Characterization of Electrospun BDMC-Loaded PLA Nanofibers with Drug Delivery Function and Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Results
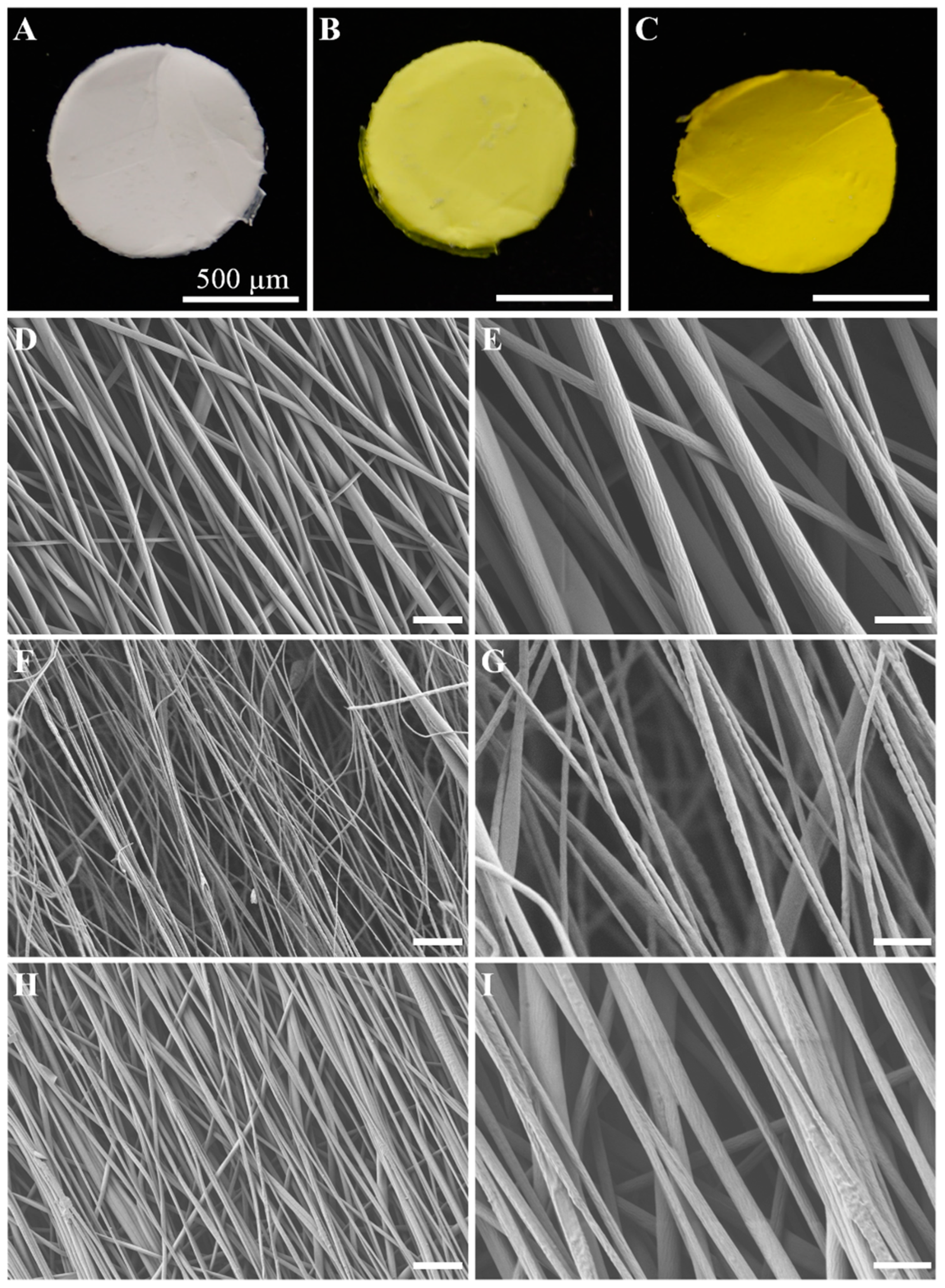
2.1. Synthesis and Morphology of the E-Spun Nanofiber
2.2. Physicochemical Characteristics
2.2.1. FTIR
2.2.2. DSC
2.2.3. TGA
2.3. In Vitro Drug Release Assay
2.4. Cell Viability
2.5. Immunocytochemistry
2.6. RT-qPCR
2.7. Inmunoblotting Assays
3. Discussion
4. Materials and Methods
4.1. Electrospun Fiber Mat on Polymer Membrane Film
4.2. Morphology Characterization by FESEM
4.3. Fourier-Transform Infrared Spectroscopy (FTIR)
4.4. Differential Scanning Calorimetry (DSC)
4.5. Thermogravimetric Analysis (TGA)
4.6. Loading Efficiency of BDMC
4.7. In Vitro Drug Release Assay
4.8. Schwann Cell Culture
4.9. MTS Cell Proliferation Assay
4.10. Immunocytochemistry
4.11. Activation of NLRP3 Inflammasome
4.12. RNA Isolation and Real-Time Quantitative PCR (RT-qPCR) Analysis
4.13. Immunoblotting Assays
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, J.; Xu, X.; Chen, X.; Liang, Q.; Bian, X.; Yang, L.; Jing, X. Biodegradable Electrospun Fibers for Drug Delivery. J. Control. Release 2003, 92, 227–231. [Google Scholar] [CrossRef]
- Trang Mai, T.T.; Thuy Nguyen, T.T.; Le, Q.D.; Nguyen, T.N.; Ba, T.C.; Nguyen, H.B.; Hoa Phan, T.B.; Tran, D.L.; Nguyen, X.P.; Park, J.S. A Novel Nanofiber Cur-Loaded Polylactic Acid Constructed by Electrospinning. Adv. Nat. Sci. Nanosci. Nanotechnol. 2012, 3, 025014. [Google Scholar] [CrossRef]
- Paolini, A.; Leoni, L.; Giannicchi, I.; Abbaszadeh, Z.; D’Oria, V.; Mura, F.; Dalla Cort, A.; Masotti, A. MicroRNAs Delivery into Human Cells Grown on 3D-Printed PLA Scaffolds Coated with a Novel Fluorescent PAMAM Dendrimer for Biomedical Applications. Sci. Rep. 2018, 8, 13888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring Recent Developments to Improve Antioxidant, Anti-Inflammatory and Antimicrobial Efficacy of Curcumin: A Review of New Trends and Future Perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Satapathy, B.K. Tuning Structural-Response of PLA/PCL Based Electrospun Nanofibrous Mats: Role of Dielectric-Constant and Electrical-Conductivity of the Solvent System. J. Biomater. Sci. Polym. Ed. 2022, 33, 1759–1793. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Fei, Y.; Wang, H.; Gao, W. Preparation and Characterization of Electrospinning PLA/Curcumin Composite Membranes. Fibers Polym. 2010, 11, 1128–1131. [Google Scholar] [CrossRef]
- Barrientos, I.J.H.; MacKenzie, G.R.; Wilson, C.G.; Lamprou, D.A.; Coats, P. Biological Performance of Electrospun Polymer Fibres. Materials 2019, 12, 363. [Google Scholar] [CrossRef] [Green Version]
- Bellan, L.M.; Craighead, H.G. Applications of Controlled Electrospinning Systems. Polym. Adv. Technol. 2011, 22, 304–309. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Kaseem, M. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Li, H.; Sureda, A.; Devkota, H.P.; Pittalà, V.; Barreca, D.; Silva, A.S.; Tewari, D.; Xu, S.; Nabavi, S.M. Curcumin, the Golden Spice in Treating Cardiovascular Diseases. Biotechnol. Adv. 2020, 38, 107343. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439807132. [Google Scholar]
