. 768. [Google Scholar]
- Eiraku, M.; Takata, N.; Ishibashi, H.; Kawada, M.; Sakakura, E.; Okuda, S.; Sekiguchi, K.; Adachi, T.; Sasai, Y. Self-Organizing Optic-Cup Morphogenesis in Three-Dimensional Culture. Nature 2011, 472, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Simunovic, M.; Brivanlou, A.H. Embryoids, Organoids and Gastruloids: New Approaches to Understanding Embryogenesis. Development 2017, 144, 976–985. [Google Scholar] [CrossRef]
- Wildgruber, M.; Swirski, F.K.; Zernecke, A. Molecular Imaging of Inflammation in Atherosclerosis. Theranostics 2013, 3, 865–884. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Ni, X.; Little, P.J.; Xu, S.; Tang, L.; Weng, J. Metformin, Macrophage Dysfunction and Atherosclerosis. Front. Immunol. 2021, 12, 682853. [Google Scholar] [CrossRef]
- Markin, A.M.; Khotina, V.A.; Zabudskaya, X.G.; Bogatyreva, A.I.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. Disturbance of Mitochondrial Dynamics and Mitochondrial Therapies in Atherosclerosis. Life 2021, 11, 165. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Bezsonov, E.E.; Orekhova, V.A.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Bharadwaj, D.; Prasad, G.; Grechko, A.V.; Sazonova, M.A.; Orekhov, A.N. Anti-Inflammatory Therapy for Atherosclerosis: Focusing on Cytokines. Int. J. Mol. Sci. 2021, 22, 7061. [Google Scholar] [CrossRef] [PubMed]
- Nedosugova, L.V.; Markina, Y.V.; Bochkareva, L.A.; Kuzina, I.A.; Petunina, N.A.; Yudina, I.Y.; Kirichenko, T.V. Inflammatory Mechanisms of Diabetes and Its Vascular Complications. Biomedicines 2022, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and Its Resolution in Atherosclerosis: Mediators and Therapeutic Opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of Inflammation in the Pathogenesis of Atherosclerosis and Therapeutic Interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.; Barrett, T.J. Monocytes and Macrophages in Atherogenesis. Curr. Opin. Lipidol. 2019, 30, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Huang, C.C.; Lu, Y.; Shirazi, S.; Gajendrareddy, P.; Ravindran, S.; Cooper, L.F. Bone Regeneration Is Mediated by Macrophage Extracellular Vesicles. Bone 2020, 141, 115627. [Google Scholar] [CrossRef]
- Blagov, A.V.; Markin, A.M.; Bogatyreva, A.I.; Tolstik, T.V.; Sukhorukov, V.N.; Orekhov, A.N. The Role of Macrophages in the Pathogenesis of Atherosclerosis. Cells 2023, 12, 522. [Google Scholar] [CrossRef]
- Altabas, V.; Biloš, L.S.K. The Role of Endothelial Progenitor Cells in Atherosclerosis and Impact of Anti-Lipemic Treatments on Endothelial Repair. Int. J. Mol. Sci. 2022, 23, 2663. [Google Scholar] [CrossRef]
- Drummer, C.; Saaoud, F.; Shao, Y.; Sun, Y.; Xu, K.; Lu, Y.; Ni, D.; Atar, D.; Jiang, X.; Wang, H.; et al. Trained Immunity and Reactivity of Macrophages and Endothelial Cells. Arter. Thromb. Vasc. Biol. 2021, 41, 1032–1046. [Google Scholar] [CrossRef]
- Feng, S.; Chen, J.W.; Shu, X.Y.; Aihemaiti, M.; Quan, J.W.; Lu, L.; Zhang, R.Y.; Yang, C.D.; Wang, X.Q. Endothelial Microparticles: A Mechanosensitive Regulator of Vascular Homeostasis and Injury under Shear Stress. Front. Cell Dev. Biol. 2022, 10, 980112. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 Signaling: An Important Molecular Mechanism of Herbal Medicine in the Treatment of Atherosclerosis via the Protection of Vascular Endothelial Cells from Oxidative Stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial Function in Cardiovascular Medicine: A Consensus Paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Harman, J.L.; Jørgensen, H.F. The Role of Smooth Muscle Cells in Plaque Stability: Therapeutic Targeting Potential. Br. J. Pharmacol. 2019, 176, 3741–3753. [Google Scholar] [CrossRef] [PubMed]
- Ramel, D.; Gayral, S.; Sarthou, M.K.; Augé, N.; Nègre-Salvayre, A.; Laffargue, M. Immune and Smooth Muscle Cells Interactions in Atherosclerosis: How to Target a Breaking Bad Dialogue? Front. Pharmacol. 2019, 10, 1276. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Childs, B.G.; Li, H.; Van Deursen, J.M. Senescent Cells: A Therapeutic Target for Cardiovascular Disease. J. Clin. Invest. 2018, 128, 1217–1228. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; Van Deursen, J.M. Senescent Cells: An Emerging Target for Diseases of Ageing. Nat. Rev. Drug. Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Stojanovic, S.D.; Fiedler, J.; Bauersachs, J.; Thum, T.; Sedding, D.G. Senescence-Induced Inflammation: An Important Player and Key Therapeutic Target in Atherosclerosis. Eur. Heart J. 2020, 41, 2983–2996. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Steinfeld, N.; Ma, C.-I.J. The Formation and Consequences of Cholesterol-Rich Deposits in Atherosclerotic Lesions. Front. Cardiovasc. Med. 2023, 10, 1148304. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Song, Z.; Chen, S.; Lin, X.; Sun, H.; Zhou, P.; Peng, Q.; Du, S.; Zheng, S.; et al. Macrophage Polarization States in Atherosclerosis. Front. Immunol. 2023, 14, 1185587. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Lv, C.; Ruan, W.; He, L.; Lu, L.; Guo, X. Rosuvastatin Exerts Anti-Atherosclerotic Effects by Improving Macrophage-Related Foam Cell Formation and Polarization Conversion via Mediating Autophagic Activities. J. Transl. Med. 2021, 19, 62. [Google Scholar] [CrossRef] [PubMed]
- Brenner, C.; Franz, W.M.; Kühlenthal, S.; Kuschnerus, K.; Remm, F.; Gross, L.; Theiss, H.D.; Landmesser, U.; Kränkel, N. DPP-4 Inhibition Ameliorates Atherosclerosis by Priming Monocytes into M2 Macrophages. Int. J. Cardiol. 2015, 199, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, E.; Keyhanfar, F.; Delbandi, A.A.; Falak, R.; Hajimiresmaiel, S.J.; Shafiei, M. Dapagliflozin Exerts Anti-Inflammatory Effects via Inhibition of LPS-Induced TLR-4 Overexpression and NF-ΚB Activation in Human Endothelial Cells and Differentiated Macrophages. Eur. J. Pharmacol. 2022, 918, 174715. [Google Scholar] [CrossRef]
- Rinne, P.; Guillamat-Prats, R.; Rami, M.; Bindila, L.; Ring, L.; Lyytikainen, L.P.; Raitoharju, E.; Oksala, N.; Lehtimaki, T.; Weber, C.; et al. Palmitoylethanolamide Promotes a Proresolving Macrophage Phenotype and Attenuates Atherosclerotic Plaque Formation. Arter. Thromb. Vasc. Biol. 2018, 38, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sheng, Z.; Liu, C.; Qian, L.; Wu, Y.; Wu, Y.; Ma, G.; Yao, Y. Kallistatin Inhibits Atherosclerotic Inflammation by Regulating Macrophage Polarization. Hum. Gene Ther. 2019, 30, 339–351. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Al-Sharea, A.; Shihata, W.A.; Veiga, C.B.; Cooney, O.D.; Fleetwood, A.J.; Flynn, M.C.; Claeson, E.; Palmer, C.S.; Lancaster, G.I.; et al. Glycolysis Is Required for LPS-Induced Activation and Adhesion of Human CD14+CD16- Monocytes. Front. Immunol. 2019, 10, 2054. [Google Scholar] [CrossRef]