- Shen, L.; Ji, H.F. The Pharmacology of Curcumin: Is It the Degradation Products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Chakraborty, S.; Karmenyan, A.; Tsai, J.W.; Chiou, A. Inhibitory effects of curcumin and cyclocurcumin in 1-methyl-4-phenylpyridinium (MPP+) induced neurotoxicity in differentiated PC12 cells. Sci. Rep. 2017, 7, 16977. [Google Scholar] [CrossRef] [Green Version]
- Cavaleri, F. Presenting a New Standard Drug Model for Turmeric and Its Prized Extract, Curcumin. Int. J. Inflamm. 2018, 2018, 5023429. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Gilani, A.-H. Therapeutic Potential of Turmeric in Alzheimer’s Disease: Curcumin or Curcuminoids? Phytother. Res. PTR 2014, 28, 517–525. [Google Scholar] [CrossRef]
- Mitra, S.; Mateti, T.; Ramakrishna, S.; Laha, A. A Review on Curcumin-Loaded Electrospun Nanofibers and Their Application in Modern Medicine. JOM 2022, 74, 3392–3407. [Google Scholar] [CrossRef]
- Ravindran, J.; Subbaraju, G.V.; Ramani, M.V.; Sung, B.; Aggarwal, B.B. Bisdemethylcurcumin and Structurally Related Hispolon Analogues of Curcumin Exhibit Enhanced Prooxidant, Anti-Proliferative and Anti-Inflammatory Activities in Vitro. Biochem. Pharmacol. 2010, 79, 1658–1666. [Google Scholar] [CrossRef] [Green Version]
- Subramani, P.A.; Panati, K.; Lebaka, V.R.; Reddy, D.D.; Narala, V.R. Nanostructures for Curcumin Delivery: Possibilities and Challenges. In Nano- and Microscale Drug Delivery Systems: Design and Fabrication; Elsevier: Amsterdam, The Netherlands, 2017; pp. 393–418. ISBN 9780323527279. [Google Scholar]
- Yeh, C.C.; Su, Y.H.; Lin, Y.J.; Chen, P.J.; Shi, C.S.; Chen, C.N.; Chang, H.I. Evaluation of the Protective Effects of Curcuminoid (Curcumin and Bisdemethoxycurcumin)-Loaded Liposomes against Bone Turnover in a Cell-Based Model of Osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 2285–2300. [Google Scholar] [CrossRef] [Green Version]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin, Tetrahydrocurcumin and Turmerones Differentially Regulate Anti-Inflammatory and Anti-Proliferative Responses through a ROS-Independent Mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Hong, H.M.; Kwon, D.D.; Pae, H.O.; Jeong, H.J. Dimethoxycurcumin, a Structural Analogue of Curcumin, Induces Apoptosis in Human Renal Carcinoma Caki Cells through the Production of Reactive Oxygen Species, the Release of Cytochrome c, and the Activation of Caspase-3. Korean J. Urol. 2010, 51, 870–878. [Google Scholar] [CrossRef] [Green Version]
- Gonz?lez-Albadalejo, J.; Sanz, D.; Claramunt, R.M.; Lavandera, J.L.; Alkorta, I.; Elguero, J. Curcumin and Curcuminoids: Chemistry, Structural Studies and Biological Properties | Curcuminay Curcuminoides: Quimica, Estudios Estructurales Ypropiedades Biologicas. An. De La Real Acad. Nac. De Farm. 2015, 81, 278–310. [Google Scholar]