- Borzȩcka, K.; Płóciennikowska, A.; Björkelund, H.; Sobota, A.; Kwiatkowska, K. CD14 Mediates Binding of High Doses of LPS but Is Dispensable for TNF-α Production. Mediat. Inflamm. 2013, 2013, 824919. [Google Scholar] [CrossRef]
- Markin, A.M.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Chakal, D.A.; Breshenkov, D.G.; Charchyan, E.R. The Role of Cytokines in Cholesterol Accumulation in Cells and Atherosclerosis Progression. Int. J. Mol. Sci. 2023, 24, 6426. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Chen, Z.; Lu, E.; Wang, Z.; Duan, J.; Tian, W.; Wang, Y.; You, L.; Zou, Y.; et al. Gαi1 and Gαi3 Regulate Macrophage Polarization by Forming a Complex Containing CD14 and Gab1. Proc. Natl. Acad. Sci. USA 2015, 112, 4731–4736. [Google Scholar] [CrossRef]
- Raby, A.-C.; Labéta, M.O. Therapeutic Boosting of the Immune Response: Turning to CD14 for Help. Curr. Pharm. Biotechnol. 2016, 17, 414–418. [Google Scholar] [CrossRef]
- Yin, K.; Tang, S.L.; Yu, X.H.; Tu, G.H.; He, R.F.; Li, J.F.; Xie, D.; Gui, Q.J.; Fu, Y.C.; Jiang, Z.S.; et al. Apolipoprotein A-I Inhibits LPS-Induced Atherosclerosis in ApoE−/− Mice Possibly via Activated STAT3-Mediated Upregulation of Tristetraprolin. Acta Pharmacol. Sin. 2013, 34, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.H.; Shim, Y.J.; Tae, Y.K.; Song, J.A.; Choi, B.K.; Park, I.S.; Min, B.H. Clusterin Stimulates the Chemotactic Migration of Macrophages through a Pertussis Toxin Sensitive G-Protein-Coupled Receptor and Gβγ-Dependent Pathways. Biochem. Biophys. Res. Commun. 2014, 445, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Tucureanu, M.M.; Rebleanu, D.; Constantinescu, C.A.; Deleanu, M.; Voicu, G.; Butoi, E.; Calin, M.; Manduteanu, I. Lipopolysaccharide-Induced Inflammation in Monocytes/Macrophages Is Blocked by Liposomal Delivery of Gi-Protein Inhibitor. Int. J. Nanomed. 2017, 13, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Luo, Y.X.; Chen, H.Z.; Liu, D.P. Mitochondria, Endothelial Cell Function, and Vascular Diseases. Front. Physiol. 2014, 5, 175. [Google Scholar] [CrossRef]
- Luan, Y.; Luan, Y.; Yuan, R.X.; Feng, Q.; Chen, X.; Yang, Y. Structure and Function of Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) and Their Role in Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2021, 2021, 4578809. [Google Scholar] [CrossRef]
- Zhunina, O.A.; Yabbarov, N.G.; Grechko, A.V.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. The Role of Mitochondrial Dysfunction in Vascular Disease, Tumorigenesis, and Diabetes. Front. Mol. Biosci. 2021, 8, 671908. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Khotina, V.A.; Sukhorukov, V.N.; Kalmykov, V.A.; Mikhaleva, L.M.; Orekhov, A.N. The Role of Mitochondrial DNA Mutations in Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 952. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef]
- Li, D.; Yang, S.; Xing, Y.; Pan, L.; Zhao, R.; Zhao, Y.; Liu, L.; Wu, M. Novel Insights and Current Evidence for Mechanisms of Atherosclerosis: Mitochondrial Dynamics as a Potential Therapeutic Target. Front. Cell Dev. Biol. 2021, 9, 673839. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Fan, L.; Zhang, W.; Wang, T.; Du, Y.; Bai, X. Baicalin Alleviates Atherosclerosis by Relieving Oxidative Stress and Inflammatory Responses via Inactivating the NF-ΚB and P38 MAPK Signaling Pathways. Biomed. Pharmacother. 2018, 97, 1673–1679. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, L.; Liu, C.; Yang, J.; Zhang, J.; Huang, L. PACS2 Is Required for Ox-LDL-Induced Endothelial Cell Apoptosis by Regulating Mitochondria-Associated ER Membrane Formation and Mitochondrial Ca2+ Elevation. Exp. Cell Res. 2019, 379, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, M.; Lu, Y.; Li, J.; Ke, Y.; Yang, J. Ilexgenin A Inhibits Mitochondrial Fission and Promote Drp1 Degradation by Nrf2-Induced PSMB5 in Endothelial Cells. Drug Dev. Res. 2019, 80, 481–489. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, W.; Gao, P.; Chen, W.; Zhou, Q. Construction of Dual Nanomedicines for the Imaging and Alleviation of Atherosclerosis. Artif. Cells Nanomed. Biotechnol. 2020, 48, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Yurdagul, A.; Kong, N.; Li, W.; Wang, X.; Doran, A.C.; Feng, C.; Wang, J.; Islam, M.A.; Farokhzad, O.C.; et al. SiRNA Nanoparticles Targeting CaMKIIγ in Lesional Macrophages Improve Atherosclerotic Plaque Stability in Mice. Sci. Transl. Med. 2020, 12, eaay1063. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-Targeted Nanomedicine for the Diagnosis and Treatment of Atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Kornmueller, K.; Vidakovic, I.; Prassl, R. Artificial High Density Lipoprotein Nanoparticles in Cardiovascular Research. Molecules 2019, 24, 2829. [Google Scholar] [CrossRef]
- Oliveira, H.C.F.; Raposo, H.F. Cholesteryl Ester Transfer Protein and Lipid Metabolism and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2020, 1276, 15–25. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; Ferranti, S.D.; Després, J.P.; Fullerton, H.J.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 2016, 133, e38–e48. [Google Scholar] [CrossRef]
- An, S.; Wang, X.; Ruck, M.A.; Rodriguez, H.J.; Kostyushev, D.S.; Varga, M.; Luu, E.; Derakhshandeh, R.; Suchkov, S.V.; Kogan, S.C.; et al. Age-Related Impaired Efficacy of Bone Marrow Cell Therapy for Myocardial Infarction Reflects a Decrease in B Lymphocytes. Mol. Ther. 2018, 26, 1685–1693. [Google Scholar] [CrossRef]
- Sultana, N.; Zhang, L.; Yan, J.; Chen, J.; Cai, W.; Razzaque, S.; Jeong, D.; Sheng, W.; Bu, L.; Xu, M.; et al. Resident C-Kit+ Cells in the Heart Are Not Cardiac Stem Cells. Nat. Commun. 2015, 6, 8701. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, A.P.; Barlucchi, L.; Torella, D.; Baker, M.; Limana, F.; Chimenti, S.; Kasahara, H.; Rota, M.; Musso, E.; Urbanek, K.; et al. Adult Cardiac Stem Cells Are Multipotent and Support Myocardial Regeneration. Cell 2003, 114, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Müller, P.; Nurzynska, D.; Casarsa, C.; Torella, D.; Nascimbene, A.; Castaldo, C.; Cascapera, S.; Böhm, M.; Quaini, F.; et al. Stem Cells in the Dog Heart Are Self-Renewing, Clonogenic, and Multipotent and Regenerate Infarcted Myocardium, Improving Cardiac Function. Proc. Natl. Acad. Sci. USA 2005, 102, 8966–8971. [Google Scholar] [CrossRef]