- Sun, X.Z.; Williams, G.R.; Hou, X.X.; Zhu, L.M. Electrospun Curcumin-Loaded Fibers with Potential Biomedical Applications. Carbohydr. Polym. 2013, 94, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar-Mohammadi, M.; Bahrami, S.H. Electrospun Curcumin Loaded Poly(ε-Caprolactone)/Gum Tragacanth Nanofibers for Biomedical Application. Int. J. Biol. Macromol. 2016, 84, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Elakkiya, T.; Malarvizhi, G.; Rajiv, S.; Natarajan, T.S. Curcumin Loaded Electrospun Bombyx Mori Silk Nanofibers for Drug Delivery. Polym. Int. 2014, 63, 100–105. [Google Scholar] [CrossRef]
- Requejo-Aguilar, R.; Alastrue-Agudo, A.; Cases-Villar, M.; Lopez-Mocholi, E.; England, R.; Vicent, M.J.; Moreno-Manzano, V. Combined Polymer-Curcumin Conjugate and Ependymal Progenitor/Stem Cell Treatment Enhances Spinal Cord Injury Functional Recovery. Biomaterials 2017, 113, 18–30. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Gao, Y.; Liu, Y.; Yu, D.; Liu, P. Electrospun Core–Sheath Nanofibers with Variable Shell Thickness for Modifying Curcumin Release to Achieve a Better Antibacterial Performance. Biomolecules 2022, 12, 1057. [Google Scholar] [CrossRef]
- Sherwood, E.R.; Toliver-Kinsky, T. Mechanisms of the Inflammatory Response. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 385–405. [Google Scholar] [CrossRef]
- Gong, Z.; Zhou, J.; Li, H.; Gao, Y.; Xu, C.; Zhao, S.; Chen, Y.; Cai, W.; Wu, J. Curcumin Suppresses NLRP3 Inflammasome Activation and Protects against LPS-Induced Septic Shock. Mol. Nutr. Food Res. 2015, 59, 2132–2142. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.D. Regulation of inflammasome activation. Immunol. Rev. 2015, 265, 6–21. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell. Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef] [Green Version]
- Xiang, P.; Chen, T.; Mou, Y.; Wu, H.; Xie, P.; Lu, G.; Gong, X.; Hu, Q.; Zhang, Y.; Ji, H. NZ Suppresses TLR4/NF-ΚB Signalings and NLRP3 Inflammasome Activation in LPS-Induced RAW264.7 Macrophages. Inflamm. Res. 2015, 64, 799–808. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; Wu, B.N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Ghonime, M.G.; Shamaa, O.R.; Das, S.; Eldomany, R.A.; Fernandes-Alnemri, T.; Alnemri, E.S.; Gavrilin, M.A.; Wewers, M.D. Inflammasome Priming by Lipopolysaccharide Is Dependent upon ERK Signaling and Proteasome Function. J. Immunol. 2014, 192, 3881–3888. [Google Scholar] [CrossRef] [Green Version]
- Shao, B.Z.; Xu, Z.Q.; Han, B.Z.; Su, D.F.; Liu, C. NLRP3 Inflammasome and Its Inhibitors: A Review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef] [Green Version]
- Tello Velasquez, J.; Nazareth, L.; Quinn, R.J.; Ekberg, J.A.K.; St John, J.A. Stimulating the Proliferation, Migration and Lamellipodia of Schwann Cells Using Low-Dose Curcumin. Neuroscience 2016, 324, 140–150. [Google Scholar] [CrossRef]
- Khadka, D.B.; Haynie, D.T. Protein- and Peptide-Based Electrospun Nanofibers in Medical Biomaterials. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1242–1262. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-Functionalized Electrospun Nanofibers for Tissue Engineering and Drug Delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Thenmozhi, S.; Dharmaraj, N.; Kadirvelu, K.; Kim, H.Y. Electrospun Nanofibers: New Generation Materials for Advanced Applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 217, 36–48. [Google Scholar] [CrossRef]
- Pankongadisak, P.; Sangklin, S.; Chuysinuan, P.; Suwantong, O.; Supaphol, P. The Use of Electrospun Curcumin-Loaded Poly(L-Lactic Acid) Fiber Mats as Wound Dressing Materials. J. Drug Deliv. Sci. Technol. 2019, 53, 101121. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Li, Q. Curcumin Accelerates the Repair of Sciatic Nerve Injury in Rats through Reducing Schwann Cells Apoptosis and Promoting Myelinization. Biomed. Pharmacother. 2017, 92, 1103–1110. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. The Effect of the Alignment of Electrospun Fibrous Scaffolds on Schwann Cell Maturation. Biomaterials 2008, 29, 653–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, S.L.; Kang, S.G.; Chin, I.J. Characterization of Cellulose Fibers Electrospun Using Ionic Liquid. Cellulose 2010, 17, 223–230. [Google Scholar] [CrossRef]
- Choktaweesap, N.; Arayanarakul, K.; Aht-Ong, D.; Meechaisue, C.; Supaphol, P. Electrospun Gelatin Fibers: Effect of Solvent System on Morphology and Fiber Diameters. Polym. J. 2007, 39, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, K.M.; Yoon, C.; Giger, R.J.; Sakamoto, J. Engineering a Platform for Nerve Regeneration with Direct Application to Nerve Repair Technology. Biomaterials 2019, 216, 119263. [Google Scholar] [CrossRef]
- Wang, W.; Itoh, S.; Konno, K.; Kikkawa, T.; Ichinose, S.; Sakai, K.; Ohkuma, T.; Watabe, K. Effects of Schwann Cell Alignment along the Oriented Electrospun Chitosan Nanofibers on Nerve Regeneration. J. Biomed. Mater. Res. Part A 2009, 91, 994–1005. [Google Scholar] [CrossRef]
- Jeliński, T.; Przybyłek, M.; Cysewski, P. Natural Deep Eutectic Solvents as Agents for Improving Solubility, Stability and Delivery of Curcumin. Pharm. Res. 2019, 36, 116. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Development of a Novel Ionic Liquid-Curcumin Complex to Enhance Its Solubility, Stability, and Activity. Chem. Commun. 2019, 55, 7737–7740. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Si, J.; Kang, C.; Chung, B.; Chung, D.; Kim, D. Facile Preparation of Water Soluble Curcuminoids Extracted from Turmeric (Curcuma longa L.) Powder by Using Steviol Glucosides. Food Chem. 2017, 214, 366–373. [Google Scholar] [CrossRef] [Green Version]
- Hoehle, S.I.; Pfeiffer, E.; Sólyom, A.M.; Metzler, M. Metabolism of Curcuminoids in Tissue Slices and Subcellular Fractions from Rat Liver. J. Agric. Food Chem. 2006, 54, 756–764. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Read, M.I.; Bland, A.R.; Majeed, M.; Jamialahmadi, T.; Sahebkar, A. Curcumin: An Inflammasome Silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The Beneficial Role of Curcumin on Inflammation, Diabetes and Neurodegenerative Disease: A Recent Update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, Inflammation, and Chronic Diseases: How Are They Linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef] [Green Version]