- Ellison, G.M.; Vicinanza, C.; Smith, A.J.; Aquila, I.; Leone, A.; Waring, C.D.; Henning, B.J.; Stirparo, G.G.; Papait, R.; Scarfò, M.; et al. Adult C-Kit(Pos) Cardiac Stem Cells Are Necessary and Sufficient for Functional Cardiac Regeneration and Repair. Cell 2013, 154, 827–842. [Google Scholar] [CrossRef]
- Podaru, M.N.; Fields, L.; Kainuma, S.; Ichihara, Y.; Hussain, M.; Ito, T.; Kobayashi, K.; Mathur, A.; D’Acquisto, F.; Lewis-McDougall, F.; et al. Reparative Macrophage Transplantation for Myocardial Repair: A Refinement of Bone Marrow Mononuclear Cell-Based Therapy. Basic. Res. Cardiol. 2019, 114, 34. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zhang, R.; Liu, Z.; Cheng, Y. The Phagocytic Role of Macrophage Following Myocardial Infarction. Heart Fail. Rev. 2023. [Google Scholar] [CrossRef]
- Troidl, C.; Möllmann, H.; Nef, H.; Masseli, F.; Voss, S.; Szardien, S.; Willmer, M.; Rolf, A.; Rixe, J.; Troidl, K.; et al. Classically and Alternatively Activated Macrophages Contribute to Tissue Remodelling after Myocardial Infarction. J. Cell. Mol. Med. 2009, 13, 3485–3496. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Unda, F.; Canavate, M.-L.; Hilario, E. Stem Cell and Regenerative Medicine. Curr. Stem Cell Res. Ther. 2009, 4, 287–297. [Google Scholar] [CrossRef]
- Korf-Klingebiel, M.; Kempf, T.; Sauer, T.; Brinkmann, E.; Fischer, P.; Meyer, G.P.; Ganser, A.; Drexler, H.; Wollert, K.C. Bone Marrow Cells Are a Rich Source of Growth Factors and Cytokines: Implications for Cell Therapy Trials after Myocardial Infarction. Eur. Heart J. 2008, 29, 2851–2858. [Google Scholar] [CrossRef]
- Ma, Y.; Mouton, A.J.; Lindsey, M.L. Cardiac Macrophage Biology in the Steady-State Heart, the Aging Heart, and Following Myocardial Infarction. Transl. Res. 2018, 191, 15–28. [Google Scholar] [CrossRef]
- Malyar, N.M.; Radtke, S.; Malyar, K.; Arjumand, J.; Horn, P.A.; Kröger, K.; Freisinger, E.; Reinecke, H.; Giebel, B.; Brock, F.E. Autologous Bone Marrow Mononuclear Cell Therapy Improves Symptoms in Patients with End-Stage Peripheral Arterial Disease and Reduces Inflammation-Associated Parameters. Cytotherapy 2014, 16, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Xie, N.; Li, W.; Yuan, B.; Shi, Y.; Wang, Y. Immunobiology of Mesenchymal Stem Cells. Cell Death Differ. 2014, 21, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Kanelidis, A.J.; Premer, C.; Lopez, J.; Balkan, W.; Hare, J.M. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circ. Res. 2017, 120, 1139–1150. [Google Scholar] [CrossRef]
- Park, B.W.; Jung, S.H.; Das, S.; Lee, S.M.; Park, J.H.; Kim, H.; Hwang, J.W.; Lee, S.; Kim, H.J.; Kim, H.Y.; et al. In Vivo Priming of Human Mesenchymal Stem Cells with Hepatocyte Growth Factor-Engineered Mesenchymal Stem Cells Promotes Therapeutic Potential for Cardiac Repair. Sci. Adv. 2020, 6, eaay6994. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, M.; Sahoo, S. Exosomes in Myocardial Repair: Advances and Challenges in the Development of Next-Generation Therapeutics. Mol. Ther. 2018, 26, 1635–1643. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic Stem Cell-Derived Exosomes Promote Endogenous Repair Mechanisms and Enhance Cardiac Function Following Myocardial Infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef]
- Tongers, J.; Losordo, D.W.; Landmesser, U. Stem and Progenitor Cell-Based Therapy in Ischaemic Heart Disease: Promise, Uncertainties, and Challenges. Eur. Heart J. 2011, 32, 1197–1206. [Google Scholar] [CrossRef]
- Bevan, G.H.; White Solaru, K.T. Evidence-Based Medical Management of Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 541–553. [Google Scholar] [CrossRef]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients with Diabetes Mellitus and Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef]
- Hamburg, N.M.; Creager, M.A. Pathophysiology of Intermittent Claudication in Peripheral Artery Disease. Circ. J. 2017, 81, 281–289. [Google Scholar] [CrossRef]
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, T.S.; McDermott, M.M. Lower Extremity Peripheral Artery Disease without Chronic Limb-Threatening Ischemia: A Review. JAMA 2021, 325, 2188–2198. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, F.G.R.; Aboyans, V.; Fowkes, F.J.I.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral Artery Disease: Epidemiology and Global Perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, W.R.; Armstrong, E.J.; Larson, C.J.; Brass, E.P. Pathogenesis of the Limb Manifestations and Exercise Limitations in Peripheral Artery Disease. Circ. Res. 2015, 116, 1527–1539. [Google Scholar] [CrossRef] [PubMed]
- Koutakis, P.; Miserlis, D.; Myers, S.A.; Kim, J.K.S.; Zhu, Z.; Papoutsi, E.; Swanson, S.A.; Haynatzki, G.; Ha, D.M.; Carpenter, L.A.; et al. Abnormal Accumulation of Desmin in Gastrocnemius Myofibers of Patients with Peripheral Artery Disease: Associations with Altered Myofiber Morphology and Density, Mitochondrial Dysfunction and Impaired Limb Function. J. Histochem. Cytochem. 2015, 63, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Raval, Z.; Losordo, D.W. Cell Therapy of Peripheral Arterial Disease: From Experimental Findings to Clinical Trials. Circ. Res. 2013, 112, 1288–1302. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Alitalo, K. Molecular Regulation of Angiogenesis and Lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef]
- Annex, B.H.; Cooke, J.P. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ. Res. 2021, 128, 1944–1957. [Google Scholar] [CrossRef]
- Semenza, G.L. Oxygen Sensing, Hypoxia-Inducible Factors, and Disease Pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Qian, H.S.; Liu, P.; Huw, L.Y.; Orme, A.; Halks-Miller, M.; Hill, S.M.; Jin, F.; Kretschmer, P.; Blasko, E.; Cashion, L.; et al. Effective Treatment of Vascular Endothelial Growth Factor Refractory Hindlimb Ischemia by a Mutant Endothelial Nitric Oxide Synthase Gene. Gene Ther. 2006, 13, 1342–1350. [Google Scholar] [CrossRef]
- Ashraf, J.V.; Zen, A.A.H. Role of Vascular Smooth Muscle Cell Phenotype Switching in Arteriogenesis. Int. J. Mol. Sci. 2021, 22, 10585. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Helisch, A. Macrophages in Collateral Arteriogenesis. Front. Physiol. 2012, 3, 353. [Google Scholar] [CrossRef] [PubMed]
- Van Royen, N.; Piek, J.J.; Buschmann, I.; Hoefer, I.; Voskuil, M.; Schaper, W. Stimulation of Arteriogenesis; a New Concept for the Treatment of Arterial Occlusive Disease. Cardiovasc. Res. 2001, 49, 543–553. [Google Scholar] [CrossRef]
- Bruce, A.C.; Kelly-Goss, M.R.; Heuslein, J.L.; Meisner, J.K.; Price, R.J.; Peirce, S.M. Monocytes Are Recruited from Venules during Arteriogenesis in the Murine Spinotrapezius Ligation Model. Arter. Thromb. Vasc. Biol. 2014, 34, 2012–2022. [Google Scholar] [CrossRef]