- Shishodia, S.; Amin, H.M.; Lai, R.; Aggarwal, B.B. Curcumin (Diferuloylmethane) Inhibits Constitutive NF-ΚB Activation, Induces G1/S Arrest, Suppresses Proliferation, and Induces Apoptosis in Mantle Cell Lymphoma. Biochem. Pharmacol. 2005, 70, 700–713. [Google Scholar] [CrossRef]
- Coelho, M.R.; Romi, M.D.; Ferreira, D.M.T.P.; Zaltman, C.; Soares-Mota, M. The Use of Curcumin as a Complementary Therapy in Ulcerative Colitis: A Systematic Review of Randomized Controlled Clinical Trials. Nutrients 2020, 12, 2296. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, Pharmaceutical, Nutraceutical, and Analytical Aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef] [Green Version]
- Naksuriya, O.; Okonogi, S.; Schiffelers, R.M.; Hennink, W.E. Curcumin Nanoformulations: A Review of Pharmaceutical Properties and Preclinical Studies and Clinical Data Related to Cancer Treatment. Biomaterials 2014, 35, 3365–3383. [Google Scholar] [CrossRef]
- Sonseca, A.; Peponi, L.; Sahuquillo, O.; Kenny, J.M.; Giménez, E. Electrospinning of Biodegradable Polylactide/Hydroxyapatite Nanofibers: Study on the Morphology, Crystallinity Structure and Thermal Stability. Polym. Degrad. Stab. 2012, 97, 2052–2059. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled Release of Curcumin from Electrospun Fiber Mats with Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]









| Gene | Primer Forward | Primer Reverse |
|---|---|---|
| NF-kβ | GGCAGAAGTCAACGCTCAG | GGTGTCGTCCCATCGTAGGT |
| TNF-α | TCATTCAAGGGCTGGTGAG | CGGCTTTGTGGAGGATTC |
| NLRP3 | GAAGATTACCCACCCGAGAA | CCCAGCAAACCTATCCACTC |
| Casp-1 | GCAAGCCAGATGTTTATCACTT | CGCCACCTTCTTTGTTCACA |
| IL-18 | ACAGCCAACGAATCCCAGAC | GACATCCTTCCATCCTTCACA |
| GAPDH | AGACAGCCGCATCTTCTTGT | CTTGCCGTGGGTAGAGTCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morillo-Bargues, M.J.; Osorno, A.O.; Guerri, C.; Pradas, M.M.; Martínez-Ramos, C. Characterization of Electrospun BDMC-Loaded PLA Nanofibers with Drug Delivery Function and Anti-Inflammatory Activity. Int. J. Mol. Sci. 2023, 24, 10340. https://doi.org/10.3390/ijms241210340
Morillo-Bargues MJ, Osorno AO, Guerri C, Pradas MM, Martínez-Ramos C. Characterization of Electrospun BDMC-Loaded PLA Nanofibers with Drug Delivery Function and Anti-Inflammatory Activity. International Journal of Molecular Sciences. 2023; 24(12):10340. https://doi.org/10.3390/ijms241210340
Chicago/Turabian StyleMorillo-Bargues, María José, Andrea Olivos Osorno, Consuelo Guerri, Manuel Monleón Pradas, and Cristina Martínez-Ramos. 2023. "Characterization of Electrospun BDMC-Loaded PLA Nanofibers with Drug Delivery Function and Anti-Inflammatory Activity" International Journal of Molecular Sciences 24, no. 12: 10340. https://doi.org/10.3390/ijms241210340
APA StyleMorillo-Bargues, M. J., Osorno, A. O., Guerri, C., Pradas, M. M., & Martínez-Ramos, C. (2023). Characterization of Electrospun BDMC-Loaded PLA Nanofibers with Drug Delivery Function and Anti-Inflammatory Activity. International Journal of Molecular Sciences, 24(12), 10340. https://doi.org/10.3390/ijms241210340