- Varberg, K.M.; Winfree, S.; Dunn, K.W.; Haneline, L.S. Kinetic Analysis of Vasculogenesis Quantifies Dynamics of Vasculogenesis and Angiogenesis In Vitro. J. Vis. Exp. 2018, 2018, e57044. [Google Scholar] [CrossRef]
- Grundmann, S.; Hoefer, I.; Ulusans, S.; Bode, C.; Oesterle, S.; Tijssen, J.G.; Piek, J.J.; Buschmann, I.; van Royen, N. Granulocyte-Macrophage Colony-Stimulating Factor Stimulates Arteriogenesis in a Pig Model of Peripheral Artery Disease Using Clinically Applicable Infusion Pumps. J. Vasc. Surg. 2006, 43, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, M.; Avraham, I.; Dor, Y.; Bachar-Lustig, E.; Itin, A.; Yung, S.; Chimenti, S.; Landsman, L.; Abramovitch, R.; Keshet, E. VEGF-Induced Adult Neovascularization: Recruitment, Retention, and Role of Accessory Cells. Cell 2006, 124, 175–189. [Google Scholar] [CrossRef]
- Yoder, M.C. Defining Human Endothelial Progenitor Cells. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 49–52. [Google Scholar] [CrossRef]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone Marrow Origin of Endothelial Progenitor Cells Responsible for Postnatal Vasculogenesis in Physiological and Pathological Neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; Van Der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science 1997, 275, 964–967. [Google Scholar] [CrossRef]
- Teraa, M.; Sprengers, R.W.; Schutgens, R.E.G.; Slaper-Cortenbach, I.C.M.; Van Der Graaf, Y.; Algra, A.; Van Der Tweel, I.; Doevendans, P.A.; Mali, W.P.T.M.; Moll, F.L.; et al. Effect of Repetitive Intra-Arterial Infusion of Bone Marrow Mononuclear Cells in Patients with No-Option Limb Ischemia: The Randomized, Double-Blind, Placebo-Controlled Rejuvenating Endothelial Progenitor Cells via Transcutaneous Intra-Arterial Supplementation (JUVENTAS) Trial. Circulation 2015, 131, 851–860. [Google Scholar] [CrossRef]
- Ziegelhoeffer, T.; Fernandez, B.; Kostin, S.; Heil, M.; Voswinckel, R.; Helisch, A.; Schaper, W. Bone Marrow-Derived Cells Do Not Incorporate into the Adult Growing Vasculature. Circ. Res. 2004, 94, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Blood Cell Progenitors: Insights into the Properties of Stem Cells. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2004, 276, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Essaadi, A.; Nollet, M.; Moyon, A.; Stalin, J.; Simoncini, S.; Balasse, L.; Bertaud, A.; Bachelier, R.; Leroyer, A.S.; Sarlon, G.; et al. Stem Cell Properties of Peripheral Blood Endothelial Progenitors Are Stimulated by Soluble CD146 via MiR-21: Potential Use in Autologous Cell Therapy. Sci. Rep. 2018, 8, 9387. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Koyanagi, M.; Dimmeler, S. Enhancing the Outcome of Cell Therapy for Cardiac Repair: Progress from Bench to Bedside and Back. Circulation 2010, 121, 325–335. [Google Scholar] [CrossRef]
- Yunir, E.; Kurniawan, F.; Rezaprasga, E.; Wijaya, I.P.; Suroyo, I.; Matondang, S.; Irawan, C.; Soewondo, P. Autologous Bone-Marrow vs. Peripheral Blood Mononuclear Cells Therapy for Peripheral Artery Disease in Diabetic Patients. Int. J. Stem Cells 2021, 14, 21–32. [Google Scholar] [CrossRef]
- Botti, C.; Maione, C.; Coppola, A.; Sica, V.; Cobellis, G. Autologous Bone Marrow Cell Therapy for Peripheral Arterial Disease. Stem Cells Cloning 2012, 5, 5–14. [Google Scholar] [CrossRef]
- Burlacu, A. Tracking the Mesenchymal Stem Cell Fate after Transplantation into the Infarcted Myocardium. Curr. Stem Cell Res. Ther. 2013, 8, 284–291. [Google Scholar] [CrossRef]
- Huntsman, H.D.; Zachwieja, N.; Zou, K.; Ripchik, P.; Valero, M.C.; De Lisio, M.; Boppart, M.D. Mesenchymal Stem Cells Contribute to Vascular Growth in Skeletal Muscle in Response to Eccentric Exercise. Am. J. Physiol. Circ. Physiol. 2013, 304, H72–H81. [Google Scholar] [CrossRef]
- Ouma, G.O.; Jonas, R.A.; Usman, M.H.U.; Mohler, E.R. Targets and Delivery Methods for Therapeutic Angiogenesis in Peripheral Artery Disease. Vasc. Med. 2012, 17, 174–192. [Google Scholar] [CrossRef]
- Murohara, T. Autologous Adipose Tissue as a New Source of Progenitor Cells for Therapeutic Angiogenesis. J. Cardiol. 2009, 53, 155–163. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Sumi, M.; Sata, M.; Toya, N.; Yanaga, K.; Ohki, T.; Nagai, R. Transplantation of Adipose Stromal Cells, but Not Mature Adipocytes, Augments Ischemia-Induced Angiogenesis. Life Sci. 2007, 80, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Traktuev, D.O.; Merfeld-Clauss, S.; Li, J.; Kolonin, M.; Arap, W.; Pasqualini, R.; Johnstone, B.H.; March, K.L. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location, and Stabilize Endothelial Networks. Circ. Res. 2008, 102, 77–85. [Google Scholar] [CrossRef]
- Kondo, K.; Shintani, S.; Shibata, R.; Murakami, H.; Murakami, R.; Imaizumi, M.; Kitagawa, Y.; Murohara, T. Implantation of Adipose-Derived Regenerative Cells Enhances Ischemia-Induced Angiogenesis. Arter. Thromb. Vasc. Biol. 2009, 29, 61–66. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science 2007, 318, 1917–1920. [Google Scholar] [CrossRef]
- Suzuki, H.; Shibata, R.; Kito, T.; Ishii, M.; Li, P.; Yoshikai, T.; Nishio, N.; Ito, S.; Numaguchi, Y.; Yamashita, J.K.; et al. Therapeutic Angiogenesis by Transplantation of Induced Pluripotent Stem Cell-Derived Flk-1 Positive Cells. BMC Cell Biol. 2010, 11, 72. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markina, Y.V.; Kirichenko, T.V.; Tolstik, T.V.; Bogatyreva, A.I.; Zotova, U.S.; Cherednichenko, V.R.; Postnov, A.Y.; Markin, A.M. Target and Cell Therapy for Atherosclerosis and CVD. Int. J. Mol. Sci. 2023, 24, 10308. https://doi.org/10.3390/ijms241210308
Markina YV, Kirichenko TV, Tolstik TV, Bogatyreva AI, Zotova US, Cherednichenko VR, Postnov AY, Markin AM. Target and Cell Therapy for Atherosclerosis and CVD. International Journal of Molecular Sciences. 2023; 24(12):10308. https://doi.org/10.3390/ijms241210308
Chicago/Turabian StyleMarkina, Yuliya V., Tatiana V. Kirichenko, Taisiya V. Tolstik, Anastasia I. Bogatyreva, Ulyana S. Zotova, Vadim R. Cherednichenko, Anton Yu. Postnov, and Alexander M. Markin. 2023. "Target and Cell Therapy for Atherosclerosis and CVD" International Journal of Molecular Sciences 24, no. 12: 10308. https://doi.org/10.3390/ijms241210308
APA StyleMarkina, Y. V., Kirichenko, T. V., Tolstik, T. V., Bogatyreva, A. I., Zotova, U. S., Cherednichenko, V. R., Postnov, A. Y., & Markin, A. M. (2023). Target and Cell Therapy for Atherosclerosis and CVD. International Journal of Molecular Sciences, 24(12), 10308. https://doi.org/10.3390/ijms241210308





